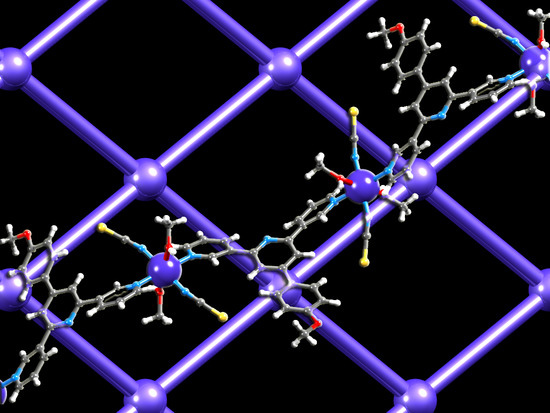
Competition in Coordination Assemblies: 1D-Coordination Polymer or 2D-Nets Based on Co(NCS)2 and 4′-(4-methoxyphenyl)-3,2′:6′,3″-terpyridine
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthesis of 2
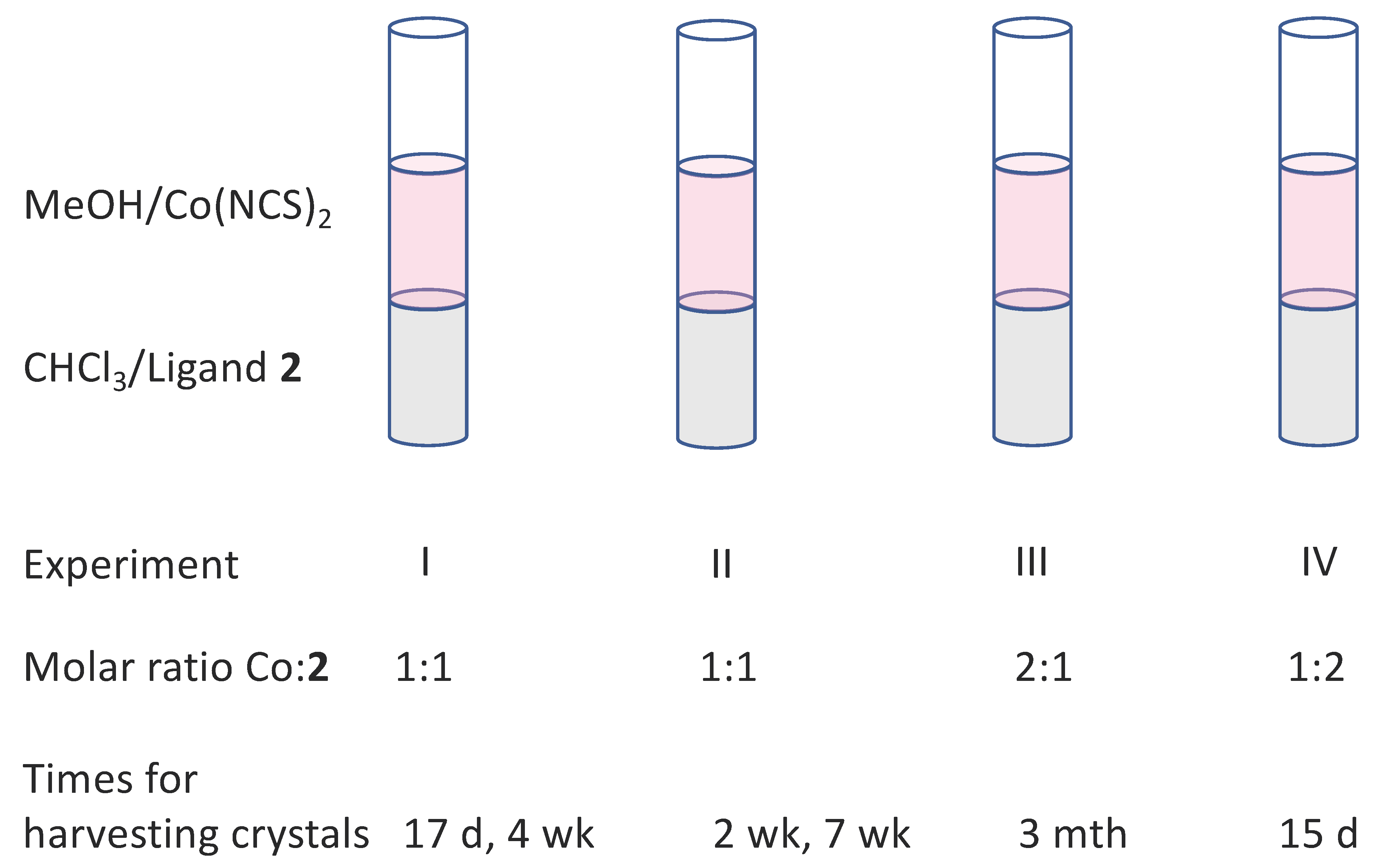
2.3. [Co(2)(NCS)2(MeOH)2]n, [{Co(2)2(NCS)2}·3MeOH]n, and [{Co(2)2(NCS)2}·2.2CHCl3]n
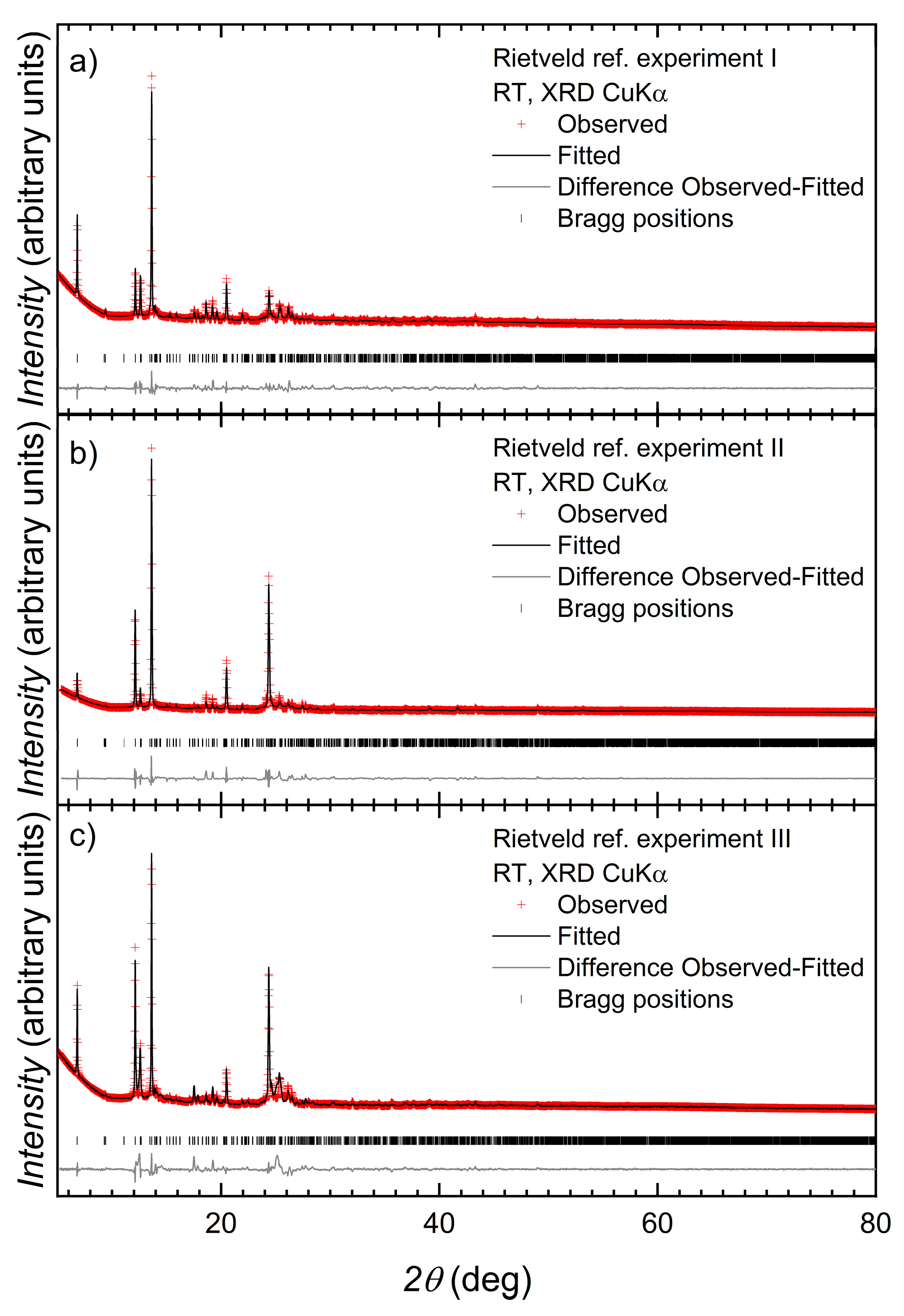
2.4. Crystallography
2.5. [Co(2)(NCS)2(MeOH)2]n
2.6. [{Co(2)2(NCS)2}·3MeOH]n
2.7. [{Co(2)2(NCS)2}·2.2CHCl3]n
3. Results and Discussion
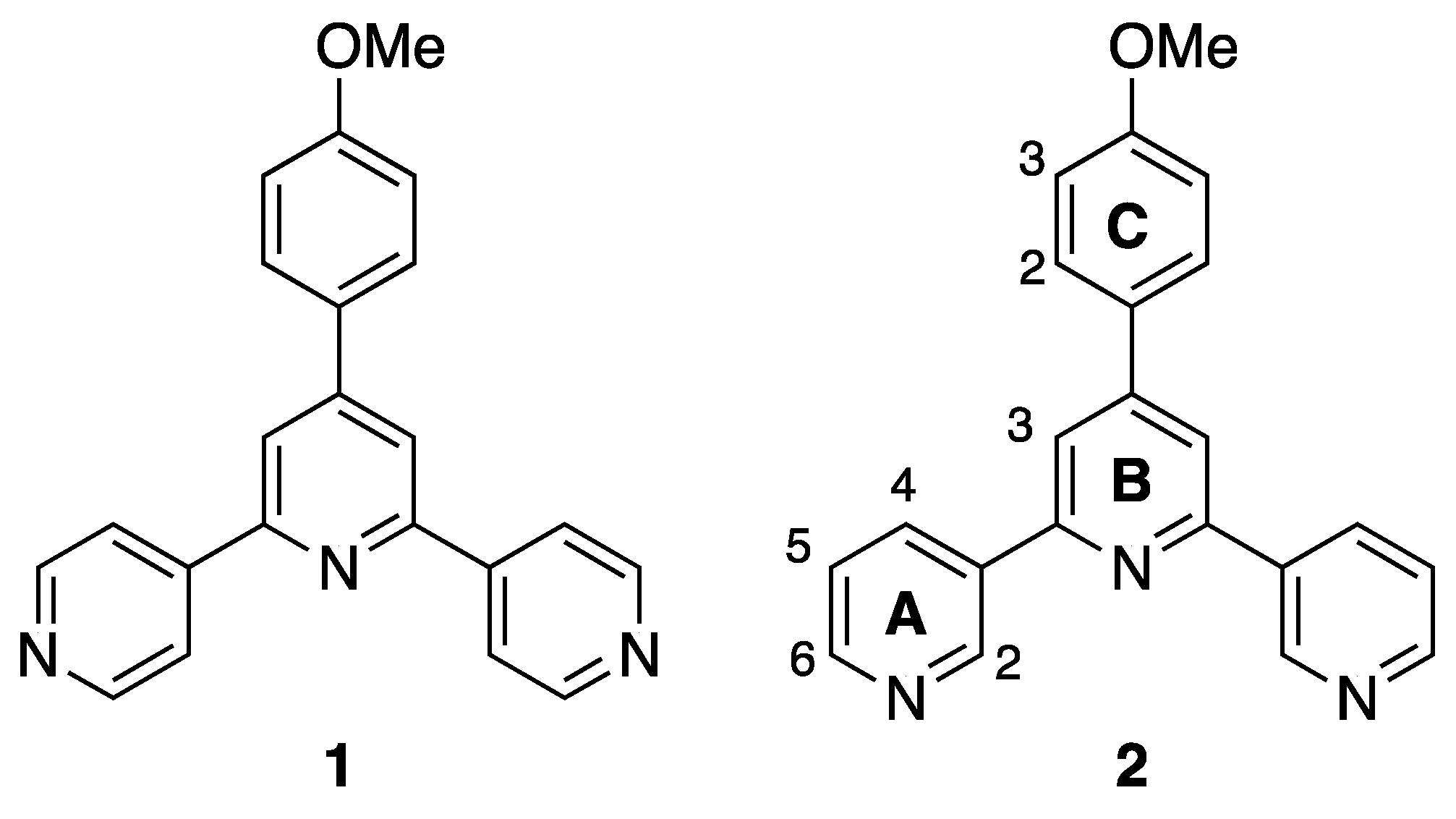
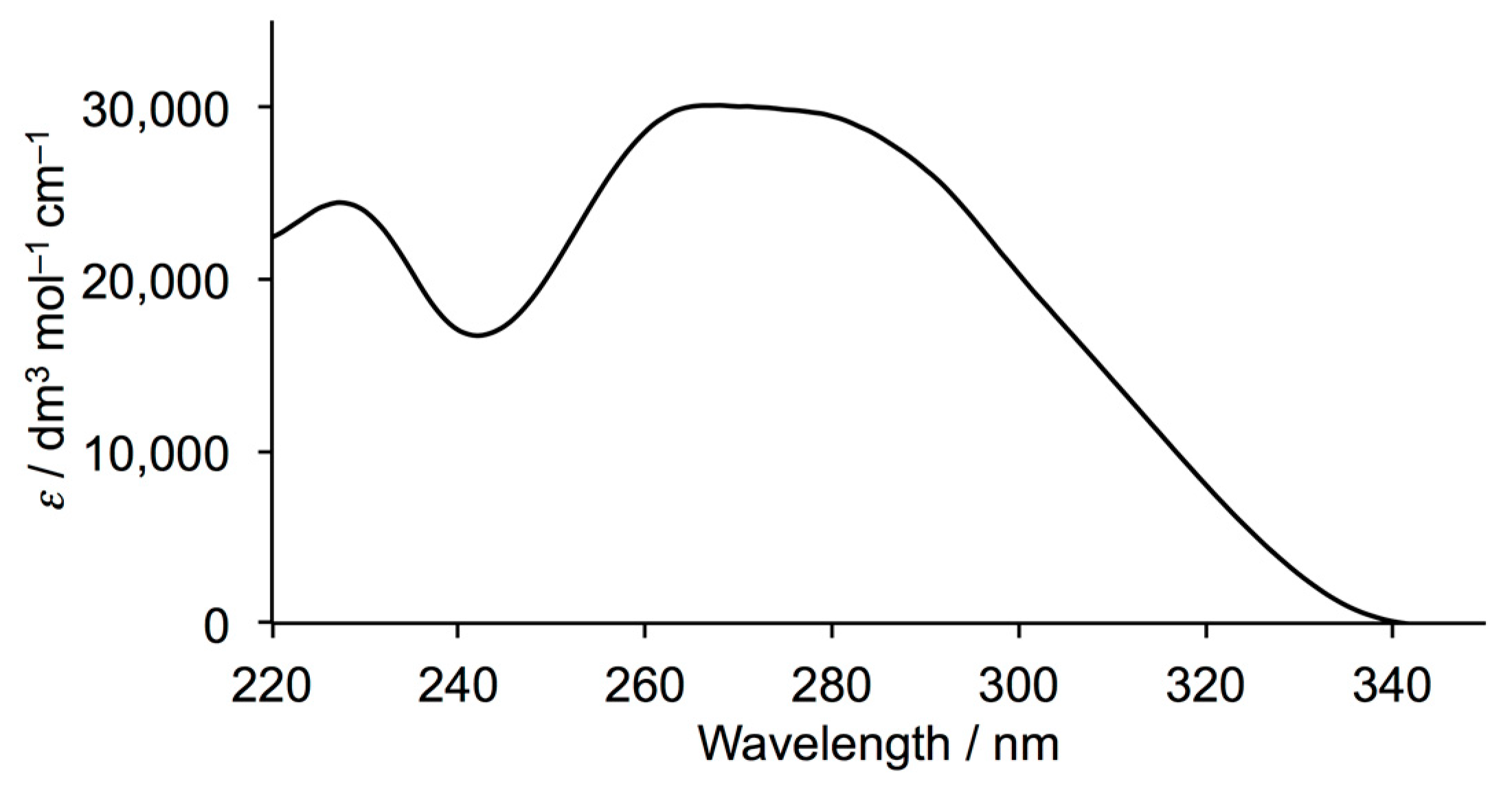
3.1. Ligand Synthesis and Characterization
3.2. Reactions of Compound 2 with Co(NCS)2
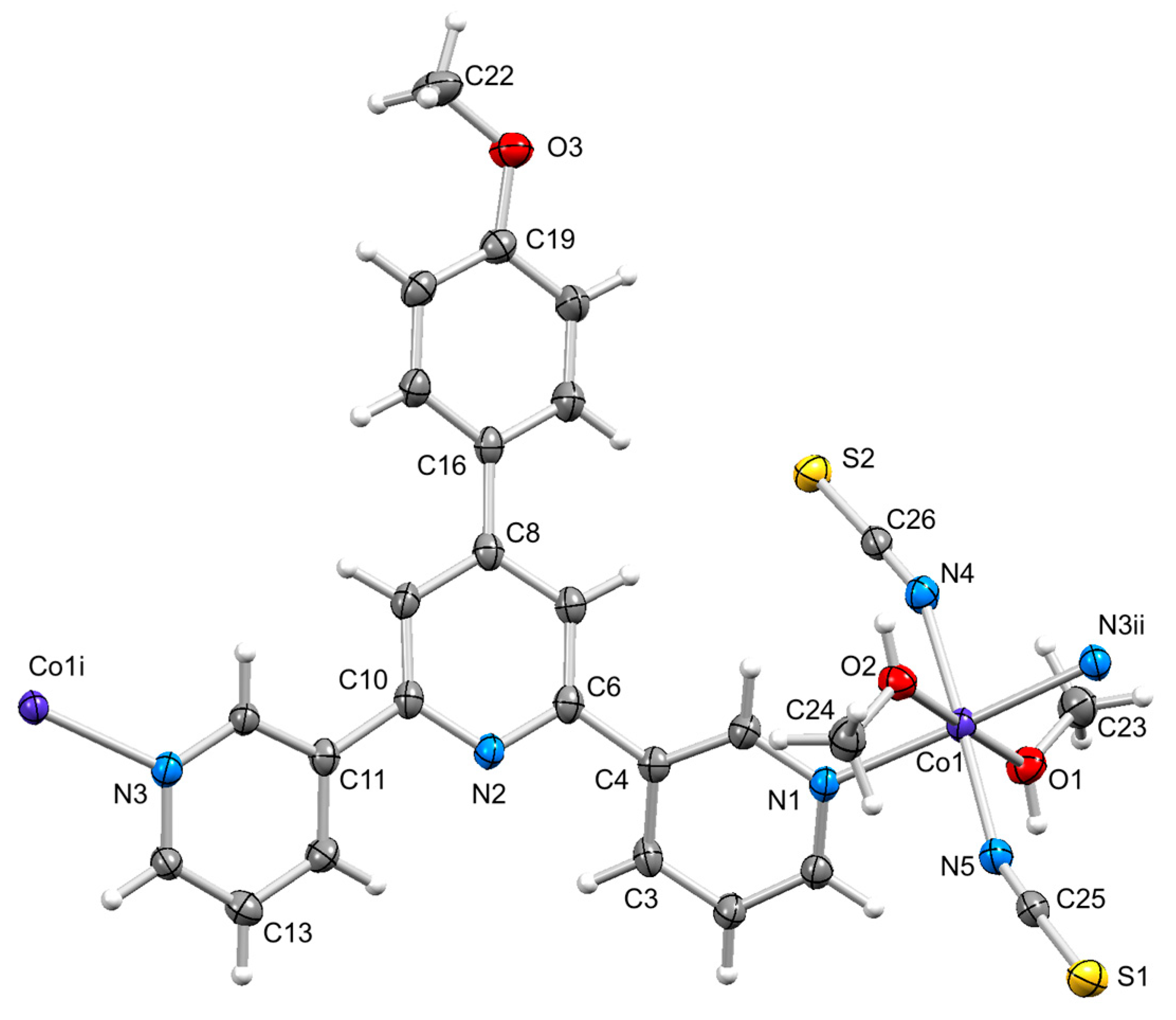
3.3. Structure of [Co(2)(NCS)2(MeOH)2]n
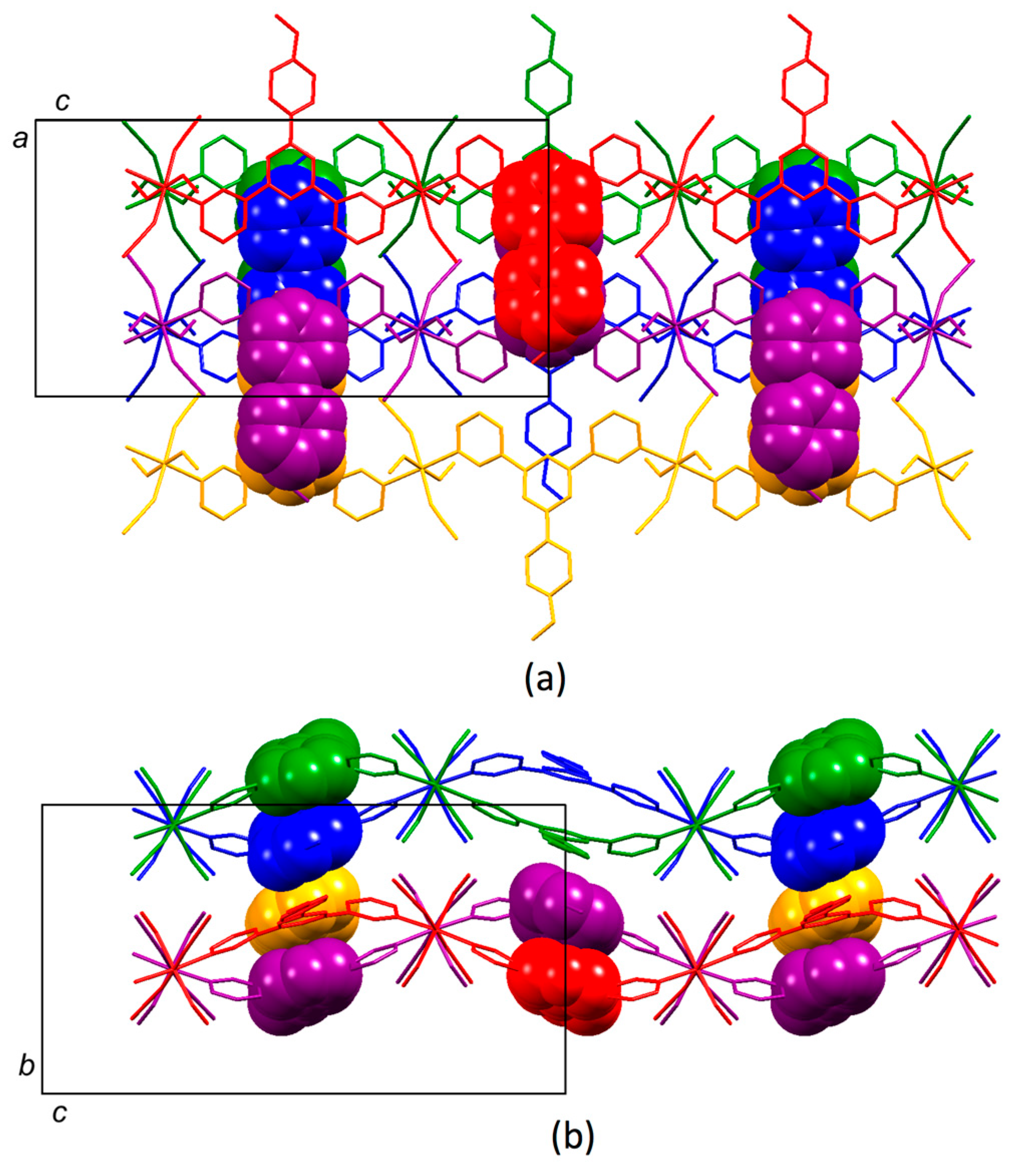
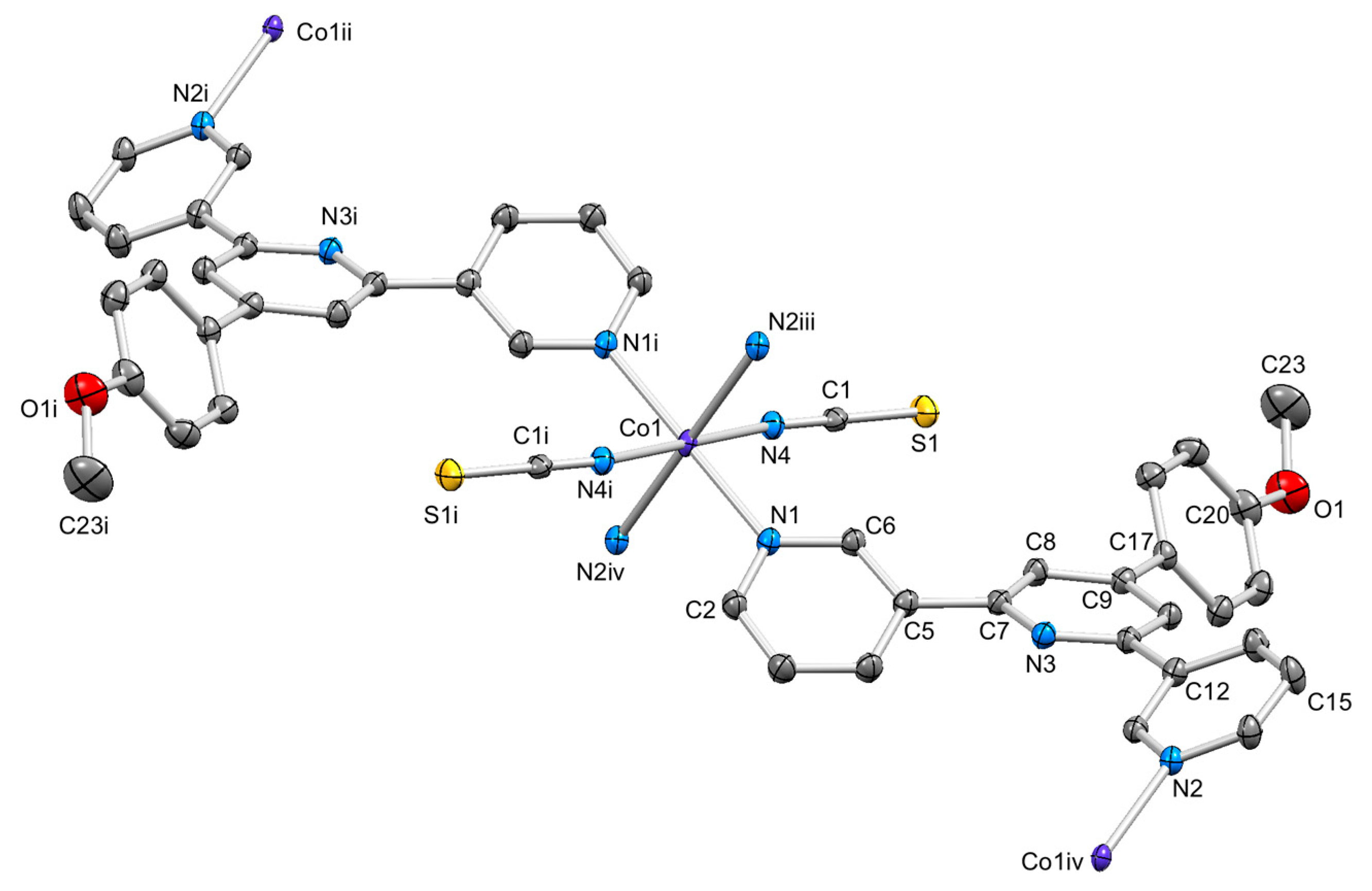
3.4. Structures of [{Co(2)2(NCS)2}·3MeOH]n and [{Co(2)2(NCS)2}·2.2CHCl3]n
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Tranchemontagne, D.J.; Mendoza-Cortés, J.L.; O’Keeffe, M.; Yaghi, O.M. Secondary building units, nets and bonding in the chemistry of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1257–1283. [Google Scholar] [CrossRef] [PubMed]
- Nangia, A.K.; Desiraju, G.R. Crystal engineering: An outlook for the future. Angew. Chem. Int. Ed. 2019, 58, 4100–4107. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xu, Q. Bimetallic Metal-organic frameworks for gas storage and separation. Cryst. Growth Des. 2017, 17, 1450–1455. [Google Scholar] [CrossRef]
- Rowsell, J.L.; Yaghi, O.M. Strategies for hydrogen storage in Metal-organic frameworks. Angew. Chem. Int. Ed. 2005, 44, 4670–4679. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, Y.; Li, D.S.; Bu, X.; Feng, P. Metal–Organic Frameworks for Separation. Adv. Mater. 2018, 30, 1705189. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, A.F.; Cao, C.S.; Zhao, B. Applications of MOFs: Recent advances in photocatalytic hydrogen production. Coord. Chem. Rev. 2019, 390, 50–75. [Google Scholar] [CrossRef]
- Chen, W.; Wu, C. Synthesis, functionalization, and applications of Metal-organic Frameworks in Biomedicine. Dalton Trans. 2018, 47, 2114–2133. [Google Scholar] [CrossRef]
- Wu, M.X.; Yang, Y.W. Metal-organic Framework (MOF)-Based Drug/Cargo Delivery and Cancer Treatment. Adv. Mater. 2017, 29, 1606134. [Google Scholar] [CrossRef]
- Abánades Lázaro, I.; Forgan, R.S. Application of Zirconium MOFs for Drug Delivery and Biomedicine. Coord. Chem. Rev. 2019, 380, 230–259. [Google Scholar] [CrossRef]
- Li, X.; Wang, B.; Cao, Y.; Zhao, S.; Wang, H.; Feng, X.; Zhou, J.; Ma, X. Water Contaminant Elimination Based on Metal-Organic Frameworks and Perspective on Their Industrial Applications. ACS Sustain. Chem. Eng. 2019, 7, 4548–4563. [Google Scholar] [CrossRef]
- Wen, J.; Fang, Y.; Zeng, G. Progress and Prospect of Adsorptive Removal of Heavy Metal Ions from Aqueous Solution using Metal-organic Frameworks: A Review of Studies from the Last Decade. Chemosphere 2018, 201, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, G.K.H. Assembly of Metal Ions and Ligands with Adaptable Coordinative Tendencies as a Route to Functional Metal-organic Solids. J. Solid State Chem. 2005, 178, 2519–2526. [Google Scholar] [CrossRef]
- Chaurin, V.; Constable, E.C.; Housecroft, C.E. What is the Coordination Number of Copper(II) in Metallosupramolecular Chemistry? New J. Chem. 2006, 30, 1740–1744. [Google Scholar] [CrossRef]
- Favre, H.A.; Powell, W.H. Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013; Royal Society of Chemistry: Cambridge, UK, 2014. [Google Scholar]
- Housecroft, C.E. 4,2′:6′,4″-Terpyridines: Diverging and Diverse Building Blocks in Coordination Polymers and Metallomacrocycles. Dalton Trans. 2014, 43, 6594–6604. [Google Scholar] [CrossRef] [PubMed]
- Housecroft, C.E. Divergent 4,2′:6′,4″- and 3,2′:6′,3″-Terpyridines as Linkers in 2- and 3-Dimensional Architectures. CrystEngComm 2015, 17, 7461–7468. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. Tetratopic Bis (4,2′:6′,4″-terpyridine) and Bis (3,2′:6′,3″-terpyridine) Ligands as 4-Connecting Nodes in 2D-Coordination Networks and 3D-Frameworks. J. Inorg. Organomet. Polym. Mater. 2018, 28, 414–427. [Google Scholar]
- Housecroft, C.E.; Constable, E.C. Ditopic and Tetratopic 4,2′:6′,4″-Terpyridines as Structural Motifs in 2D- and 3D-Coordination Assemblies. Chimia 2019, 73, 462. [Google Scholar] [CrossRef]
- Wang, J.; Hanan, G.S. A Facile Route to Sterically Hindered and Non-hindered 4′-Aryl-2,2′:6′,2″-terpyridines. Synlett 2005, 2005, 1251–1254. [Google Scholar] [CrossRef]
- Klein, Y.M.; Constable, E.C.; Housecroft, C.E.; Zampese, J.A.; Crochet, A. Greasy tails switch 1D-coordination [Zn2(OAc)4(4′-(4-ROC6H4)-4,2′:6′,4″-tpy)]n polymers to discrete [Zn2(OAc)4(4′-(4-ROC6H4)-4,2′:6′,4″-tpy)2] complexes. CrystEngComm 2014, 16, 9915–9929. [Google Scholar] [CrossRef]
- Long, D.L.; Blake, A.J.; Champness, N.R.; Wilson, C.; Schröder, M. Constructing terbium Co-ordination Polymers of 4,4′-Bipyridine-N, N’-dioxide by Means of Diffusion Solvent Mixtures. Chem. Eur. J. 2002, 8, 2026–2033. [Google Scholar] [CrossRef]
- Corbett, P.T.; Leclaire, J.; Vial, L.; West, K.R.; Wietor, J.L.; Sanders, J.K.M.; Otto, S. Dynamic Combinatorial Chemistry. Chem. Rev. 2006, 106, 3652–3711. [Google Scholar] [CrossRef] [PubMed]
- Baum, G.; Constable, E.C.; Fenske, D.; Housecroft, C.E.; Kulke, T. Chiral 1,2-Ethanediyl-spaced Quaterpyridines Give a Library of Cyclic and Double helicates with copper(I). Chem. Commun. 1999, 195–196. [Google Scholar] [CrossRef]
- Tuna, F.; Hamblin, J.; Jackson, A.; Clarkson, G.; Alcock, N.W.; Hannon, M.J. Metallo-supramolecular Libraries: Triangles, Polymers and Double-helicates Assembled by Copper(I) Coordination to Directly Linked Bis-pyridylimine Ligands. Dalton Trans. 2003, 2141–2148. [Google Scholar] [CrossRef]
- Schmittel, M. From Self-sorted Coordination Libraries to Networking Nanoswitches for Catalysis. Chem. Commun. 2015, 51, 14956–14968. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, X.; Wang, J.L.; Moorefield, C.N.; Wesdemiotis, C.; Newkome, G.R. From Supramolecular Triangle to Heteroleptic Rhombus: A Simple Bridge Can Make a Difference. Chem. Commun. 2012, 48, 9873–9875. [Google Scholar] [CrossRef] [PubMed]
- Köberl, M.; Cokoja, M.; Herrmann, W.A.; Kühn, F.E. From Molecules to Materials: Molecular Paddle-wheel Synthons of Macromolecules, Cage Compounds and Metal-organic Frameworks. Dalton Trans. 2011, 40, 6834–6859. [Google Scholar] [CrossRef] [PubMed]
- Klein, Y.M.; Constable, E.C.; Housecroft, C.E.; Prescimone, A. Assembling Coordination Ladders with 4′-(4-methoxyphenyl)-4,2′:6′,4″-Terpyridine as Rails and Rungs. Inorg. Chem. Commun. 2014, 49, 41–43. [Google Scholar] [CrossRef]
- Klein, Y.M.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. 2-Dimensional Networks Assembled Using 4′-Functionalized 4,2′:6′,4″-Terpyridines and Co(NCS)2. Polyhedron 2016, 103, 58–65. [Google Scholar] [CrossRef]
- Mondal, A.K.; Khatua, S.; Tomar, K.; Konar, S. Field-Induced Single-Ion-Magnetic Behavior of Octahedral CoII in a Two-dimensional Coordination Polymer. Eur. J. Inorg. Chem. 2016, 2016, 3545–3552. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Bruno, I.J.; Cole, J.C.; Edgington, P.R.; Kessler, M.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. New Software for Searching the Cambridge Structural Database and Visualising Crystal Structures. Acta Cryst. 2002, 58, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Khatua, S.; Goswami, S.; Biswas, S.; Tomar, K.; Jena, H.S.; Konar, S. Stable Multiresponsive Luminescent MOF for Colorimetric Detection of Small Molecules in Selective and Reversible Manner. Chem. Mater. 2015, 27, 5349–5360. [Google Scholar] [CrossRef]
- Khatua, S.; Bar, A.K.; Konar, S. Tuning Proton Conductivity by Interstitial Guest Change in Size-Adjustable Nanopores of a CuI-MOF: A Potential Platform for Versatile Proton Carriers. Chem. Eur. J. 2016, 22, 16277–16285. [Google Scholar] [CrossRef] [PubMed]
- Bruker axs. Software for the Integration of CCD Detector System Bruker Analytical X-ray Systems; Bruker AXS: Madison, WI, USA.
- Sheldrick, G.M. ShelXT-Integrated space-group and crystal-structure determination. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with ShelXL. Acta Cryst. 2015, 27, 3–8. [Google Scholar]
- Palatinus, L.; Chapuis, G. Superflip—A Computer Program for the Solution of Crystal Structures by Charge Flipping in Arbitrary Dimensions. J. Appl. Cryst. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Palatinus, L.; Prathapa, S.J.; van Smaalen, S. EDMA: A Computer Program for Topological Analysis of Discrete Electron Densities. J. Appl. Cryst. 2012, 45, 575–580. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and Analysis of Crystal Structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Spek, A.L. Platon Squeeze: A Tool for the Calculation of the Disordered Solvent Contribution to the Calculated Structure Factors. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Rietveld, H.M. A Profile Refinement Method for Nuclear and Magnetic Structures. J. Appl. Cryst. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent Advances in Magnetic Structure Determination by Neutron Powder Diffraction. Physica B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Roisnel, T.; Rodríguez-Carvajal, J. WinPLOTR: A Windows tool for powder diffraction patterns analysis Materials Science Forum. In Materials Science Forum, Proceedings of the Seventh European Powder Diffraction Conference (EPDIC 7), Barcelona, Spain, 20–23 May 2000; Scitec Publications: New York, NY, USA, 2001; pp. 118–123. [Google Scholar]
- Gozzo, F.; De Caro, L.; Giannini, C.; Guagliardi, A.; Schmitt, B.; Prodi, A. The instrumental resolution function of synchrotron radiation powder diffractometers in the presence of focusing optics. J. Appl. Cryst. 2006, 39, 347–357. [Google Scholar] [CrossRef]
- Courbion, G.; Ferey, G. Na2Ca3Al2F14: A New Example of a Structure with “Independent F−”—A New Method of Comparison Between Fluorides and Oxides of Different Formula. J. Solid State Chem. 1988, 76, 426–431. [Google Scholar] [CrossRef]
- Rocco, D.; Housecroft, C.E.; Constable, E.C. Synthesis of Terpyridines: Simple Reactions—What Could Possibly Go Wrong? Molecules 2019, 24, 1799. [Google Scholar] [CrossRef]
- Seddon, K.R. Pseudopolymorph: A Polemic. Cryst. Growth Des. 2004, 4, 1087. [Google Scholar] [CrossRef]
- Nangia, A. Pseudopolymorph: Retain This Widely Accepted Term. Cryst. Growth Des. 2006, 6, 2–4. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E.; Neuburger, M.; Vujovic, S.; Zampese, J.A.; Zhang, G. Cobalt(II) Coordination Polymers with 4′-Substituted 4,2′:6′,4″- and 3,2′:6′,3″-Terpyridines: Engineering a Switch from Planar to Undulating Chains and Sheets. CrystEngComm 2012, 14, 3554–3563. [Google Scholar] [CrossRef]
- Janiak, C. A Critical Account on π–π Stacking in Metal Complexes with Aromatic Nitrogen-containing Ligands. Dalton Trans. 2000, 3885–3896. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Klein, Y.M.; Prescimone, A.; Karpacheva, M.; Constable, E.C.; Housecroft, C.E. Sometimes the same, sometimes different: Understanding self-assembly algorithms in coordination networks. Polymers 2018, 10, 1369. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocco, D.; Prescimone, A.; Klein, Y.M.; Gawryluk, D.J.; Constable, E.C.; Housecroft, C.E. Competition in Coordination Assemblies: 1D-Coordination Polymer or 2D-Nets Based on Co(NCS)2 and 4′-(4-methoxyphenyl)-3,2′:6′,3″-terpyridine. Polymers 2019, 11, 1224. https://doi.org/10.3390/polym11071224
Rocco D, Prescimone A, Klein YM, Gawryluk DJ, Constable EC, Housecroft CE. Competition in Coordination Assemblies: 1D-Coordination Polymer or 2D-Nets Based on Co(NCS)2 and 4′-(4-methoxyphenyl)-3,2′:6′,3″-terpyridine. Polymers. 2019; 11(7):1224. https://doi.org/10.3390/polym11071224
Chicago/Turabian StyleRocco, Dalila, Alessandro Prescimone, Y. Maximilian Klein, Dariusz J. Gawryluk, Edwin C. Constable, and Catherine E. Housecroft. 2019. "Competition in Coordination Assemblies: 1D-Coordination Polymer or 2D-Nets Based on Co(NCS)2 and 4′-(4-methoxyphenyl)-3,2′:6′,3″-terpyridine" Polymers 11, no. 7: 1224. https://doi.org/10.3390/polym11071224
APA StyleRocco, D., Prescimone, A., Klein, Y. M., Gawryluk, D. J., Constable, E. C., & Housecroft, C. E. (2019). Competition in Coordination Assemblies: 1D-Coordination Polymer or 2D-Nets Based on Co(NCS)2 and 4′-(4-methoxyphenyl)-3,2′:6′,3″-terpyridine. Polymers, 11(7), 1224. https://doi.org/10.3390/polym11071224