Aminomethylated Calix[4]resorcinarenes as Modifying Agents for Glycidyl Methacrylate (GMA) Rigid Copolymers Surface
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Information
2.2. Synthesis of the Starting Materials
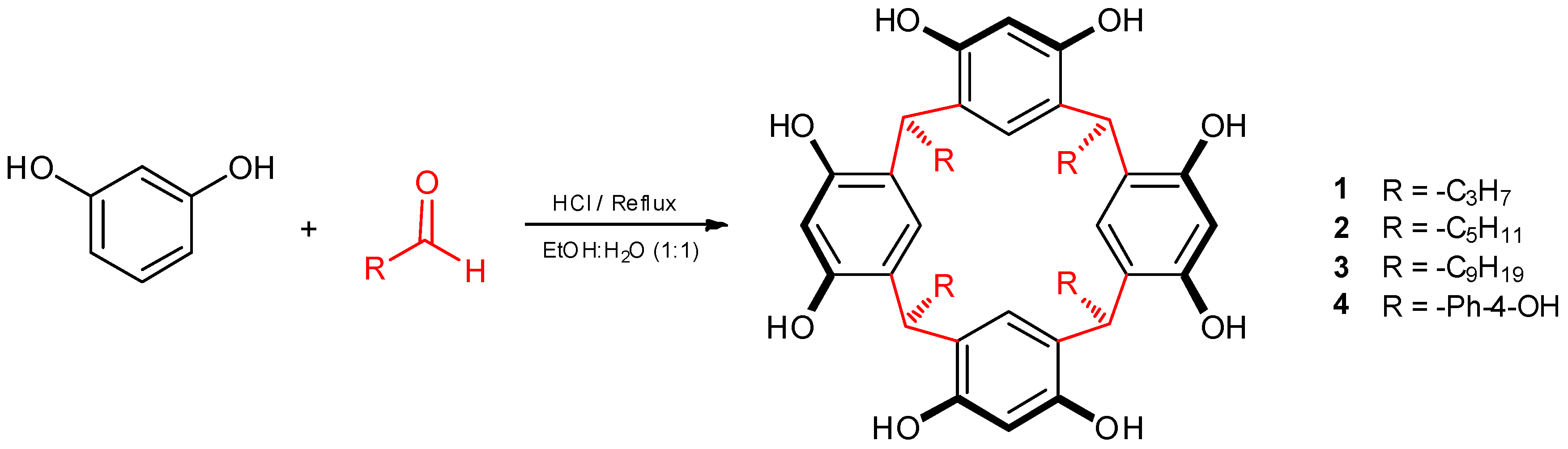
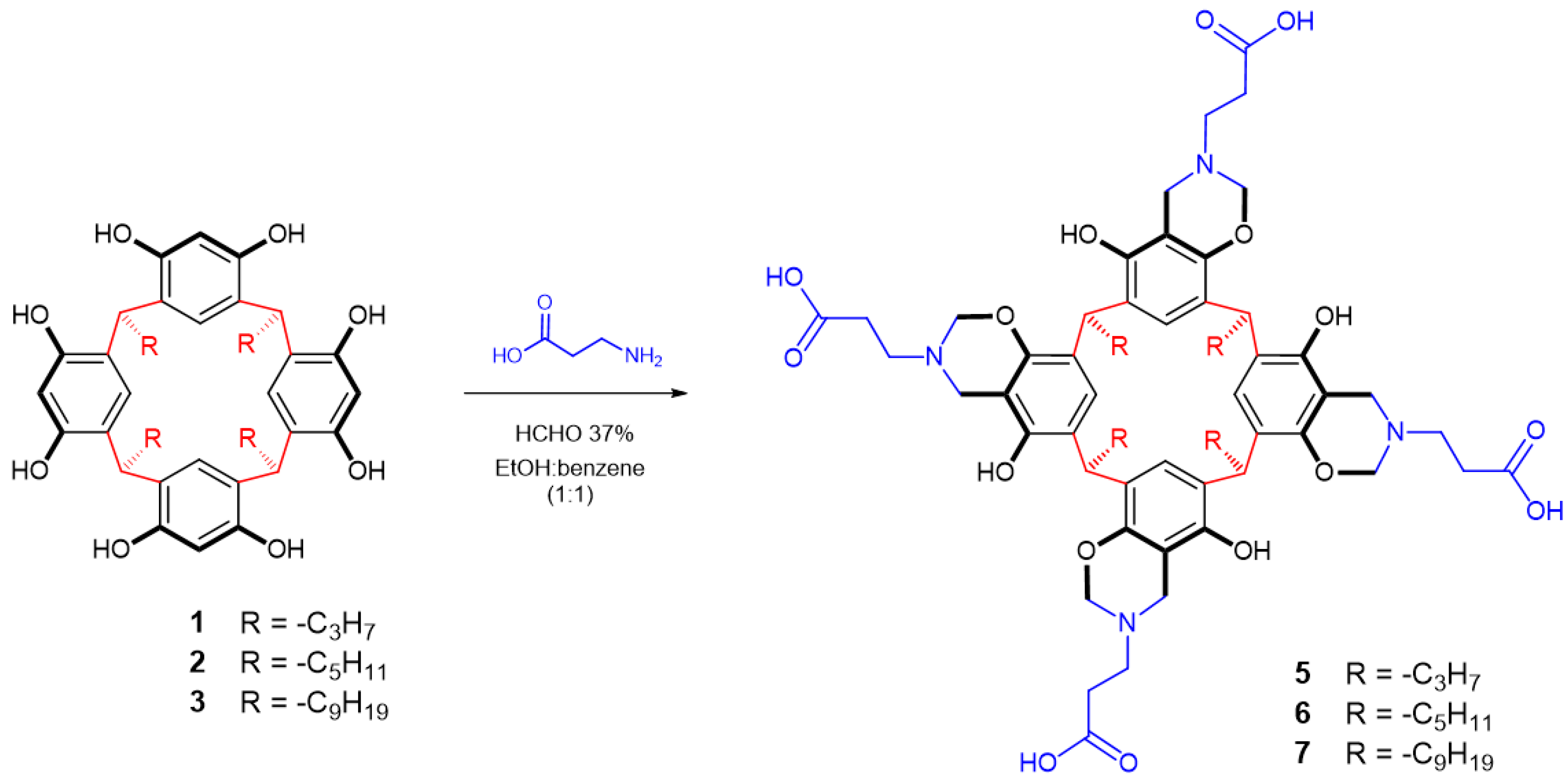
2.3. Reaction of Calix[4]resorcinarenes 1–3 with β-alanine
2.4. Reaction of Calix[4]resorcinarene 4 with l-proline
2.5. Preparation of Copolymers
2.5.1. Poly(GMA–co–EDMA)
2.5.2. Poly(BMA–co–EDMA–co–GMA)
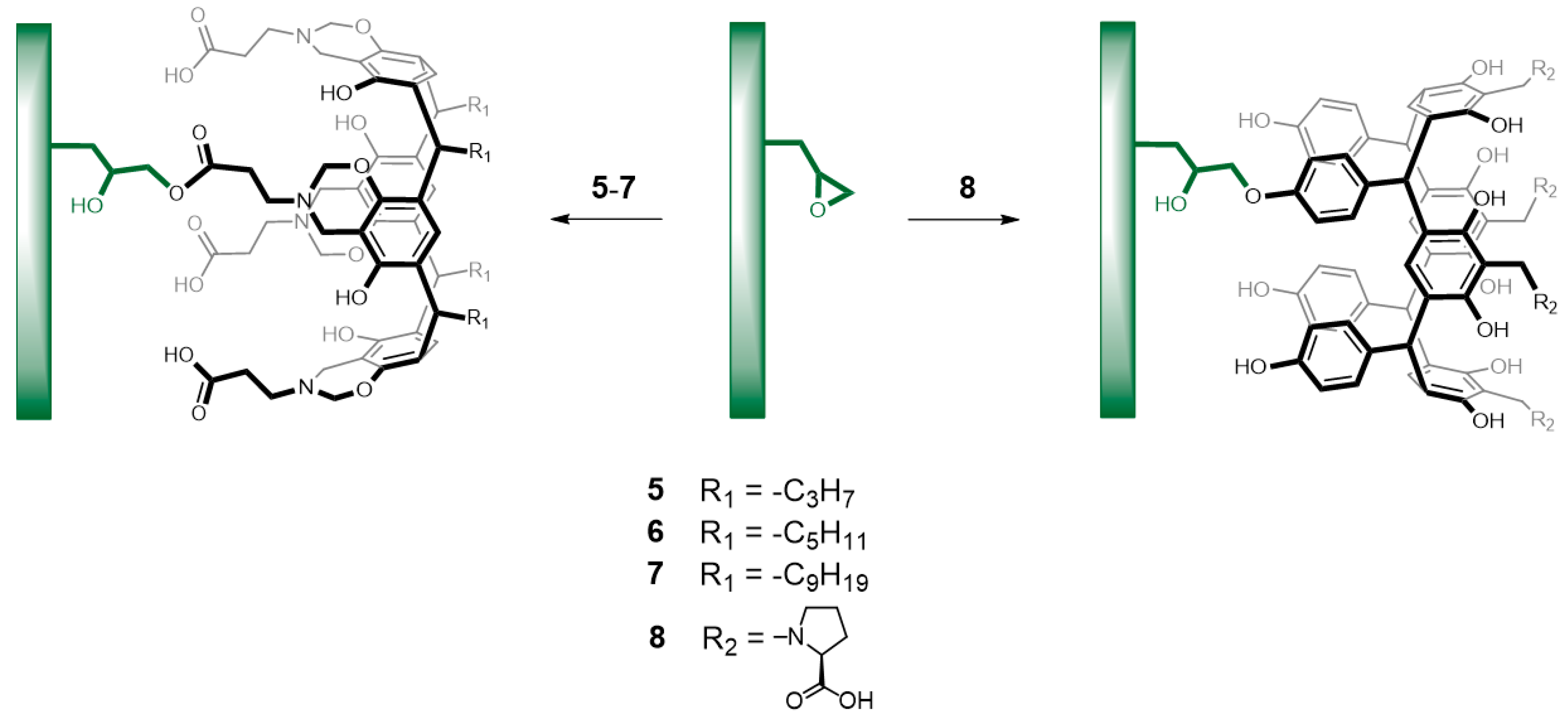
2.6. Surface Modification of Copolymers with Aminomethylated-Calix[4]resorcinarenes 5–7
2.7. Surface Modification of Copolymers with Aminomethylated-Calix[4]resorcinarene 8
2.8. Interaction of Peptides with Copolymers
3. Results
3.1. Synthesis of Calix[4]resorcinarenes and Aminomethylated Derivates
3.2. Copolymerization and Surface Modification
3.3. Interaction of the Functionalized Surfaces with Peptides
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Díaz-Bao, M.; Barreiro, R.; Miranda, J.; Cepeda, A.; Regal, P. Recent Advances and Uses of Monolithic Columns for the Analysis of Residues and Contaminants in Food. Chromatography 2015, 2, 79–95. [Google Scholar] [CrossRef]
- Andrade-Eiroa, A.; Canle, M.; Leroy-Cancellieri, V.; Cerdà, V. Solid-phase extraction of organic compounds: A critical review (Part I). Trends Anal. Chem. 2016, 80, 641–654. [Google Scholar] [CrossRef]
- Andrade-Eiroa, A.; Canle, M.; Leroy-Cancellieri, V.; Cerdà, V. Solid-phase extraction of organic compounds: A critical review. part ii. Trends Anal. Chem. 2016, 80, 655–667. [Google Scholar] [CrossRef]
- Spietelun, A.; Pilarczyk, M.; Kloskowski, A.; Namiesnik, J. Current trends in solid-phase microextraction (SPME) fibre coatings. Chem. Soc. Rev. 2010, 39, 4524–4537. [Google Scholar] [CrossRef] [PubMed]
- Fumes, B.H.; Silva, M.R.; Andrade, F.N.; Nazario, C.E.D.; Lanças, F.M. Recent advances and future trends in new materials for sample preparation. Trends Anal. Chem. 2015, 71, 9–25. [Google Scholar] [CrossRef]
- Mokhtari, B.; Pourabdollah, K.; Dalali, N. Analytical applications of calixarenes from 2005 up-to-date. J. Incl. Phenom. Macrocycl. Chem. 2011, 69, 1–55. [Google Scholar] [CrossRef]
- Li, M.; Tarawally, M.; Liu, X.; Liu, X.; Guo, L.; Yang, L.; Wang, G. Application of cyclodextrin-modified gold nanoparticles in enantioselective monolith capillary electrochromatography. Talanta 2013, 109, 1–6. [Google Scholar] [CrossRef]
- Sanabria, E.; Maldonado, M. Calixarenes: Generalities and Their Role in Improving the Solubility, Biocompatibility, Stability, Bioavailability, Detection, and Transport of Biomolecules. Biomolecules 2019, 9, 90. [Google Scholar] [CrossRef]
- Li, N.; Harrison, R.G.; Lamb, J.D. Application of resorcinarene derivatives in chemical separations. J. Incl. Phenom. Macrocycl. Chem. 2014, 78, 39–60. [Google Scholar] [CrossRef]
- O’Farrell, C.M.; Chudomel, J.M.; Collins, J.M.; Dignam, C.F.; Wenzel, T.J. Water-Soluble Calix[4]resorcinarenes with Hydroxyproline Groups as Chiral NMR Solvating Agents. J. Org. Chem. 2008, 73, 2843–2851. [Google Scholar] [CrossRef]
- Wenzel, T.J. Calixarenes and calix[4]resorcinarenes as chiral NMR solvating agents. J. Incl. Phenom. Macrocycl. Chem. 2014, 78, 1–14. [Google Scholar] [CrossRef]
- Beyeh, N.K.; Weimann, D.P.; Kaufmann, L.; Schalley, C.A.; Rissanen, K. Ion-pair recognition of tetramethylammonium salts by halogenated resorcinarenes. Chem. A Eur. J. 2012, 18, 5552–5557. [Google Scholar] [CrossRef] [PubMed]
- Salorinne, K.; Weimann, D.P.; Schalley, C.A.; Nissinen, M. Resorcinarene podand with amine-functionalized side arms - Synthesis, structure, and binding properties of a neutral anion receptor. Eur. J. Org. Chem. 2009, 2009, 6151–6159. [Google Scholar] [CrossRef]
- Hayashida, O.; Uchiyama, M. Cyclophane-based tetra(resorcinarene) as a host for both histone and hydrophobic molecular guests. Tetrahedron Lett. 2006, 47, 4091–4094. [Google Scholar] [CrossRef]
- Deakyne, C.A.; Fowler, D.A.; Atwood, J.L. Molecular capsules based on metal complexes with resorcin [4]arenes and pyrogallol[4]arenes. PATAI’S Chem. Funct. Groups 2012, 1–33. [Google Scholar] [CrossRef]
- Biros, S.M.; Rebek, J.J. Structure and binding properties of water-soluble cavitands and capsules. Chem. Soc. Rev. 2007, 36, 93–104. [Google Scholar] [CrossRef]
- Palmer, L.C.; Rebek, J.J. The ins and outs of molecular encapsulation. Org. Biomol. Chem. 2004, 2, 3051–3059. [Google Scholar] [CrossRef]
- Tan, D.A.; Mocerino, M. Distal functionalisation of C4 symmetric tetramethoxyresorcinarene by selective lithiation. J. Incl. Phenom. Macrocycl. Chem. 2018, 91, 71–80. [Google Scholar] [CrossRef]
- Russo, M.; Meo, P. Lo Binding abilities of a chiral calix[4]resorcinarene: a polarimetric investigation on a complex case of study. Beilstein J. od Org. Chem. 2017, 13, 2698–2709. [Google Scholar] [CrossRef]
- Helttunen, K.; Tero, T.-R.; Nissinen, M. Influence of lower rim C-methyl group on crystal forms and metal complexation of resorcinarene bis-crown-5. Cryst. Eng. Comm. 2015, 17, 3667–3676. [Google Scholar] [CrossRef]
- Jose, T.; Cañellas, S.; Pericàs, M.A.; Kleij, A.W. Polystyrene-supported bifunctional resorcinarenes as cheap, metal-free and recyclable catalysts for epoxide/CO2 coupling reactions. Green Chem. 2017, 19, 5488–5493. [Google Scholar] [CrossRef]
- Pollok, C.H.; Zhang, Q.; Tiefenbacher, K.; Merten, C. Chirality induction from a chiral guest to the hydrogen bonding network of its hexameric resorcinarene host capsule. ChemPhysChem. 2017, 18, 1987–1991. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Guo, H.; Vicens, J. Mini-review: calixarene liquid crystals. J. Incl. Phenom. Macrocycl. Chem. 2014, 80, 177–186. [Google Scholar] [CrossRef]
- Jain, V.K.; Kanaiya, P.H. Chemistry of calix[4]resorcinarenes. Russ. Chem. Rev. 2011, 80, 75–102. [Google Scholar] [CrossRef]
- Nordborg, A.; Hilder, E.F. Recent advances in polymer monoliths for ion-exchange chromatography. Anal. Bioanal. Chem. 2009, 394, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Soh, S.; Zhao, J.; Yong, E.; Gong, Y. Preparation and Application of Methylcalix[4]resorcinarene-bonded Silica Particles as Chiral Stationary Phase in High-performance Liquid Chromatography. Chirality 2011, 23, E91–E97. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.M.; Wang, X.; Soh, S.F.; Tan, S.; Zhao, J.; Yong, E.L.; Lee, H.K.; Gong, Y. Preparation and Application of Mixed Octadecylsilyl- and (3-(C-methylcalix[4]resorcinarene)-hydroxypropoxy)-propylsilyl-Appended Silica Particles as Stationary Phase for High-Performance Liquid Chromatography. Instrum. Sci. Technol. 2012, 40, 100–111. [Google Scholar] [CrossRef]
- Sokoließ, T.; Menyes, U.; Roth, U.; Jira, T. Separation of cis- and trans-isomers of thioxanthene and dibenz[b,e]oxepin derivatives on calixarene- and resorcinarene-bonded high-performance liquid chromatography stationary phases. J. Chromatogr. A 2002, 948, 309–319. [Google Scholar] [CrossRef]
- Groarke, R.J.; Brabazon, D. Methacrylate polymer monoliths for separation applications. Materials 2016, 9, 446. [Google Scholar] [CrossRef]
- Castillo-Aguirre, A.A.; Velásquez-Silva, B.A.; Palacio, C.; Baez, F.; Rivera-Monroy, Z.J.; Maldonado, M. Surface modification of poly(GMA-co-EDMA-co-MMA) with resorcarenes. J. Braz. Chem. Soc. 2018, 29, 1965–1972. [Google Scholar] [CrossRef]
- Castillo-Aguirre, A.; Rivera-Monroy, Z.; Maldonado, M. Selective o-alkylation of the crown conformer of tetra(4-hydroxyphenyl)calix[4]resorcinarene to the corresponding tetraalkyl ether. Molecules 2017, 22, 1660. [Google Scholar] [CrossRef] [PubMed]
- Sanabria, E.; Esteso, M.; Pérez, A.; Vargas, E.; Maldonado, M. Synthesis and Characterization of Two Sulfonated Resorcinarenes: A New Example of a Linear Array of Sodium Centers and Macrocycles. Molecules 2015, 20, 9915–9928. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Aguirre, A.A.; Rivera-Monroy, Z.J.; Maldonado, M. Analysis by RP-HPLC and Purification by RP-SPE of the C -Tetra(p-hydroxyphenyl)resorcinolarene Crown and Chair Stereoisomers. J. Anal. Methods Chem. 2019, 2019, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Silva, A.; Cortés, B.; Rivera-Monroy, Z.; Pérez-Redondo, A.; Maldonado, M. Crystal structure and dynamic NMR studies of octaacetyl-tetra(propyl)calix[4]resorcinarene. J. Mol. Struct. 2017, 1137, 380–386. [Google Scholar] [CrossRef]
- Maldonado, M.; Sanabria, E.; Batanero, B.; Esteso, M. Apparent molal volume and viscosity values for a new synthesized diazoted resorcin[4]arene in DMSO at several temperatures. J. Mol. Liq. 2017, 231, 142–148. [Google Scholar] [CrossRef]
- Kashapov, R.R.; Zakharova, L.Y.; Saifutdinova, M.N.; Kochergin, Y.S.; Gavrilova, E.L.; Sinyashin, O.G. Construction of a water-soluble form of amino acid C-methylcalix[4]resorcinarene. J. Mol. Liq. 2015, 208, 58–62. [Google Scholar] [CrossRef]
- Airola, K.; Böhmer, V.; Paulus, E.F.; Rissanen, K.; Schmidt, C.; Thondorf, I.; Vogt, W. Selective Derivatisation of Resorcarenes: 1. The Regioselective Formation of Tetra-Benzoxazine Derivatives. Tetrahedron 1997, 53, 10709–10724. [Google Scholar] [CrossRef]
- Okanda, F.M.; El Rassi, Z. Affinity monolithic capillary columns for glycomics/proteomics: 1. Polymethacrylate monoliths with immobilized lectins for glycoprotein separation by affinity capillary electrochromatography and affinity nano-liquid chromatography in either a single colum. Electrophoresis 2006, 27, 1020–1030. [Google Scholar] [CrossRef]
- Merhar, M.; Podgornik, A.; Barut, M.; Žigon, M.; Štrancar, A. Methacrylate monoliths prepared from various hydrophobic and hydrophilic monomers-Structural and chromatographic characteristics. J. Sep. Sci. 2003, 26, 322–330. [Google Scholar] [CrossRef]
- Nasirtabrizi, M.H.; Khodabandlou, S.; Zargin, L.; Parchehbaf Jadid, A. Modification of copolymers using nucleophilic reactions between glycidyl methacrylate and 9-anthracene carboxylic acid. Int. J. Ind. Chem. 2014, 5, 1–8. [Google Scholar] [CrossRef]
- García, M.A.; Hernandez, O.S.; Martínez, G.M.; Klimova, E.; Klimova, T.; Romero, A.M.; Hernandez, O.S.; Martínez, G.M.; Klimova, E.; Klimova, T.; et al. Synthesis of Tetrabenzoxazines and Their Supramolecular Complexes with Fullerene C60. Fullerenes Nanotub. Carbon Nanostruct. 2006, 13, 171–181. [Google Scholar] [CrossRef]
- Vovk, A.I.; Shivanyuk, A.M.; Bugas, R.V.; Muzychka, O.V.; Melnyk, A.K. Antioxidant and antiradical activities of resorcinarene tetranitroxides. Bioorg. Med. Chem. Lett. 2009, 19, 1314–1317. [Google Scholar] [CrossRef] [PubMed]

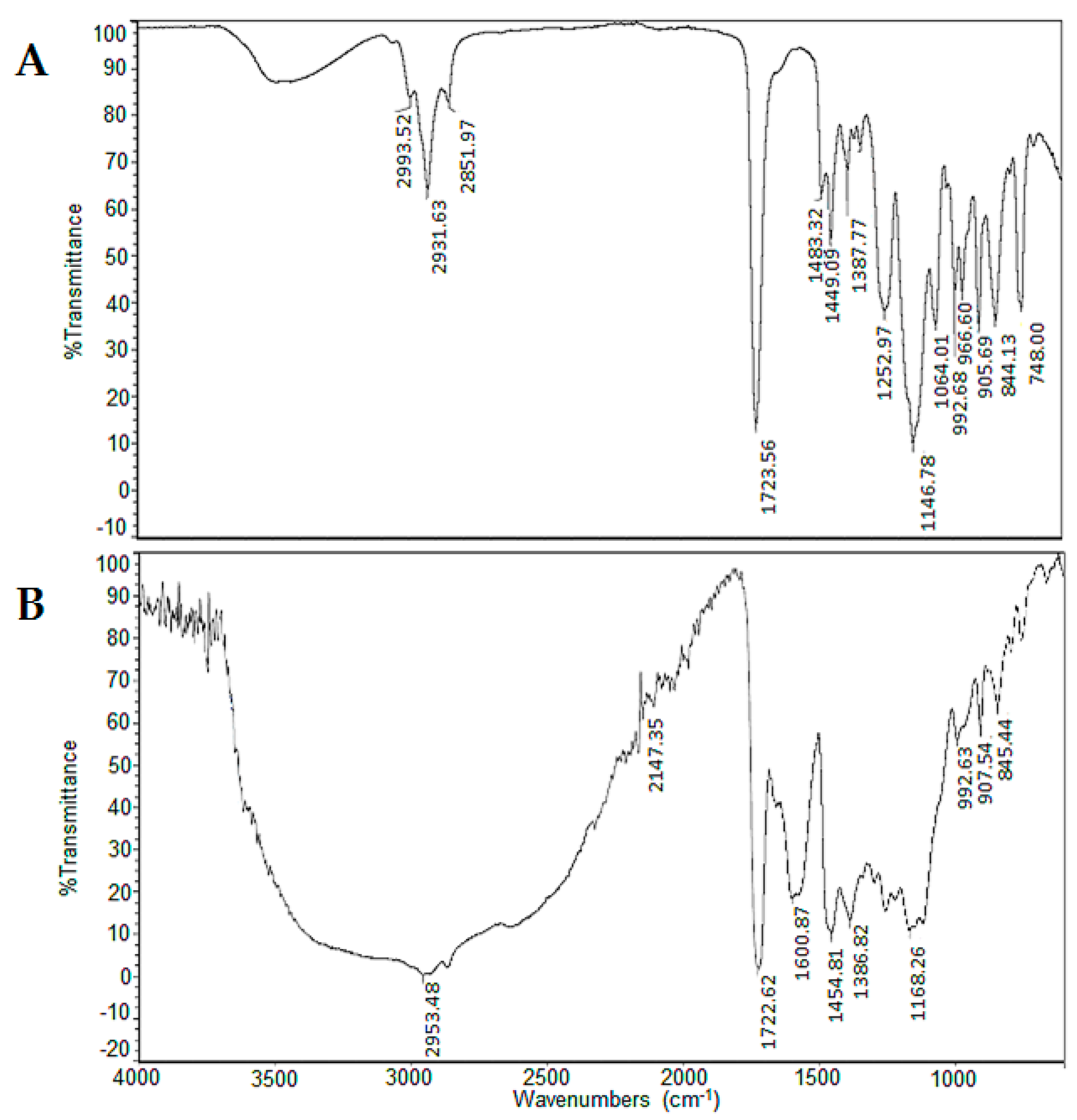

| Copolymer | IR(ATR) (cm−1) | Elemental Analysis | Substitution c | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OH | a C–H | b C–H | C=O | a C–H | C–N | C–O | C–O–C | C | H | N | µmol/g | |
| I | 2853 | 1723 | 1144 | 907 | 62.41 | 7.86 | - | - | ||||
| II | 3259 | 2954 | 2907 | 1723 | 1600 | 1255 | 1147 | --- | 58.96 | 6.88 | 0.62 | 111 |
| III | 3432 | 2998 | 2854 | 1723 | 1607 | 1255 | 1146 | --- | 60.28 | 7.06 | 0.84 | 150 |
| IV | 3220 | 2923 | 2853 | 1723 | 1603 | 1255 | 1147 | --- | 61.78 | 7.62 | 1.33 | 238 |
| V | 2852 | 1724 | 1147 | 906 | 59.68 | 7.09 | - | - | ||||
| VI | 3260 | 2953 | 2890 | 1723 | 1601 | 1255 | 1168 | --- | 54.49 | 6.42 | 1.79 | 320 |
| VII | 3280 | 2928 | 2854 | 1723 | 1559 | 1255 | 1170 | --- | 59.82 | 6.94 | 2.21 | 396 |
| VIII | 3270 | 2923 | 2869 | 1723 | 1602 | 1255 | 1149 | --- | 63.41 | 7.90 | 2.37 | 423 |
| IX | 3377 | 2969 | 2869 | 1723 | 1599 | 1220 | 1150 | --- | 53.30 | 6.52 | 2.19 | 391 |
| AcEYSFEFSY | KAEAEAKA | |||
|---|---|---|---|---|
| pH | 2.5 | 7.0 | 2.5 | 7.0 |
| 6-Poly(BMA–co–EDMA–co–GMA) | 32% | 58% | 16% | 22% |
| Poly(BMA–co–EDMA–co–GMA) a | 1% | 1% | 1% | 1% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velásquez-Silva, B.A.; Castillo-Aguirre, A.; Rivera-Monroy, Z.J.; Maldonado, M. Aminomethylated Calix[4]resorcinarenes as Modifying Agents for Glycidyl Methacrylate (GMA) Rigid Copolymers Surface. Polymers 2019, 11, 1147. https://doi.org/10.3390/polym11071147
Velásquez-Silva BA, Castillo-Aguirre A, Rivera-Monroy ZJ, Maldonado M. Aminomethylated Calix[4]resorcinarenes as Modifying Agents for Glycidyl Methacrylate (GMA) Rigid Copolymers Surface. Polymers. 2019; 11(7):1147. https://doi.org/10.3390/polym11071147
Chicago/Turabian StyleVelásquez-Silva, Betty Astrid, Alver Castillo-Aguirre, Zuly Jenny Rivera-Monroy, and Mauricio Maldonado. 2019. "Aminomethylated Calix[4]resorcinarenes as Modifying Agents for Glycidyl Methacrylate (GMA) Rigid Copolymers Surface" Polymers 11, no. 7: 1147. https://doi.org/10.3390/polym11071147
APA StyleVelásquez-Silva, B. A., Castillo-Aguirre, A., Rivera-Monroy, Z. J., & Maldonado, M. (2019). Aminomethylated Calix[4]resorcinarenes as Modifying Agents for Glycidyl Methacrylate (GMA) Rigid Copolymers Surface. Polymers, 11(7), 1147. https://doi.org/10.3390/polym11071147






