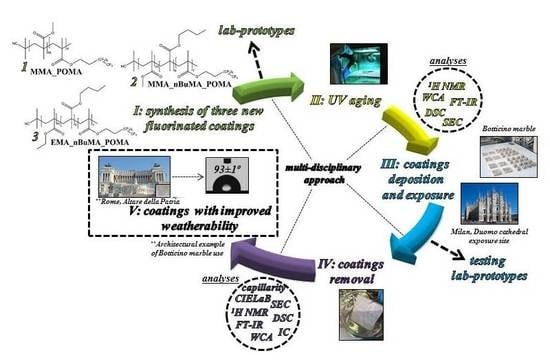
Towards Novel Fluorinated Methacrylic Coatings for Cultural Heritage: A Combined Polymers and Surfaces Chemistry Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MMA_POMA, MMA_nBuMA_POMA and EMA_nBuMA_POMA
2.3. Polymer Foils Preparation and Relative Characterization before and after UV Test
2.4. Application of Polymer Coatings onto Botticino Marble and External Exposure
2.5. Coatings Removal from Botticino Samples and Further Polymers and Stones Characterization
3. Results and Discussion
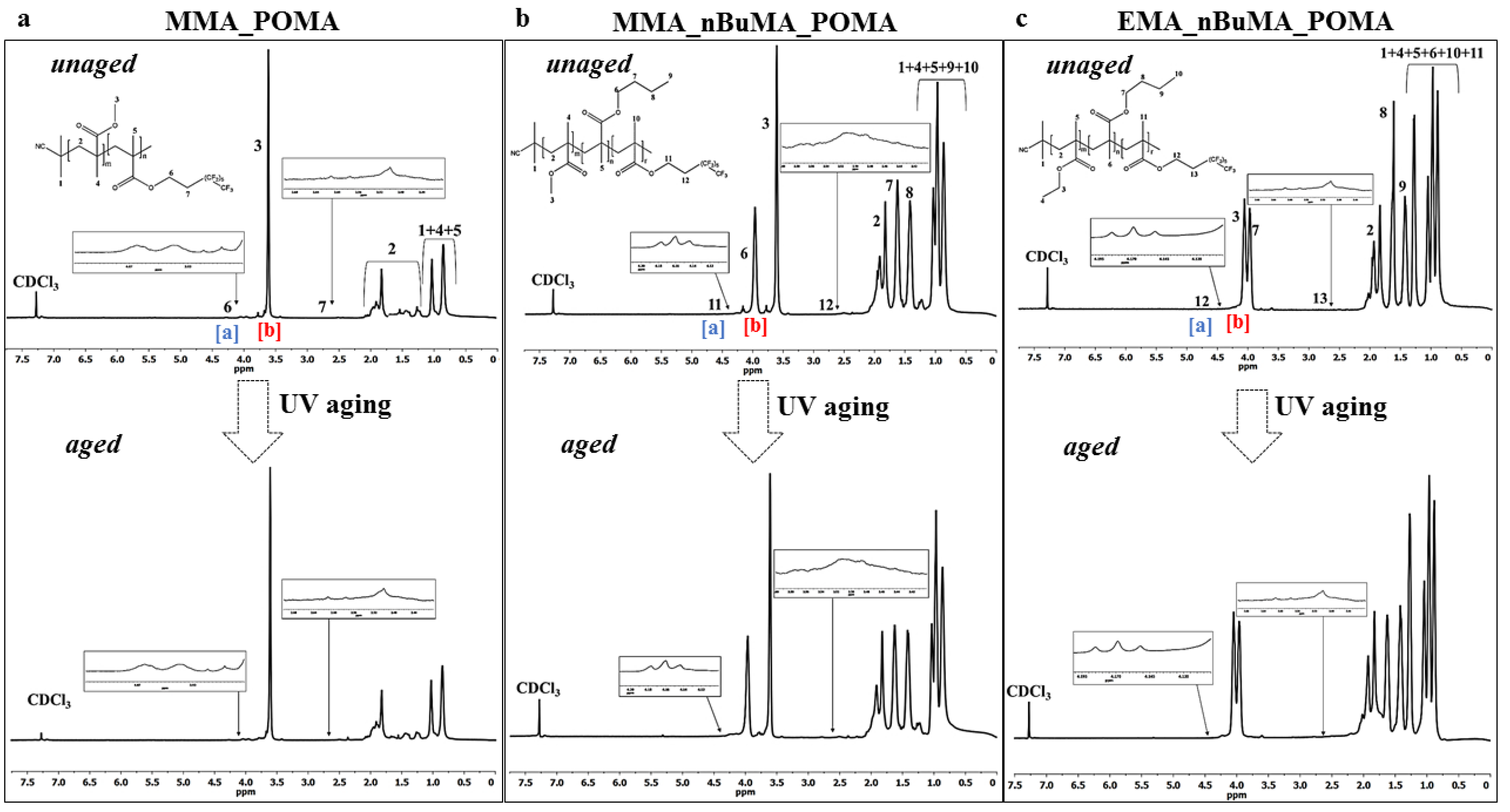
3.1. Synthesis of MMA_POMA, MMA_nBuMA_POMA and EMA_nBuMA_POMA Resins and Their Characterization before and after the UV Aging Test
3.2. Characterization of Botticino Coated Samples and Exposure in a Real Polluted Environment
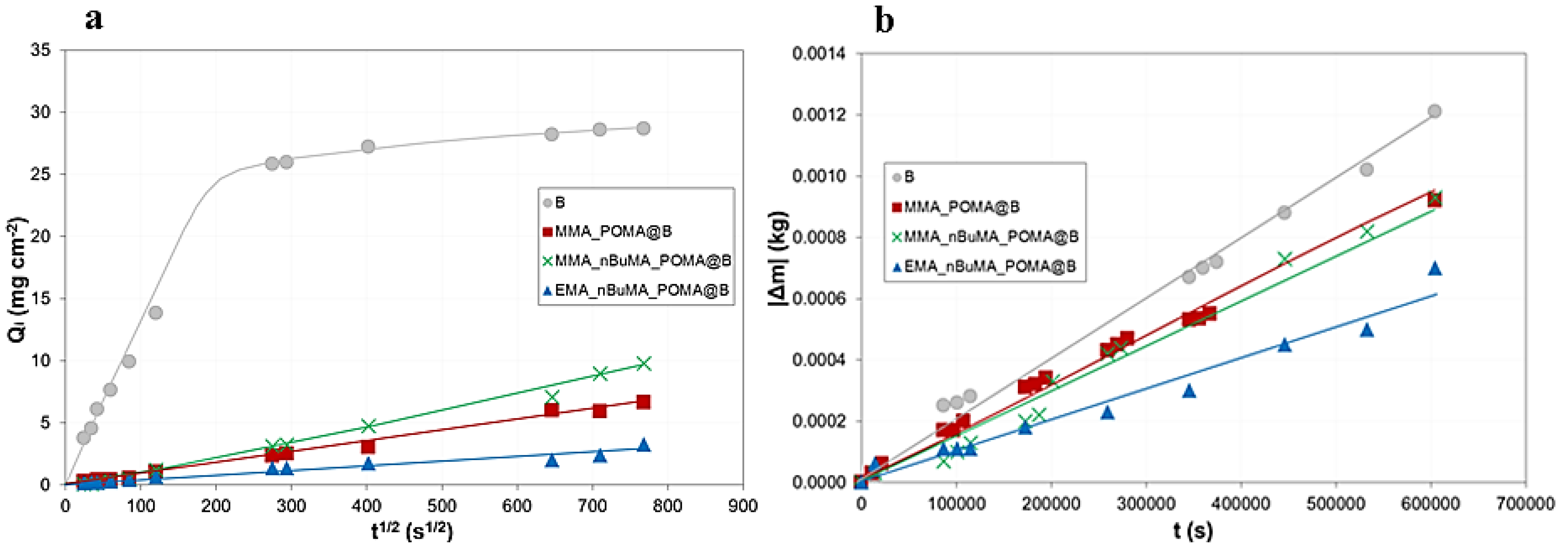
3.2.1. Surface Properties, Water Capillarity and Vapour Permeability of Treated Marble
3.2.2. Real External Exposure Test
3.3. Coatings Removal from Botticino Samples: Polymers and Stones Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Camuffo, D. Microclimate for Cultural Heritage: Conservation, Restoration, and Maintenance of Indoor and Outdoor Monuments; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 978-0-444-63296-8. [Google Scholar]
- Varotsos, C.; Tzanis, C.; Cracknell, A. The enhanced deterioration of the cultural heritage monuments due to air pollution. Environ. Sci. Pollut. Res. 2009, 16, 590–592. [Google Scholar] [CrossRef] [PubMed]
- Charola, A.E. Salts in the Deterioration of Porous Materials: An Overview. J. Am. Inst. Conserv. 2018, 39, 327–343. [Google Scholar] [CrossRef]
- De Ferri, L.; Lottici, P.P.; Lorenzi, A.; Montenero, A.; Salvioli-Mariani, E. Study of silica nanoparticles—Polysiloxane hydrophobic treatments for stone-based monument protection. J. Cult. Herit. 2011, 12, 356–363. [Google Scholar] [CrossRef]
- Bonazza, A.; Sabbioni, C.; Ghedini, N. Quantitative data on carbon fractions in interpretation of black crusts and soiling on European built heritage. Atmos. Environ. 2005, 39, 2607–2618. [Google Scholar] [CrossRef]
- Piacenti, F. Chemistry for the conservation of the cultural heritage. Sci. Total Environ. 1994, 143, 113–120. [Google Scholar] [CrossRef]
- Iñigo, A.C.; García-Talegón, J.; Vicente-Tavera, S.; Martín-González, S.; Casado-Marín, S.; Vargas-Muñoz, M.; Pérez-Rodríguez, J.L. Colour and ultrasound propagation speed changes by different ageing of freezing/thawing and cooling/heating in granitic materials. Cold Reg. Sci. Technol. 2013, 85, 71–78. [Google Scholar] [CrossRef]
- Fernandes, P. Applied microbiology and biotechnology in the conservation of stone cultural heritage materials. Appl. Microbiol. Biotechnol. 2006, 73, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.J.; Gomez-Heras, M.; McCabe, S. Understanding the decay of stone-built cultural heritage. Prog. Phys. Geogr. 2008, 32, 439–461. [Google Scholar] [CrossRef]
- Melo, M.J.; Bracci, S.; Camaiti, M.; Chiantore, O.; Piacenti, F. Photodegradation of acrylic resins used in the conservation of stone. Polym. Degrad. Stab. 1999, 66, 23–30. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Karapanagiotis, I.; Tsakalof, A.; Zuburtikudis, I.; Kolinkeová, B.; Panayiotou, C. Superhydrophobic films for the protection of outdoor cultural heritage assets. Appl. Phys. A Mater. Sci. Process. 2009, 97, 351–360. [Google Scholar] [CrossRef]
- Cappelletti, G.; Fermo, P.; Camiloni, M. Smart hybrid coatings for natural stones conservation. Prog. Org. Coat. 2015, 78. [Google Scholar] [CrossRef]
- Dillon, C.E.; Lagalante, A.F.; Wolbers, R.C. Acrylic emulsion paint films: The effect of solution pH, conductivity, and ionic strength on film swelling and surfactant removal. Stud. Conserv. 2014, 59, 52–62. [Google Scholar] [CrossRef]
- Ma, C.; Wang, H.; Zhang, H.; Liu, X.; Chen, H. Preparation and Properties of Fluorinated Poly(ethyl methacrylate-co-butyl acrylate). Polym. Sci. Ser. B 2019, 61, 163–169. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Q.; Liu, T.; Zhang, B. The preservation damage of hydrophobic polymer coating materials in conservation of stone relics. Prog. Org. Coat. 2013, 76, 1127–1134. [Google Scholar] [CrossRef]
- Cocca, M.; D’Arienzo, L.; D’Orazio, L.; Gentile, G.; Martuscelli, E. Polyacrylates for conservation: Chemico-physical properties and durability of different commercial products. Polym. Test. 2004, 23, 333–342. [Google Scholar] [CrossRef]
- Karapanagiotis, I.; Manoudis, P.N.; Savva, A.; Panayiotou, C. Superhydrophobic polymer-particle composite films produced using various particle sizes. Surf. Interface Anal. 2012, 44, 870–875. [Google Scholar] [CrossRef]
- Bernett, M.K.; Zisman, W.A. Wetting properties of acrylic and methacrylic polymers containing fluorinated side chains. J. Phys. Chem. 1962, 66, 1207–1208. [Google Scholar] [CrossRef]
- Imae, T. Fluorinated polymers. Curr. Opin. Colloid Interface Sci. 2003, 8, 307–314. [Google Scholar] [CrossRef]
- Papadopoulou, S.K.; Dritsas, G.; Karapanagiotis, I.; Zuburtikudis, I.; Panayiotou, C. Surface characterization of poly(2,2,3,3,3-pentafluoropropyl methacrylate) by inverse gas chromatography and contact angle measurements. Eur. Polym. J. 2010, 46, 202–208. [Google Scholar] [CrossRef]
- Alessandrini, G.; Aglietto, M.; Castelvetro, V.; Ciardelli, F.; Peruzzi, R.; Toniolo, L. Comparative evaluation of fluorinated and unfluorinated acrylic copolymers as water-repellent coating materials for stone. J. Appl. Polym. Sci. 2000, 76, 962–977. [Google Scholar] [CrossRef]
- Mazzola, M.; Frediani, P.; Bracci, S.; Salvini, A. New strategies for the synthesis of partially fluorinated acrylic polymers as possible materials for the protection of stone monuments. Eur. Polym. J. 2003, 39, 1995–2003. [Google Scholar] [CrossRef]
- Keller, L.; Decker, C.; Zahouily, K.; Benfarhi, S.; Le Meins, J.M.; Miehe-Brendle, J. Synthesis of polymer nanocomposites by UV-curing of organoclay-acrylic resins. Polymer 2004, 45, 7437–7447. [Google Scholar] [CrossRef]
- Sabatini, V.; Cattò, C.; Cappelletti, G.; Cappitelli, F.; Antenucci, S.; Farina, H.; Ortenzi, M.A.; Camazzola, S.; Di Silvestro, G. Protective features, durability and biodegration study of acrylic and methacrylic fluorinated polymer coatings for marble protection. Prog. Org. Coat. 2018, 114, 47–57. [Google Scholar] [CrossRef]
- Sabatini, V.; Farina, H.; Montarsolo, A.; Pargoletti, E.; Ortenzi, M.A.; Cappelletti, G. Fluorinated Polyacrylic Resins for the Protection of Cultural Heritages: The Effect of Fluorine on Hydrophobic Properties and Photochemical Stability. Chem. Lett. 2018, 47, 280–283. [Google Scholar] [CrossRef]
- Sabatini, V.; Rimoldi, L.; Tripaldi, L.; Meroni, D.; Farina, H.; Ortenzi, M.; Ardizzone, S. TiO2-SiO2-PMMA Terpolymer Floating Device for the Photocatalytic Remediation of Water and Gas Phase Pollutants. Catalysts 2018, 8, 568. [Google Scholar] [CrossRef]
- Sabatini, V.; Pargoletti, E.; Longoni, M.; Farina, H.; Ortenzi, M.A.; Cappelletti, G. Stearyl methacrylate co-polymers: Towards new polymer coatings for mortars protection. Appl. Surf. Sci. 2019, 488, 213–220. [Google Scholar] [CrossRef]
- Clerici, A.; Meda, A. Confronto tra le caratteristiche meccaniche di diversi livelli di estrazione del Botticino Classico. G. Geol. Appl. 2005, 2, 307–312. [Google Scholar] [CrossRef]
- Fermo, P.; Cappelletti, G.; Cozzi, N.; Padeletti, G.; Kaciulis, S.; Brucale, M.; Merlini, M. Hydrophobizing coatings for cultural heritage. A detailed study of resin/stone surface interaction. Appl. Phys. A Mater. Sci. Process. 2014, 116, 341–348. [Google Scholar] [CrossRef]
- UNI 10925:2001 Beni Culturali—Materiali Lapidei Naturali ed Artificiali—Metodologia Per l’Irraggiamento Con Luce Solare Artificiale ICS: [91.100.15]. Available online: https://www.unirc.it/documentazione/materiale_didattico/1463_2016_426_26713.pdf (accessed on 13 April 2016).
- Cappelletti, G.; Fermo, P.; Pino, F.; Pargoletti, E.; Pecchioni, E.; Fratini, F.; Ruffolo, S.A.; La Russa, M.F. On the role of hydrophobic Si-based protective coatings in limiting mortar deterioration. Environ. Sci. Pollut. Res. 2015, 22, 17733–17743. [Google Scholar] [CrossRef]
- Forrest, C. International Law and the Protection of Cultural Heritage; Taylor & Francis: Milton Park, UK, 2012; ISBN 9780203865194. [Google Scholar]
- Ruggles, D.F.; Silverman, H. From Tangible to Intangible Heritage; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 978-1-4419-0071-5. [Google Scholar]
- UNI EN, 1936 Determination of Apparent Density and Total Open Porosity 2001. Available online: https://www.scribd.com/document/276530524/BS-en-1936-1999-Natural-Stone-Test-Methods-Determination-of-Real-Density-and-Apparent-Density-and-of-Total-and-Open-Porosity-1 (accessed on 13 February 1999).
- Pino, F.; Fermo, P.; La Russa, M.; Ruffolo, S.; Comite, V.; Baghdachi, J.; Pecchioni, E.; Fratini, F.; Cappelletti, G. Advanced mortar coatings for cultural heritage protection. Durability towards prolonged UV and outdoor exposure. Environ. Sci. Pollut. Res. 2017, 24, 12608–12617. [Google Scholar] [CrossRef]
- Fermo, P.; Turrion, R.G.; Rosa, M.; Omegna, A. A new approach to assess the chemical composition of powder deposits damaging the stone surfaces of historical monuments. Environ. Sci. Pollut. Res. 2015, 22, 6262–6270. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Kim, J.-B.; Bruening, M.L.; Baker, G.L. Functionalization of Surfaces by Water-Accelerated Atom-Transfer Radical Polymerization of Hydroxyethyl Methacrylate and Subsequent Derivatization. Macromolecules 2002, 35, 1175–1179. [Google Scholar] [CrossRef]
- Del Grosso, C.A.; Poulis, J.A.; de la Rie, E.R. The photo-stability of acrylic tri-block copolymer blends for the consolidation of cultural heritage. Polym. Degrad. Stab. 2019, 159, 31–42. [Google Scholar] [CrossRef]
- Chiantore, O.; Trossarelli, L.; Lazzari, M. Photooxidative degradation of acrylic and methacrylic polymers. Polymer 2000, 41, 1657–1668. [Google Scholar] [CrossRef]
- MacCallum, N.G.J.R. Journal of Polymer Science Part A: General Papers. J. Polym. Sci. Part A Gen. Pap. 1964, 2, 983–1000. [Google Scholar] [CrossRef]
- Soeriyadi, A.H.; Trouillet, V.; Bennet, F.; Bruns, M.; Whittaker, M.R.; Boyer, C.; Barker, P.J.; Davis, T.P.; Barner-Kowollik, C. A detailed surface analytical study of degradation processes in (meth)acrylic polymers. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1801–1811. [Google Scholar] [CrossRef]
- Sabatini, V.; Farina, H.; Montarsolo, A.; Ardizzone, S.; Ortenzi, M.A. Novel Synthetic Approach to Tune the Surface Properties of Polymeric Films: Ionic Exchange Reaction between Sulfonated Polyarylethersulfones and Ionic Liquids. Polym. Plast. Technol. Eng. 2017, 56. [Google Scholar] [CrossRef]
- Anton, B.D. Surface-Fluorinated Coatings. Adv. Mater. 1998, 5, 1197–1205. [Google Scholar] [CrossRef]
- Ocak, Y.; Sofuoglu, A.; Tihminlioglu, F.; Böke, H. Protection of marble surfaces by using biodegradable polymers as coating agent. Prog. Org. Coat. 2009, 66, 213–220. [Google Scholar] [CrossRef]
- La Russa, M.F.; Ruffolo, S.A.; Rovella, N.; Belfiore, C.M.; Palermo, A.M.; Guzzi, M.T.; Crisci, G.M. Multifunctional TiO2 Coatings for Cultural Heritage. Prog. Org. Coat. 2012, 74, 186–191. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Tsakalof, A.; Karapanagiotis, I.; Zuburtikudis, I.; Panayiotou, C. Fabrication of super-hydrophobic surfaces for enhanced stone protection. Surf. Coat. Technol. 2009, 203, 1322–1328. [Google Scholar] [CrossRef]
- Rodrigues, J.D.; Grossi, A. Indicators and ratings for the compatibility assessment of conservation actions. J. Cult. Herit. 2007, 8, 32–43. [Google Scholar] [CrossRef]
- Aslanidou, D.; Karapanagiotis, I.; Lampakis, D. Waterborne superhydrophobic and superoleophobic coatings for the protection of marble and sandstone. Materials 2018, 11, 585. [Google Scholar] [CrossRef] [PubMed]
- Toniolo, L.; Poli, T.; Castelvetro, V.; Manariti, A.; Chiantore, O.; Lazzari, M. Tailoring new fluorinated acrylic copolymers as protective coatings for marble. J. Cult. Herit. 2002, 3, 309–316. [Google Scholar] [CrossRef]
- Lettieri, M.; Masieri, M.; Morelli, A.; Pipoli, M.; Frigione, M. Oleo/Hydrophobic Coatings Containing Nano-Particles for the Protection of Stone Materials Having Different Porosity. Coatings 2018, 8, 429. [Google Scholar] [CrossRef]
- Tidblad, J.; Kucera, V.; Ferm, M.; Kreislova, K.; Brüggerhoff, S.; Doytchinov, S.; Screpanti, A.; Grøntoft, T.; Yates, T.; De La Fuente, D.; et al. Effects of air pollution on materials and cultural heritage: ICP materials celebrates 25 years of research. Int. J. Corros. 2012, 2012. [Google Scholar] [CrossRef]
- Ausset, P.; Crovisier, J.L.; Del Monte, M.; Furlan, V.; Girardet, F.; Hammecker, C.; Jeannette, D.; Lefevre, R.A. Experimental study of limestone and sandstone sulphation in polluted realistic conditions: The Lausanne Atmospheric Simulation Chamber (LASC). Atmos. Environ. 1996, 30, 3197–3207. [Google Scholar] [CrossRef]
- Comite, V.; Fermo, P. The effects of air pollution on cultural heritage: The case study of Santa Maria delle Grazie al Naviglio Grande (Milan) ⋆. Eur. Phys. J. Plus 2018, 133. [Google Scholar] [CrossRef]
- Bernardoni, V.; Vecchi, R.; Valli, G.; Piazzalunga, A.; Fermo, P. PM10 source apportionment in Milan (Italy) using time-resolved data. Sci. Total Environ. 2011, 409, 4788–4795. [Google Scholar] [CrossRef]
- Bernardoni, V.; Elser, M.; Valli, G.; Valentini, S.; Bigi, A.; Fermo, P.; Piazzalunga, A.; Vecchi, R. Size-segregated aerosol in a hot-spot pollution urban area: Chemical composition and three-way source apportionment. Environ. Pollut. 2017, 231, 601–611. [Google Scholar] [CrossRef]
- Gulotta, D.; Bertoldi, M.; Bortolotto, S.; Fermo, P.; Piazzalunga, A.; Toniolo, L. The Angera stone: A challenging conservation issue in the polluted environment of Milan (Italy). Environ. Earth Sci. 2013, 69, 1085–1094. [Google Scholar] [CrossRef]
- Fermo, P.; Goidanich, S.; Comite, V.; Toniolo, L.; Gulotta, D. Study and Characterization of Environmental Deposition on Marble and Surrogate Substrates at a Monumental Heritage Site. Geosciences 2018, 8, 349. [Google Scholar] [CrossRef]
- Comite, V.; de Buergo, M.Á.; Barca, D.; Belfiore, C.M.; Bonazza, A.; La Russa, M.F.; Pezzino, A.; Randazzo, L.; Ruffolo, S.A. Damage monitoring on carbonate stones: Field exposure tests contributing to pollution impact evaluation in two Italian sites. Constr. Build. Mater. 2017, 152, 907–922. [Google Scholar] [CrossRef]
- Vecchi, R.; Bernardoni, V.; Fermo, P.; Lucarelli, F.; Mazzei, F.; Nava, S.; Prati, P.; Piazzalunga, A.; Valli, G. 4-hours resolution data to study PM10 in a “hot spot” area in Europe. Environ. Monit. Assess. 2009, 154, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, M.; Chiantore, O. Thermal ageing of paraloid acrylic protective polymers. Polymer 2000, 41, 6447–6455. [Google Scholar] [CrossRef]
- Castelvetro, V.; Aglietto, M.; di Mirabello, L.M.; Toniolo, L.; Peruzzi, R.; Chiantore, O. Adapting the properties of new fluorinated acrylic polymers to suit the conservation of ancient monuments. Surf. Coat. Int. 1998, 81, 551–556. [Google Scholar] [CrossRef]
- Van Herk, A.; Kaczmarek, H.; Kamin, A. Photooxidative degradation of poly (alkyl methacrylate) s. Eur. Polym. J. 2000, 36, 767–777. [Google Scholar]





| Samples | (Da) | D | Tg (°C) | θ (°) Air-Side | θ (°) Mold-Side | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| UV Aging | ||||||||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| MMA_POMA | 27000 | 25000 | 2.3 | 2.4 | 89 | 87 | 72 ± 2 | 74 ± 3 | 93 ± 1 | 91 ± 1 |
| MMA_nBuMA_POMA | 27000 | 22100 | 2.6 | 2.7 | 62 | 64 | 75 ± 1 | 73 ± 2 | 94 ± 2 | 94 ± 3 |
| EMA_nBuMA_POMA | 26800 | 23400 | 2.6 | 2.8 | 48 | 47 | 69 ± 2 | 72 ± 4 | 96 ± 4 | 92 ± 4 |
| Samples | WCA (°) | ΔE*(Treated-Bare) | Qft (mg × cm−2) | CA (mg × cm−2 × s−1/2) | % RVP | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outdoor Exposure | ||||||||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| B | 40 ± 6 | 70 ± 7 | − | − | 28.7 | 32.7 | 0.143 | 0.102 | − | − |
| MMA_POMA@B | 90 ± 3 | 77 ± 7 | 3.2 | 3.1 | 6.6 | 5.6 | 0.003 | 0.005 | 24 | 30 |
| MMA_nBuMA_POMA@B | 82 ± 1 | 83 ± 4 | 3.0 | 2.8 | 9.8 | 1.3 | 0.005 | 0.005 | 23 | 25 |
| EMA_nBuMA_POMA@B | 82 ± 2 | 80 ± 4 | 1.4 | 7.1 | 3.3 | 4.2 | 0.010 | 0.016 | 42 | 53 |
| Samples | Anions (µg × cm−2) | |
|---|---|---|
| NO3− | SO42− | |
| B | 20 ± 5 | 24 ± 8 |
| MMA_POMA@B | 21 ± 5 | 7 ± 4 |
| MMA_nBuMA_POMA@B | 44 ± 7 | 2 ± 1 |
| EMA_nBuMA_POMA@B | 19 ± 4 | 9 ± 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabatini, V.; Pargoletti, E.; Comite, V.; Ortenzi, M.A.; Fermo, P.; Gulotta, D.; Cappelletti, G. Towards Novel Fluorinated Methacrylic Coatings for Cultural Heritage: A Combined Polymers and Surfaces Chemistry Study. Polymers 2019, 11, 1190. https://doi.org/10.3390/polym11071190
Sabatini V, Pargoletti E, Comite V, Ortenzi MA, Fermo P, Gulotta D, Cappelletti G. Towards Novel Fluorinated Methacrylic Coatings for Cultural Heritage: A Combined Polymers and Surfaces Chemistry Study. Polymers. 2019; 11(7):1190. https://doi.org/10.3390/polym11071190
Chicago/Turabian StyleSabatini, Valentina, Eleonora Pargoletti, Valeria Comite, Marco Aldo Ortenzi, Paola Fermo, Davide Gulotta, and Giuseppe Cappelletti. 2019. "Towards Novel Fluorinated Methacrylic Coatings for Cultural Heritage: A Combined Polymers and Surfaces Chemistry Study" Polymers 11, no. 7: 1190. https://doi.org/10.3390/polym11071190
APA StyleSabatini, V., Pargoletti, E., Comite, V., Ortenzi, M. A., Fermo, P., Gulotta, D., & Cappelletti, G. (2019). Towards Novel Fluorinated Methacrylic Coatings for Cultural Heritage: A Combined Polymers and Surfaces Chemistry Study. Polymers, 11(7), 1190. https://doi.org/10.3390/polym11071190