Enhancing the Interfacial Adhesion with Rubber Matrix by Grafting Polydopamine-Carbon Nanotubes onto Poly(p-phenylene terephthalamide) Fibers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The “One-Step” Method of Surface Modification of PPTA Fibers
2.3. The “Two-Step” Method of Surface Modification of PPTA Fibers
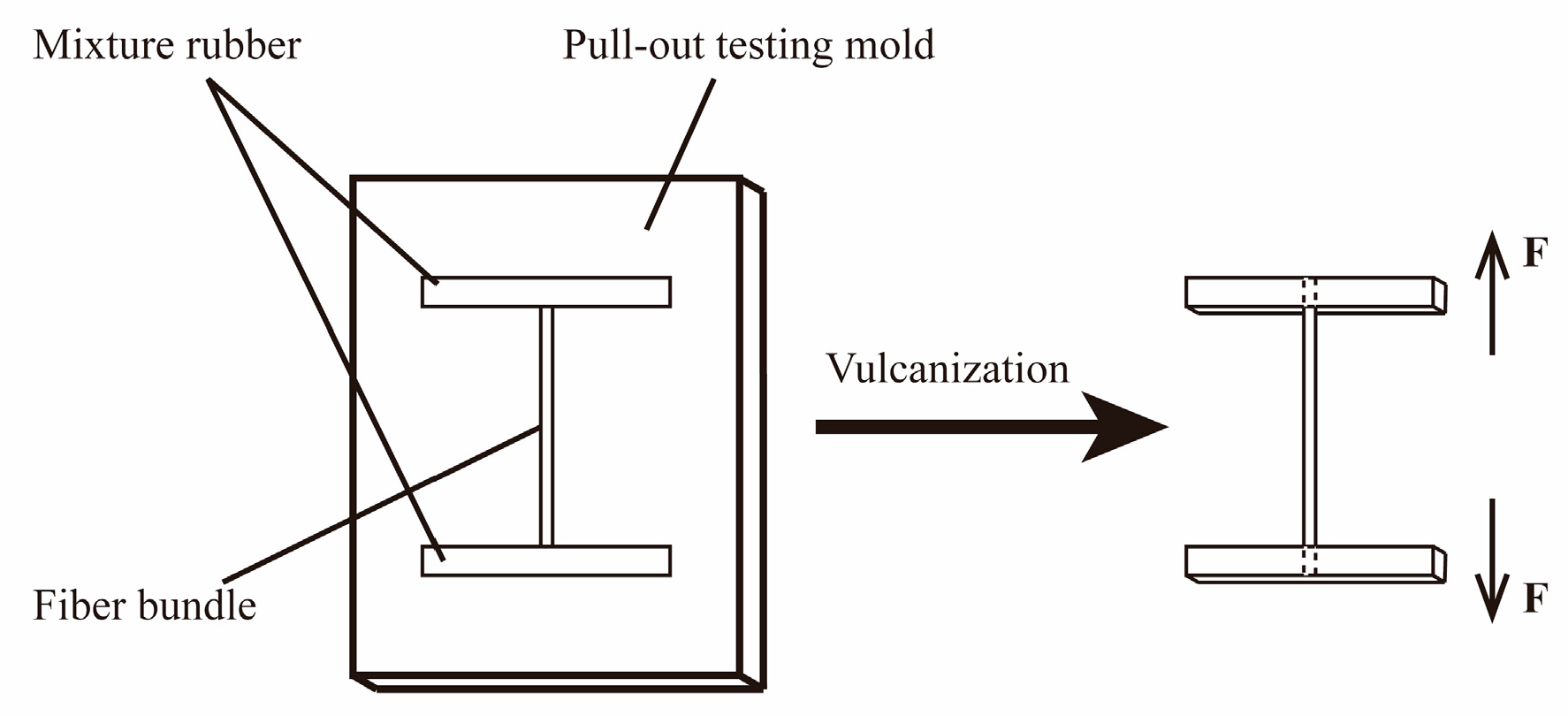
2.4. Preparation of PPTA Fibers/Rubber Composites
2.5. Characterizations
3. Results and Discussion
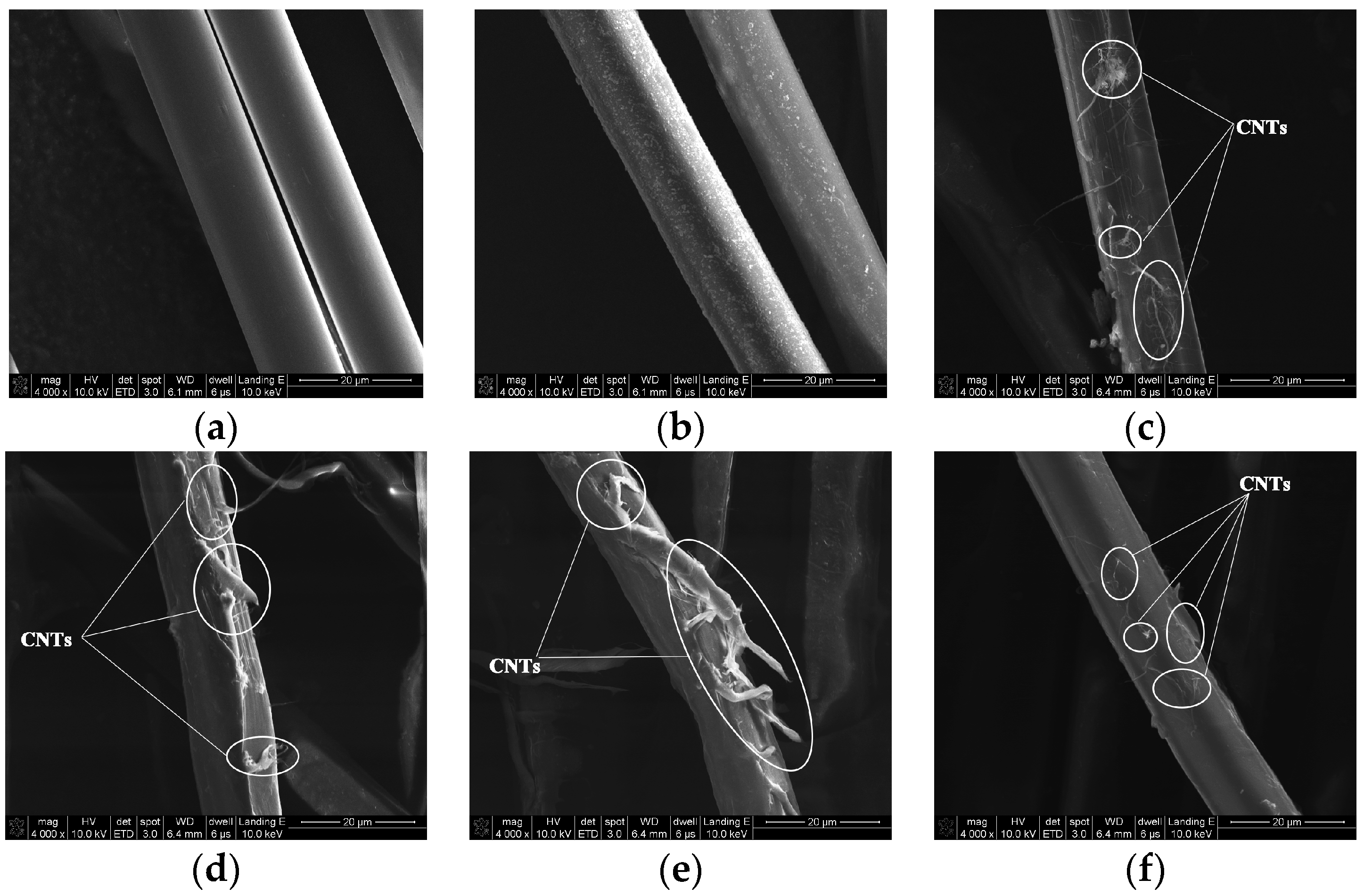
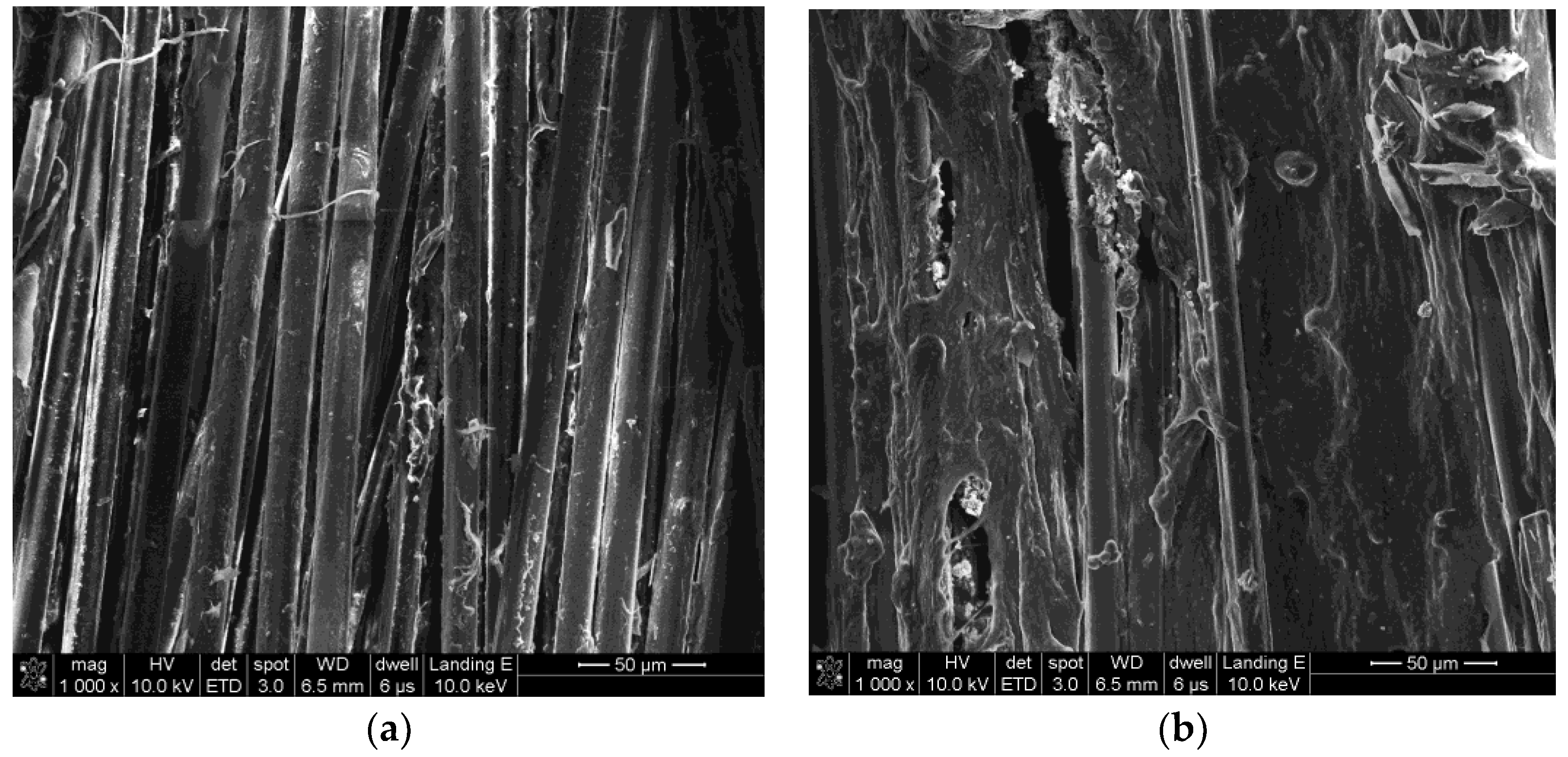
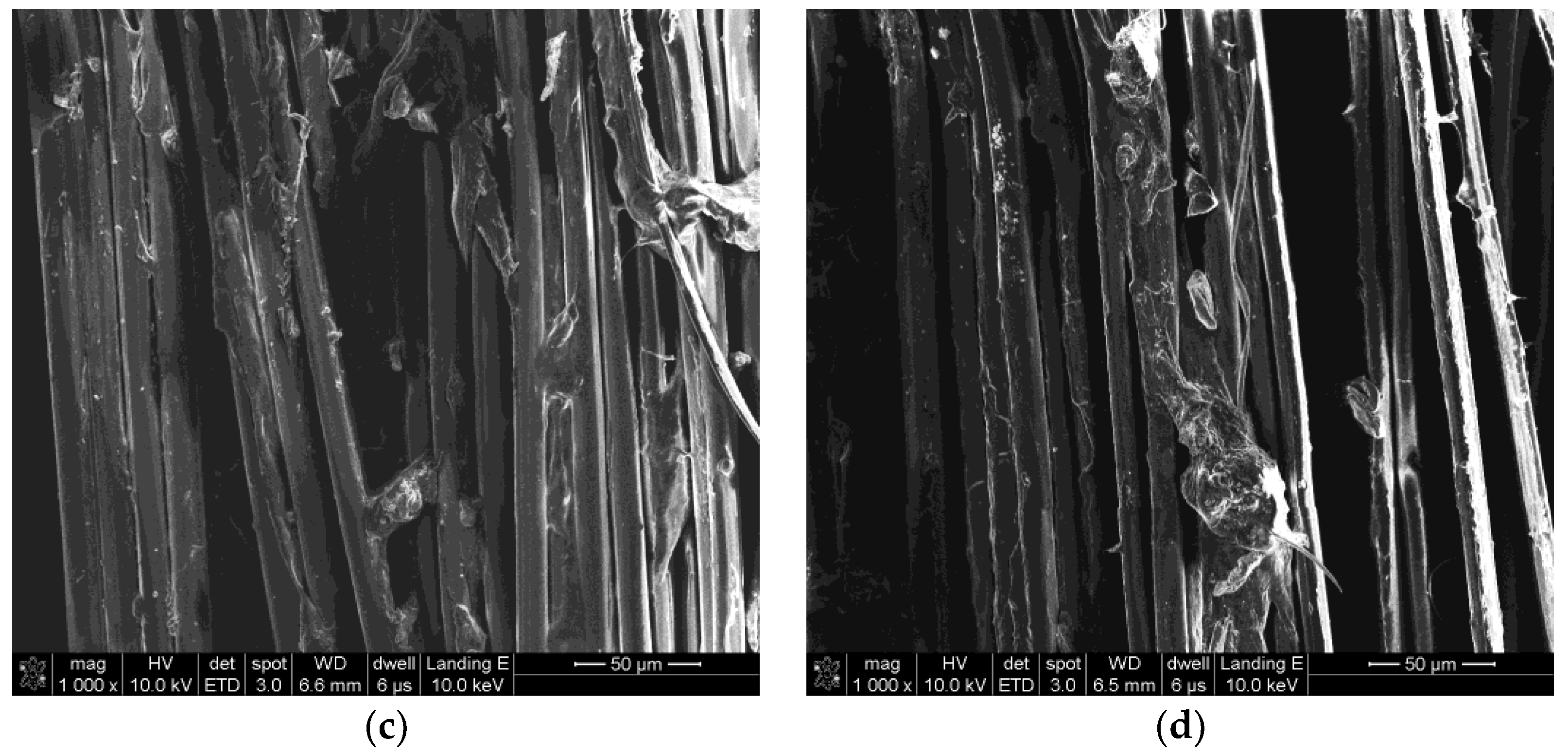
3.1. Surface Morphologies of PPTA Fibers
3.2. Chemical Structures of PPTA Fibers
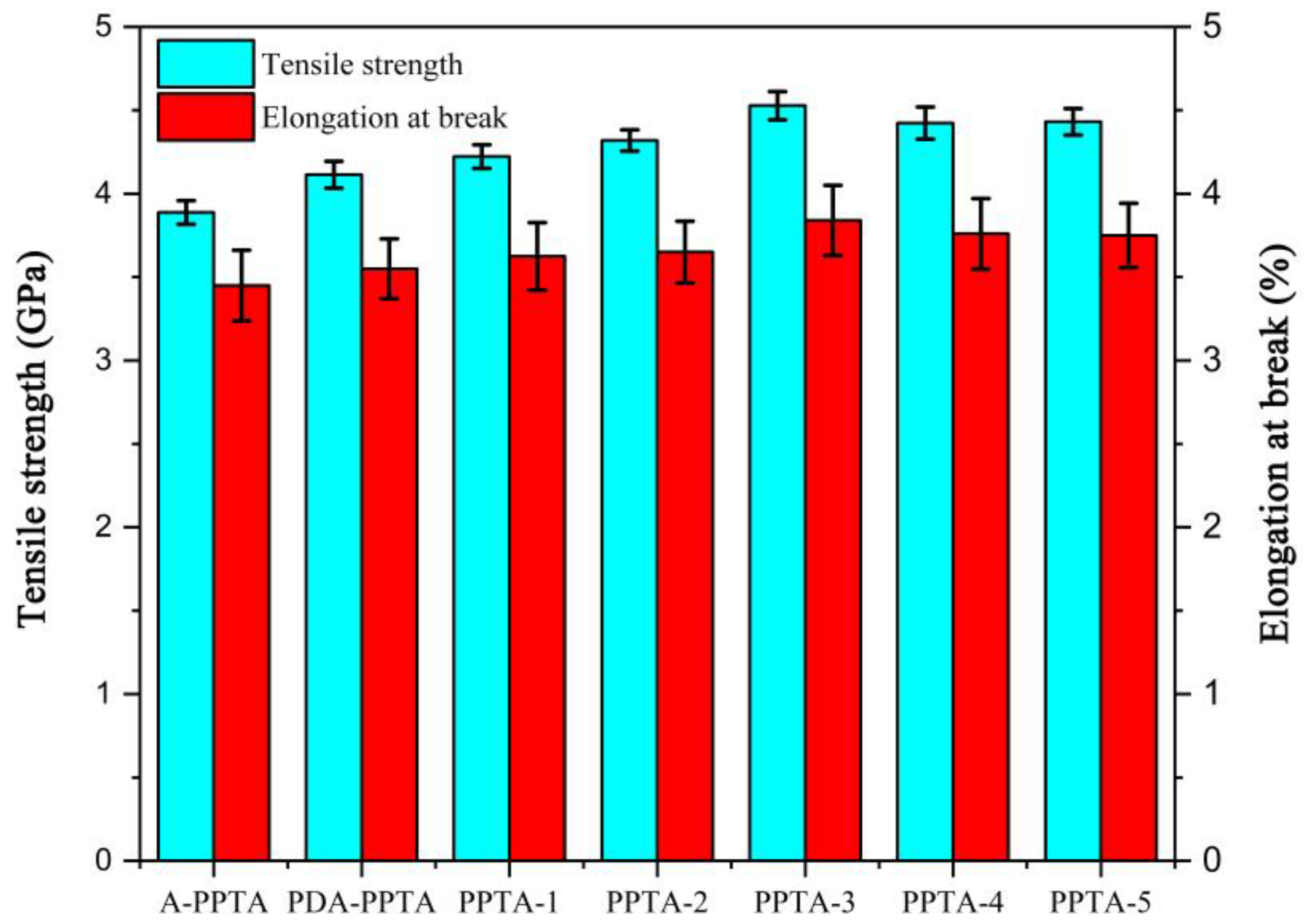
3.3. The Single-Filament Tensile Strength of PPTA Fibers
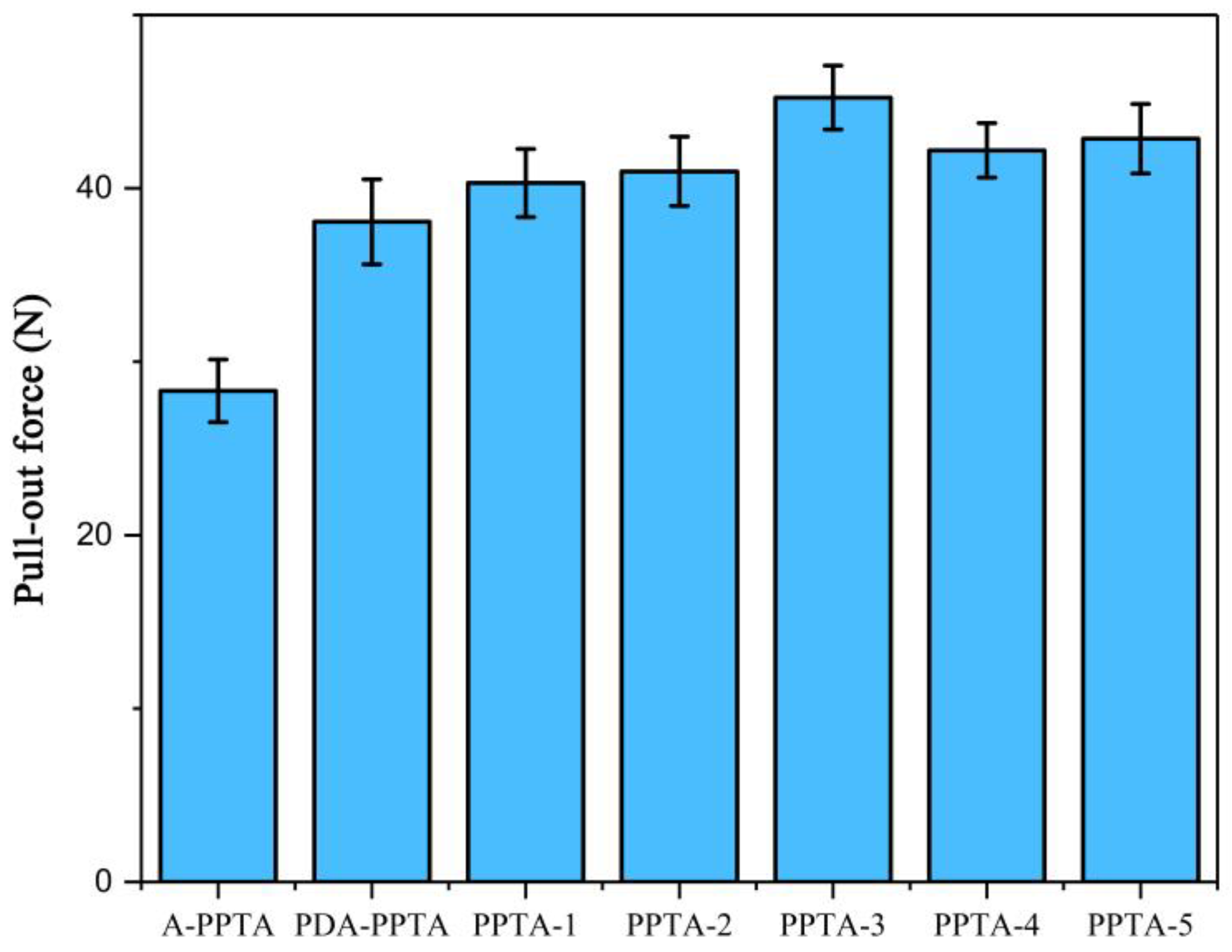
3.4. The Adhesive Property between PPTA Fibers and Rubber
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, J.H.; Yang, X.X.; Huang, L.H. Numerical prediction of mechanical properties of rubber composites reinforced by aramid fiber under large deformation. Compos. Struct. 2018, 201, 29–37. [Google Scholar] [CrossRef]
- Mustafa, E. 7-Aramid fibers. In Fiber Technology for Fiber-Reinforced Composites, 1st ed.; Özgür Seydibeyoğlu, M., Mohanty, A.K., Misra, M., Eds.; Woodhead Publishing Inc.: Cambridge, UK, 2017; pp. 153–167. [Google Scholar]
- Wang, Y.F.; Qu, R.J.; Pan, F.W.; Jia, X.H.; Sun, C.M.; Ji, C.N.; Zhang, Y.; An, K.; Mu, Y.L. Preparation and characterization of thiol- and amino-functionalized polysilsesquioxane coated poly (p-phenylenetherephthal amide) fibers and their adsorption properties towards Hg(II). Chem. Eng. J. 2017, 317, 187–203. [Google Scholar] [CrossRef]
- Jin, H.; Wang, Y. Synthesis and characterization of the novel meta-modified aramid fibers with liquid crystalline properties. Polym. Compos. 2012, 33, 1620–1627. [Google Scholar] [CrossRef]
- Liu, L.; Huang, Y.D.; Zhang, Z.Q.; Jiang, Z.X.; Wu, L.N. Ultrasonic treatment of aramid fiber surface and its effect on the interface of aramid/epoxy composites. Appl. Surf. Sci. 2008, 254, 2594–2599. [Google Scholar] [CrossRef]
- Kondo, Y.; Miyazaki, K.; Takayanagi, K.; Sakurai, K. Surface treatment of PET fiber by EB-irradiation-induced graft polymerization and its effect on adhesion in natural rubber matrix. Eur. Polym. J. 2008, 44, 1567–1576. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Huang, Y.D.; Liu, L.; Cai, K.L. Effects of γ-ray radiation grafting on aramid fibers and its composites. Appl. Surf. Sci. 2008, 254, 3153–3161. [Google Scholar] [CrossRef]
- Kondo, Y.; Miyazaki, K.; Yamaguchi, Y.; Sasaki, T.; Irie, S.; Sakurai, K. Mechanical properties of fiber reinforced styrene–butadiene rubbers using surface-modified UHMWPE fibers under EB irradiation. Eur. Polym. J. 2006, 42, 1008–1014. [Google Scholar] [CrossRef]
- Fan, W.; Tian, H.X.; Wang, H.H.; Zhang, T.; Yang, X.; Yu, Y.; Meng, X.; Yu, X.; Yuan, L.; Xu, B.; et al. Enhanced interfacial adhesion of aramid fiber III reinforced epoxy composites via low temperature plasma treatment. Polym. Test. 2018, 72, 147–156. [Google Scholar] [CrossRef]
- Wang, C.X.; Du, M.; Lv, J.C.; Zhou, Q.Q.; Ren, Y.; Liu, G.L.; Gao, D.W.; Jin, L.M. Surface modification of aramid fiber by plasma induced vapor phase graft polymerization of acrylic acid. I. Influence of plasma conditions. Appl. Surf. Sci. 2015, 349, 333–342. [Google Scholar] [CrossRef]
- Park, S.J.; Seo, M.K.; Ma, T.J.; Lee, D.R. Effect of chemical treatment of Kevlar fibers on mechanical interfacial properties of composites. J. Colloid. Interface. Sci. 2002, 252, 249–255. [Google Scholar] [CrossRef]
- Zhao, J. Effect of surface treatment on the structure and properties of para-aramid fibers by phosphoric acid. Fiber. Polym. 2013, 14, 59–64. [Google Scholar] [CrossRef]
- Li, S.N.; Gu, A.J.; Liang, G.Z.; Yuan, L.; Xue, J. A facile and green preparation of poly (glycidyl methacrylate) coated aramide fibers. J. Mater. Chem. 2012, 22, 8960–8968. [Google Scholar] [CrossRef]
- Fan, G.N.; Zhao, J.C.; Zhang, Y.K.; Guo, Z. Grafting modification of Kevlar fiber using horseradish peroxidase. Polym. Bull. 2006, 56, 507–515. [Google Scholar] [CrossRef]
- Zheng, C.; Han, Y.T.; Luo, L.B.; Liu, X. Grafting degradable coordination polymer on aramid fiber surface to improve its interfacial properties. Mater. Lett. 2018, 233, 102–106. [Google Scholar]
- Wang, B.; Duan, Y.G.; Zhang, J.J. Titanium dioxide nanoparticles-coated aramid fiber showing enhanced interfacial strength and UV resistance properties. Mater. Des. 2016, 103, 330–338. [Google Scholar] [CrossRef]
- Yu, M.F.; Lourie, O.; Dyer, M.J.; Moloni, K.; Kelly, T.F.; Ruoff, R.S. Strength and breaking mechanism of multiwalled carbon nanotubes under tensile load. Science 2000, 287, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Z.; Sun, H.Y.; Gao, C. Superstructured assembly of nanocarbons: Fullerenes, nanotubes, and graphene. Chem. Rev. 2015, 115, 7046–7117. [Google Scholar] [CrossRef]
- Chen, W.; Qian, X.M.; He, X.Q.; Liu, Z.Y.; Liu, J.P. Surface modification of Kevlar by grafting carbon nanotubes. J. Appl. Polym. Sci. 2011, 123, 1983–1990. [Google Scholar] [CrossRef]
- He, X.D.; Zhang, F.H.; Wang, R.G.; Liu, W. Preparation of a carbon nanotube/carbon fiber multi-scale reinforcement by grafting multi-walled carbon nanotubes onto the fibers. Carbon 2007, 45, 2559–2563. [Google Scholar] [CrossRef]
- Rodríguez-Uicab, O.; Avilés, F.; Gonzalez-Chi, P.I.; Canché-Escamilla, G.; Duarte-Aranda, S.; Yazdani-Pedram, M.; Toro, P.; Gamboa, F.; Mazo, M.A.; Nistal, A.; et al. Deposition of carbon nanotubes onto aramid fibers using as-received and chemically modified fibers. Appl. Surf. Sci. 2016, 385, 379–390. [Google Scholar] [CrossRef]
- Ehlert, G.J.; Sodano, H.A. Fiber strain sensors from carbon nanotubes self-assembled on aramid fibers. J. Intel. Mater. Syst. Struct. 2014, 25, 2117–2121. [Google Scholar] [CrossRef]
- Alimohammadi, F.; Parvinzadeh Gashti, M.; Mozaffari, A. Polyvinylpyrrolidone/Carbon Nanotube/Cotton Functional Nanocomposite: Preparation and Characterization of Properties. Fibers Polym. 2018, 19, 1940–1947. [Google Scholar] [CrossRef]
- Ebrahimi, I.; Gashti, M.P. Polypyrrole-MWCNT-Ag composites for electromagnetic shielding: Comparison between chemical deposition and UV-reduction approaches. J. Phys. Chem. Solids 2018, 118, 80–87. [Google Scholar] [CrossRef]
- Gashti, M.P.; Navid, M.Y.; Rahimi, M.H. Effects of coating of nano- and microemulsion silicones on thermal properties and flammability of polyethylene terephthalate textile. Pigment Resin Technol. 2013, 42, 34–44. [Google Scholar] [CrossRef]
- Gashti, M.P.; Moradian, S.; Rashidi, A.; Yazdanshenas, M.E. Dispersibility of hydrophilic and hydrophobic nano-silica particles in polyethylene terephthalate films: Evaluation of morphology and thermal properties. Polym. Polym. Compos. 2014, 23, 285–296. [Google Scholar] [CrossRef]
- Yang, X.; Tu, Q.Z.; Shen, X.M.; Zhu, P.; Li, Y.; Zhang, S. A Novel Method for Deposition of Multi-Walled Carbon Nanotubes onto Poly(p-Phenylene Terephthalamide) Fibers to Enhance Interfacial Adhesion with Rubber Matrix. Polymers 2019, 11, 374. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, B.P.; Messersmith, P.B. A reversible wet/dry adhesive inspired by mussels and geckos. Nature 2007, 448, 338–341. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 5849. [Google Scholar] [CrossRef]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile conjugation of biomolecu les onto surfaces via mussel adhesive protein inspired coatings. Adv. Mater. 2009, 21, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Zhang, S.; Yang, L.; Liu, M.; Li, H.; Zhang, Y.; Yao, S. Multifunctional Electrochemical Platforms Based on the Michael Addition/Schiff Base Reaction of Polydopamine Modified Reduced Graphene Oxide: Construction and Application. ACS Appl. Mater. Interfaces 2015, 7, 17935–17946. [Google Scholar] [CrossRef]
- Fu, L.H.; Shi, Y.G.; Wang, K.; Zhou, P.; Liu, M.Y.; Wan, Q.; Tao, L.; Zhang, X.Y.; Wei, Y. Biomimic modification of graphene oxide. New J. Chem. 2015, 39, 8172–8178. [Google Scholar] [CrossRef]
- Xu, L.Q.; Yang, W.J.; Neoh, K.G.; Kang, E.-T.; Fu, G.D. Dopamine-Induced Reduction and Functionalization of Graphene Oxide Nanosheets. Macromolecules 2010, 43, 8336–8339. [Google Scholar] [CrossRef]
- Sa, R.; Yan, Y.; Wei, Z.; Zhang, L.; Wang, W.; Tian, M. Surface modification of aramid fibers by bio-inspired poly(dopamine) and epoxy functionalized silane grafting. ACS Appl. Mater. Interfaces 2014, 6, 21730–21738. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Liu, Y.; Wang, Y.; Xie, Z.; Dong, Q.; Dong, M.; Liu, H.; Shao, Q.; Lu, N.; Murugadoss, V.; et al. Amino graphene oxide/dopamine modified aramid fibers: Preparation, epoxy nanocomposites and property analysis. Polymer 2019, 168, 131–137. [Google Scholar] [CrossRef]
- Chen, S.S.; Cao, Y.W.; Feng, J.C. Polydopamine as an efficient and robust platform to functionalize carbon fiber for high-performance polymer composites. ACS Appl. Mater. Interfaces 2014, 6, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.M.; Liu, B.H.; Kong, H.J. Layer-by-Layer Self-Assembly Strategy for Surface Modification of Aramid Fibers to Enhance Interfacial Adhesion to Epoxy Resin. Polymers 2018, 10, 820. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhang, H.; Liu, M.; Deng, F.; Huang, H.; Wan, Q.; Li, Z.; Wang, K.; He, X.; Zhang, X.; et al. A bioinspired strategy for surface modification of silica nanoparticles. Appl. Surf. Sci. 2015, 357, 1996–2003. [Google Scholar] [CrossRef]
- Zhang, X.; Zeng, G.; Tian, J.; Wan, Q.; Huang, Q.; Wang, K.; Zhang, Q.; Liu, M.; Deng, F.; Wei, Y. PEGylation of carbon nanotubes via mussel inspired chemistry: Preparation, characterization and biocompatibility evaluation. Appl. Surf. Sci. 2015, 351, 425–432. [Google Scholar] [CrossRef]
- Ma, P.C.; Kim, J.K.; Tang, B.Z. Functionalization of carbon nanotubes using a silane coupling agent. Carbon 2006, 44, 3232–3238. [Google Scholar] [CrossRef]
- Yu, R.Q.; Chen, L.W.; Liu, Q.P.; Lin, J.; Tan, K.L.; Ng, S.C.; Chan, H.S.O.; Xu, G.Q.; Andy Hor, T.A. Platinum deposition on carbon nanotubes via chemical modification. Chem. Mater. 1998, 10, 718–722. [Google Scholar] [CrossRef]
- Sharma, S.; Pathak, A.; Singh, V.N.; Teotia, S.; Dhakate, S.R.; Singh, B.P. Excellent mechanical properties of long length multiwalled carbon nanotube bridged Kevlar fabric. Carbon 2018, 137, 104–117. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, Q.; Zhang, X.M.; Zhang, W.; Jia, H.; Ji, Q.; Yang, F.; Rui, X. Rational design of multifunctional properties for styrene-butadience rubber reinforced by modified Kevlar nanofibers. Compos. Part B Eng. 2019, 166, 196–203. [Google Scholar] [CrossRef]
- Wang, L.; Shi, Y.; Chen, S.; Wang, W.; Tian, M.; Ning, N.; Zhang, L. Highly efficient mussel-like inspired modification of aramid fibers by UV-accelerated catechol/polyamine deposition followed chemical grafting for high-performance polymer composites. Chem. Eng. J. 2017, 314, 583–593. [Google Scholar] [CrossRef]
- Sa, R.; Yan, Y.; Wang, L.; Li, Y.; Zhang, L.; Ning, N.; Wang, W.; Tian, M. Improved adhesion properties of poly-p-phenyleneterephthamide fibers with a rubber matrix via UV-initiated grafting modification. RSC Adv. 2015, 114, 94351–94360. [Google Scholar] [CrossRef]
- Zhang, R.L.; Gao, B.; Ma, Q.H.; Zhang, J.; Cui, H.Z.; Liu, L. Directly grafting graphene oxide onto carbon fiber and the effect on the mechanical properties of carbon fiber composites. Mater. Des. 2016, 93, 364–369. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Miller, D.J.; Freeman, B.D.; Paul, D.R.; Bielawski, C.W. Elucidating the structure of poly (dopamine). Langmuir 2012, 28, 6428–6435. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Huang, Y.D.; Liu, L.; Bai, Y.P.; Xu, L.W. Formation of a carbon fiber/polyhedral oligomeric silsesquioxane/carbon nanotube hybrid reinforcement and its effect on the interfacial properties of carbon fiber/epoxy composites. Carbon 2011, 49, 2624–2632. [Google Scholar] [CrossRef]





| Materials | Parts per Hundreds of Rubber |
|---|---|
| Styrene-butadiene rubber | 60 |
| Natural rubber | 40 |
| Antioxidant (4010NA) | 1.5 |
| Carbon black | 20 |
| White carbon black | 15 |
| Zinc oxide | 5 |
| Stearic acid | 2.5 |
| Aromatic oil | 10 |
| Coumarone indene resin | 10 |
| Rubber adhesive (RA) | 1 |
| Rubber adhesive (RS) | 1 |
| Accelerant (CZ) | 5 |
| Sulphur | 1 |
| Total | 172 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Tu, Q.; Shen, X.; Yin, Q.; Pan, M.; Jiang, C.; Hu, C. Enhancing the Interfacial Adhesion with Rubber Matrix by Grafting Polydopamine-Carbon Nanotubes onto Poly(p-phenylene terephthalamide) Fibers. Polymers 2019, 11, 1231. https://doi.org/10.3390/polym11081231
Yang X, Tu Q, Shen X, Yin Q, Pan M, Jiang C, Hu C. Enhancing the Interfacial Adhesion with Rubber Matrix by Grafting Polydopamine-Carbon Nanotubes onto Poly(p-phenylene terephthalamide) Fibers. Polymers. 2019; 11(8):1231. https://doi.org/10.3390/polym11081231
Chicago/Turabian StyleYang, Xuan, Qunzhang Tu, Xinmin Shen, Qin Yin, Ming Pan, Chengming Jiang, and Caibing Hu. 2019. "Enhancing the Interfacial Adhesion with Rubber Matrix by Grafting Polydopamine-Carbon Nanotubes onto Poly(p-phenylene terephthalamide) Fibers" Polymers 11, no. 8: 1231. https://doi.org/10.3390/polym11081231
APA StyleYang, X., Tu, Q., Shen, X., Yin, Q., Pan, M., Jiang, C., & Hu, C. (2019). Enhancing the Interfacial Adhesion with Rubber Matrix by Grafting Polydopamine-Carbon Nanotubes onto Poly(p-phenylene terephthalamide) Fibers. Polymers, 11(8), 1231. https://doi.org/10.3390/polym11081231






