Novel Ruthenium-Silver PTA-Based Polymers and Their Behavior in Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
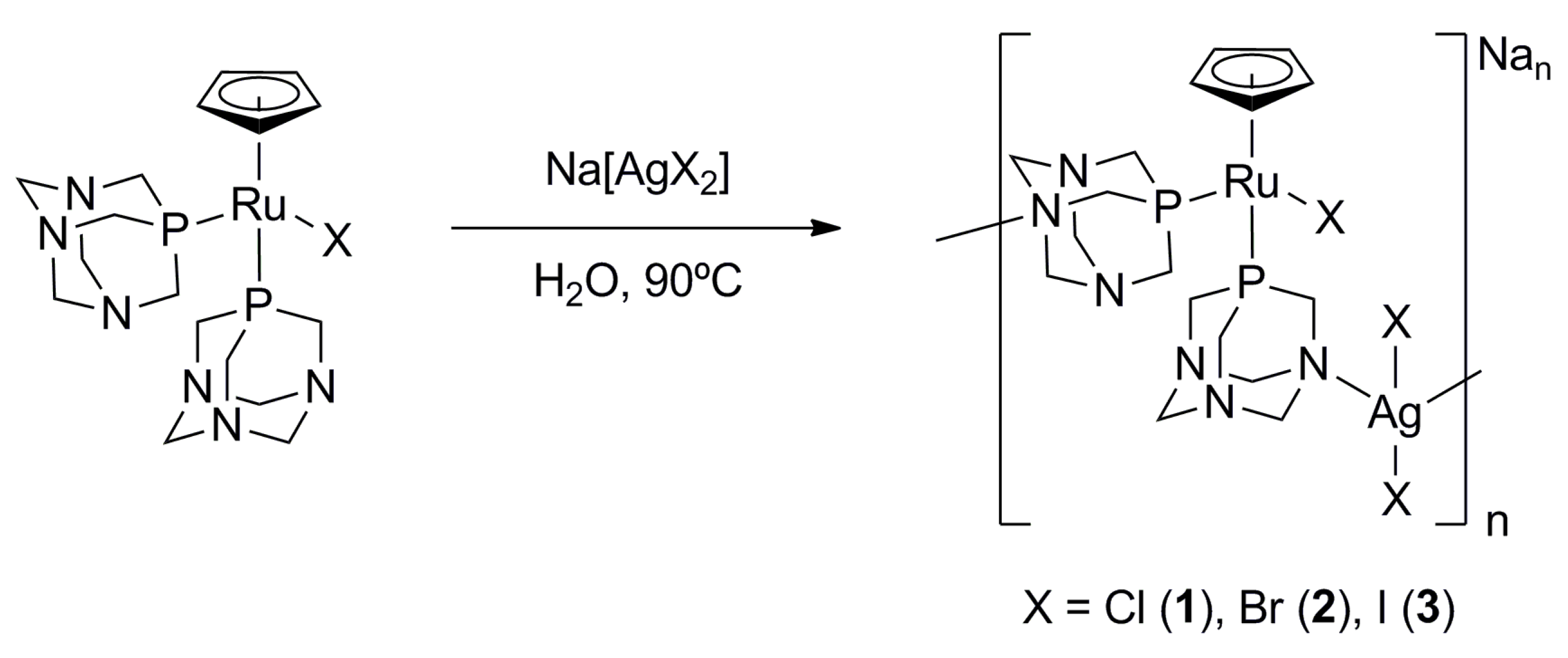
3.1. Synthesis of the Coordination Polymers
3.1.1. Synthesis of System-1): Na[RuCpCl(PTA)-μ-(PTA)-1κP:2κ2N-AgCl2]n
3.1.2. Synthesis of System-2 and System-3: Na[RuCpBr(PTA)-![Polymers 11 01249 i001 Polymers 11 01249 i001]() -(PTA)-1|P:2|2N-AgBr2]n (System-2), Na[RuCpI(PTA)-
-(PTA)-1|P:2|2N-AgBr2]n (System-2), Na[RuCpI(PTA)-![Polymers 11 01249 i001 Polymers 11 01249 i001]() -(PTA)-1|P:2|2N-AgI2]n (System-3)
-(PTA)-1|P:2|2N-AgI2]n (System-3)
3.2. Chemical Characterization
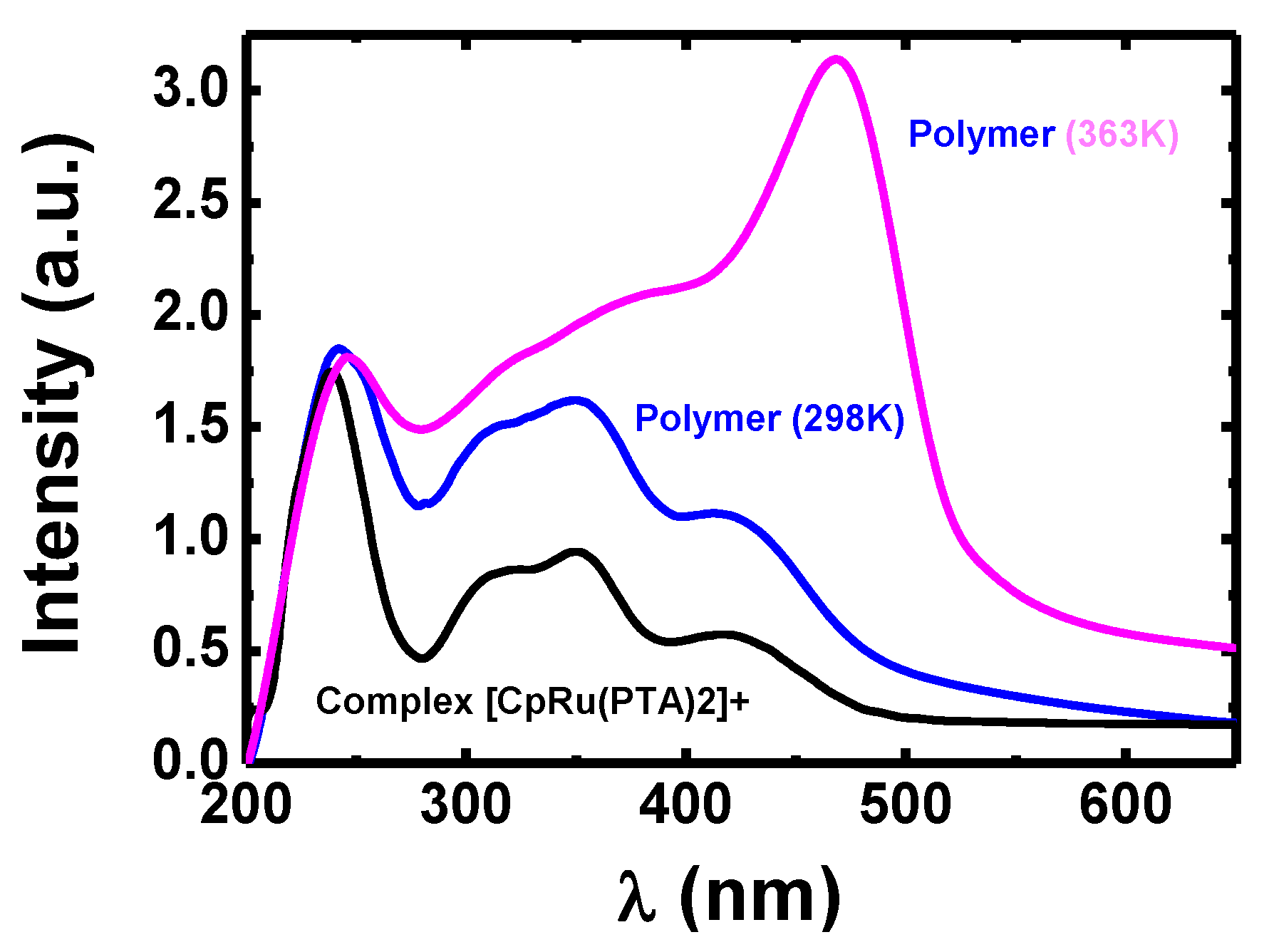
3.3. Optical Properties
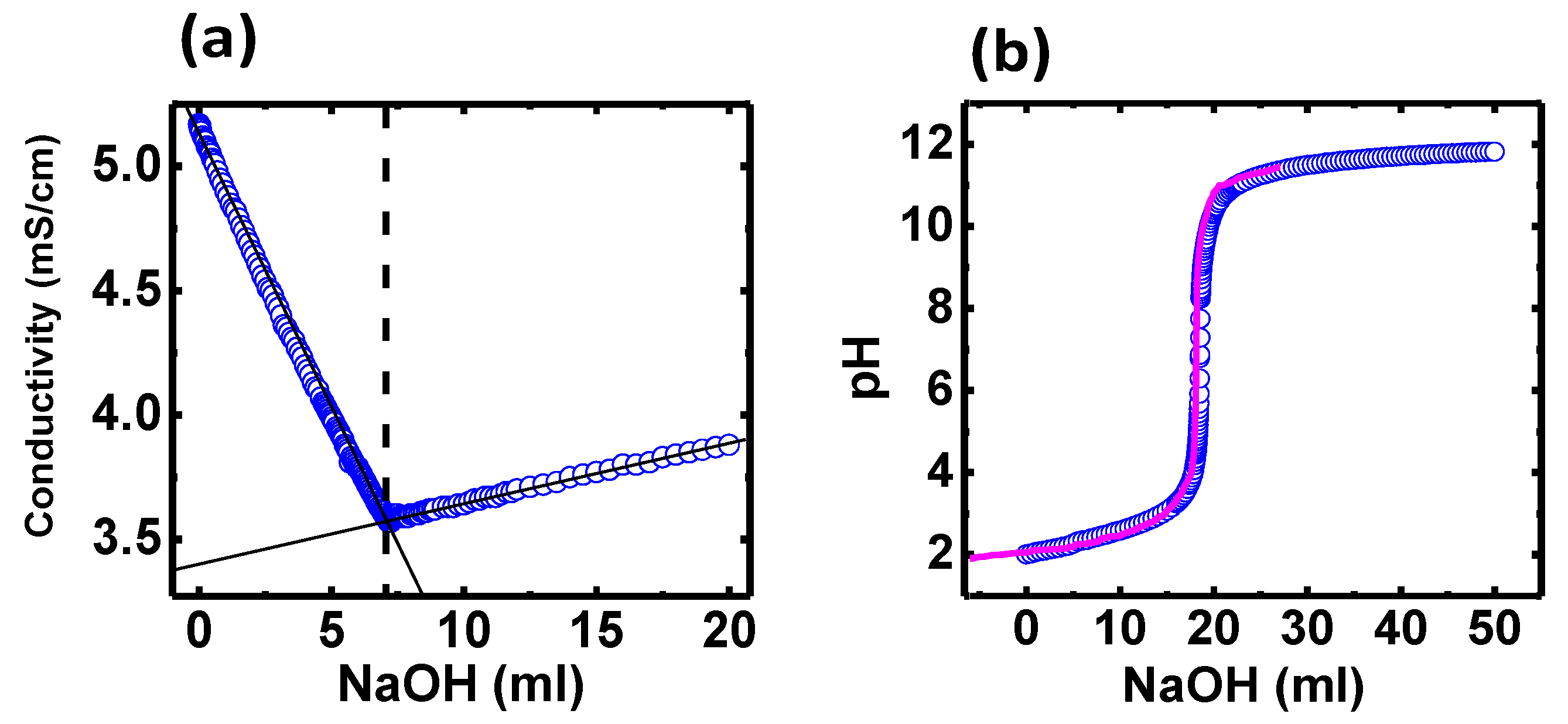
3.4. Non-Ionic Character of the Polymer
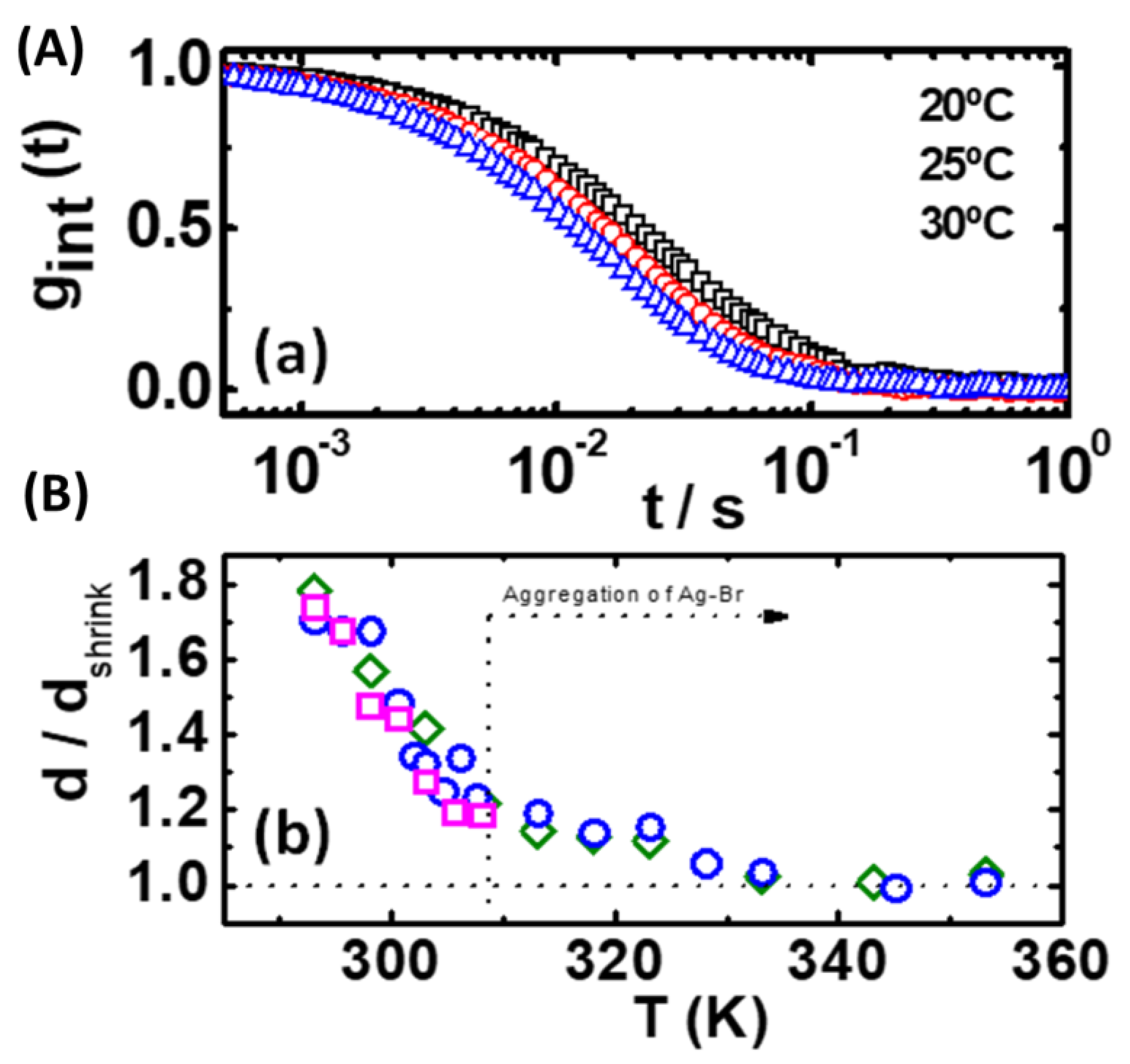
3.5. Polymer Self-Assembling
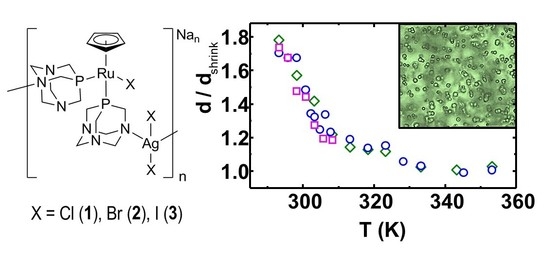
3.6. Polymer De-Swelling
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lidrissi, C.; Romerosa, A.; Saoud, M.; Serrano-Ruiz, M.; Gonsalvi, L.; Peruzzini, M. Stable, water-soluble Pta-based Ru–Ag organometallic polymers. Angew. Chem. Int. Ed. 2005, 44, 2568–2572. [Google Scholar] [CrossRef] [PubMed]
- Frost, B.J.; Mebi, C.A.; Gingrich, P.W. Boron-nitrogen adducts of 1,3,5-triaza-7-phosphaadamantane (PTA): Synthesis, reactivity, and molecular structure. Eur. J. Inorg. Chem. 2006, 2006, 1182–1189. [Google Scholar] [CrossRef]
- Serrano Ruiz, M.; Romerosa, A.; Sierra-Martin, B.; Fernandez-Barbero, A. A water soluble diruthenium-gold organometallic microgel. Angew. Chem. Int. Ed. 2008, 47, 8665–8669. [Google Scholar] [CrossRef] [PubMed]
- Scalambra, F.; Serrano-Ruiz, M.; Romerosa, A. First water-soluble backbone Ru–Ru–Ni heterometallic organometallic polymer. Macromol. Rapid Commun. 2015, 36, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Scalambra, F.; Serrano-Ruiz, M.; Gudat, D.; Romerosa, A. Amorphization of a Ru–Ru–Cd-Coordination Polymer at Low Pressure. ChemistrySelect 2016, 1, 901–905. [Google Scholar] [CrossRef]
- Scalambra, F.; Serrano-Ruiz, M.; Romerosa, A. Water driven formation of nano-channels: Unusual solid-state structural transformation of a heterometallic polymer. Dalton Trans. 2018, 47, 3588–3595. [Google Scholar] [CrossRef] [PubMed]
- Spokoyny, A.M.; Kim, D.; Sumrein, A.; Mirkin, C.A. Infinite coordination polymer nano- and microparticle structures. Chem. Soc. Rev. 2009, 38, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Mohr, F.; Falvello, L.R.; Laguna, M. A silver(I) coordination polymer containing tridentate N- and P-coordinating 1,3,5-triaza-7-phosphaadamantane (PTA) ligands. Eur. J. Inorg. Chem. 2006, 2006, 3152–3154. [Google Scholar] [CrossRef]
- Frost, B.J.; Bautista, C.M.; Huang, R.; Shearer, J. Manganese complexes of 1,3,5-triaza-7-phosphaadamantane (PTA): The first nitrogen-bound transition-metal complex of PTA. Inorg. Chem. 2006, 45, 3481–3483. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Filipowicz, M.; Guedes Da Silva, M.F.C.; Kłak, J.; Smoleński, P.; Pombeiro, A.J.L. Unprecedented mixed-valence Cu(I)/Cu(II) complex derived from N-methyl-1,3,5-triaza-7-phosphaadamantane: Synthesis, structural features, and magnetic properties. Organometallics 2012, 31, 7921–7925. [Google Scholar] [CrossRef]
- Scalambra, F.; Serrano-Ruiz, M.; Romerosa, A. Water and catalytic isomerization of linear allylic alcohols by [RuCp(H2O-κO)(PTA)2]+(PTA = 1,3,5-triaza-7-phosphaadamantane). Dalton Trans. 2017, 46, 5864–5871. [Google Scholar] [CrossRef]
- Romerosa, A.; Campos-Malpartida, T.; Lidrissi, C.; Saoud, M.; Serrano-Ruiz, M.; Peruzzini, M.; Garrido-Cárdenas, J.A.; García-Maroto, F. Synthesis, characterization, and DNA binding of new water-soluble cyclopentadienyl ruthenium(II) complexes incorporating phosphines. Inorg. Chem. 2006, 45, 1289–1298. [Google Scholar] [CrossRef]
- Akbayeva, D.N.; Gonsalvi, L.; Oberhauser, W.; Peruzzini, M.; Vizza, F.; Brüggeller, P.; Romerosa, A.; Sava, G.; Bergamo, A. Synthesis, catalytic properties and biological activity of new water soluble ruthenium cyclopentadienyl PTA complexes [(C5R5)RuCl(PTA)2] (R = H, Me; PTA = 1,3,5-triaza-7-phosphaadamantane). Chem. Commun. 2003, 264–265. [Google Scholar] [CrossRef]
- Serrano-Ruiz, M.; Lorenzo-Luis, P.; Romerosa, A. Easy synthesis and water solubility of ruthenium complexes containing PPh3, mTPPMS, PTA and mPTA, (mTPPMS = meta-triphenyphosphine monosulfonate, PTA = 1,3,5-triaza-7-phosphaadamantane, mPTA = N-methyl-1,3,5-triaza-7-phosphaadamantane). Inorg. Chim. Acta 2017, 455, 528–534. [Google Scholar] [CrossRef]
- Sierra-Martin, B.; Serrano-Ruiz, M.; García-Sakai, V.; Scalambra, F.; Romerosa, A.; Fernandez-Barbero, A. Self-organization and swelling of ruthenium-metal coordination polymers with PTA (metal = Ag, Au, Co). Polymers 2018, 10, 528. [Google Scholar] [CrossRef]
- Serrano-Ruiz, M.; Imberti, S.; Bernasconi, L.; Jadagayeva, N.; Scalambra, F.; Romerosa, A. Study of the interaction of water with the aqua-soluble dimeric complex [RuCp(PTA)2-μ-CN-1κC:2κ(2)N-RuCp(PTA)2](CF3SO3) (PTA = 1,3,5-triaza-7-phosphaadamantane) by neutron and X-ray diffraction in solution. Chem. Commun. 2014, 50, 11587–11590. [Google Scholar] [CrossRef]
- Gossens, C.; Dorcier, A.; Dyson, P.J.; Rothlisberger, U. pKa estimation of ruthenium(II)-arene PTA complexes and their hydrolysis products via a DFT/continuum electrostatics approach. Organometallics 2007, 26, 3969–3975. [Google Scholar] [CrossRef]
- Scalambra, F.; Holzmann, N.; Bernasconi, L.; Imberti, S.; Romerosa, A. Water Participation in Catalysis: An Atomistic Approach to Solvent Effects in the Catalytic Isomerization of Allylic Alcohols. ACS Catal. 2018, 8, 3812–3819. [Google Scholar] [CrossRef]
- Saeed, M.A.; Wong, B.M.; Fronczek, F.R.; Venkatraman, R.; Hossain, M.A. Formation of an amine-water cyclic pentamer: A new type of water cluster in a polyazacryptand. Cryst. Growth Des. 2010, 10, 1486–1488. [Google Scholar] [CrossRef]
- Denton, A.R.; Tang, Q. Counterion-induced swelling of ionic microgels. J. Chem. Phys. 2016, 145, 164901. [Google Scholar] [CrossRef]
- Capriles-González, D.; Sierra-Martín, B.; Fernández-Nieves, A.; Fernández-Barbero, A. Coupled deswelling of multiresponse microgels. J. Phys. Chem. B 2008, 112, 12195–12200. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sierra-Martin, B.; Serrano-Ruiz, M.; Scalambra, F.; Fernandez-Barbero, A.; Romerosa, A. Novel Ruthenium-Silver PTA-Based Polymers and Their Behavior in Water. Polymers 2019, 11, 1249. https://doi.org/10.3390/polym11081249
Sierra-Martin B, Serrano-Ruiz M, Scalambra F, Fernandez-Barbero A, Romerosa A. Novel Ruthenium-Silver PTA-Based Polymers and Their Behavior in Water. Polymers. 2019; 11(8):1249. https://doi.org/10.3390/polym11081249
Chicago/Turabian StyleSierra-Martin, Benjamin, Manuel Serrano-Ruiz, Franco Scalambra, Antonio Fernandez-Barbero, and Antonio Romerosa. 2019. "Novel Ruthenium-Silver PTA-Based Polymers and Their Behavior in Water" Polymers 11, no. 8: 1249. https://doi.org/10.3390/polym11081249
APA StyleSierra-Martin, B., Serrano-Ruiz, M., Scalambra, F., Fernandez-Barbero, A., & Romerosa, A. (2019). Novel Ruthenium-Silver PTA-Based Polymers and Their Behavior in Water. Polymers, 11(8), 1249. https://doi.org/10.3390/polym11081249