Component Adjustment of Poly(arylene ether nitrile) with Sulfonic and Carboxylic Groups for Dielectric Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SCPEN Copolymers
2.3. Preparation of SCPEN Films
2.4. Characterization
3. Results and Discussion
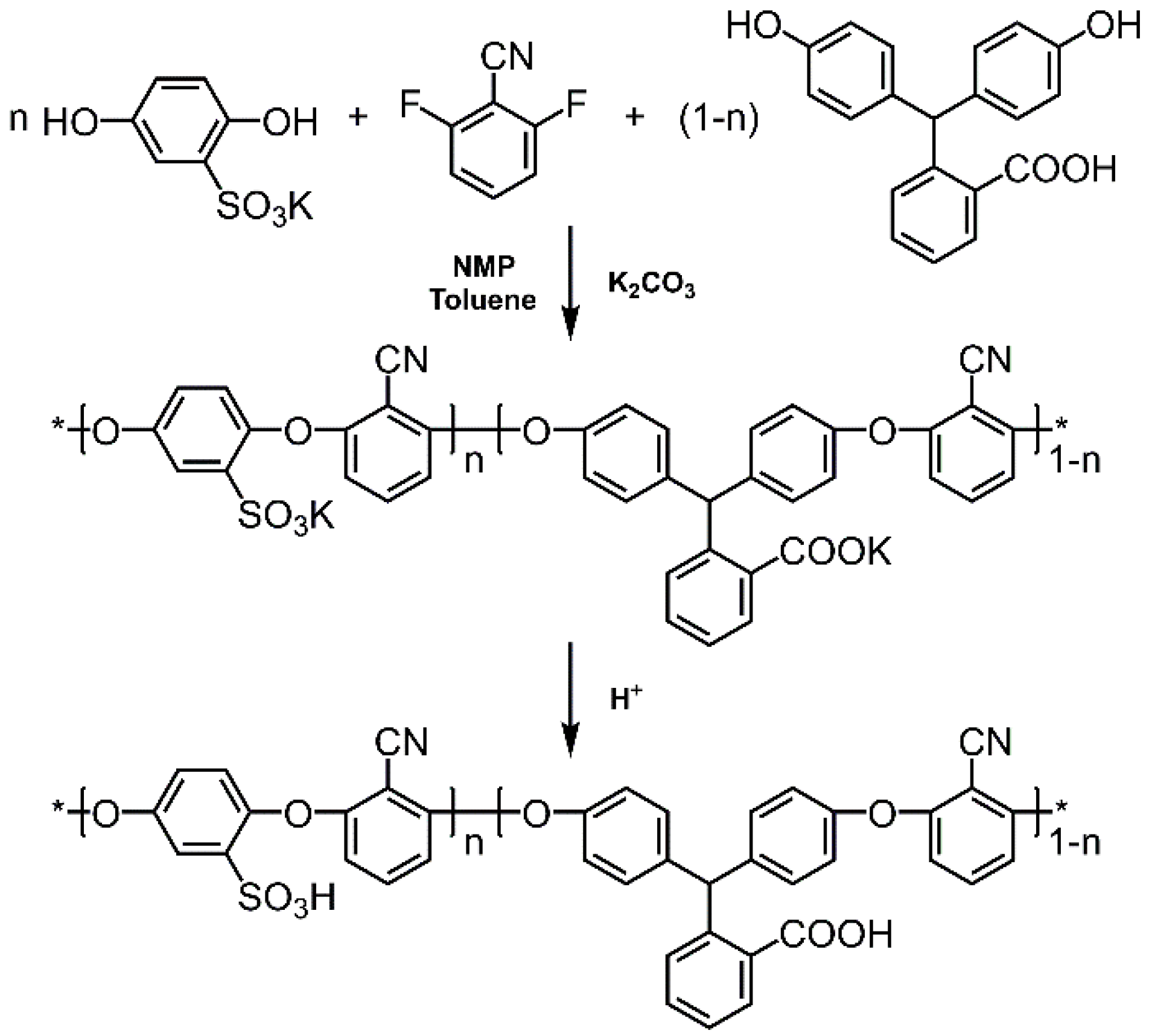
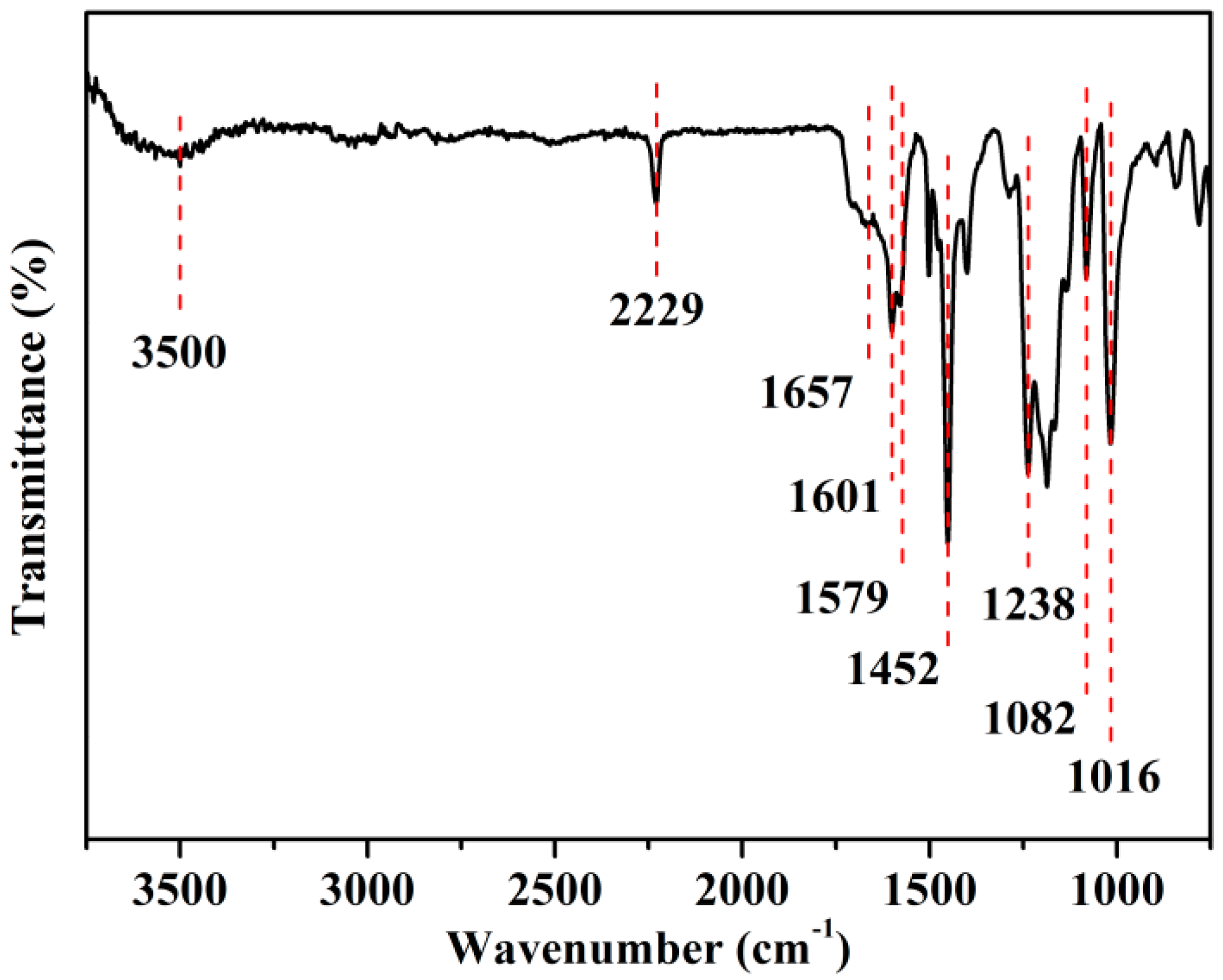
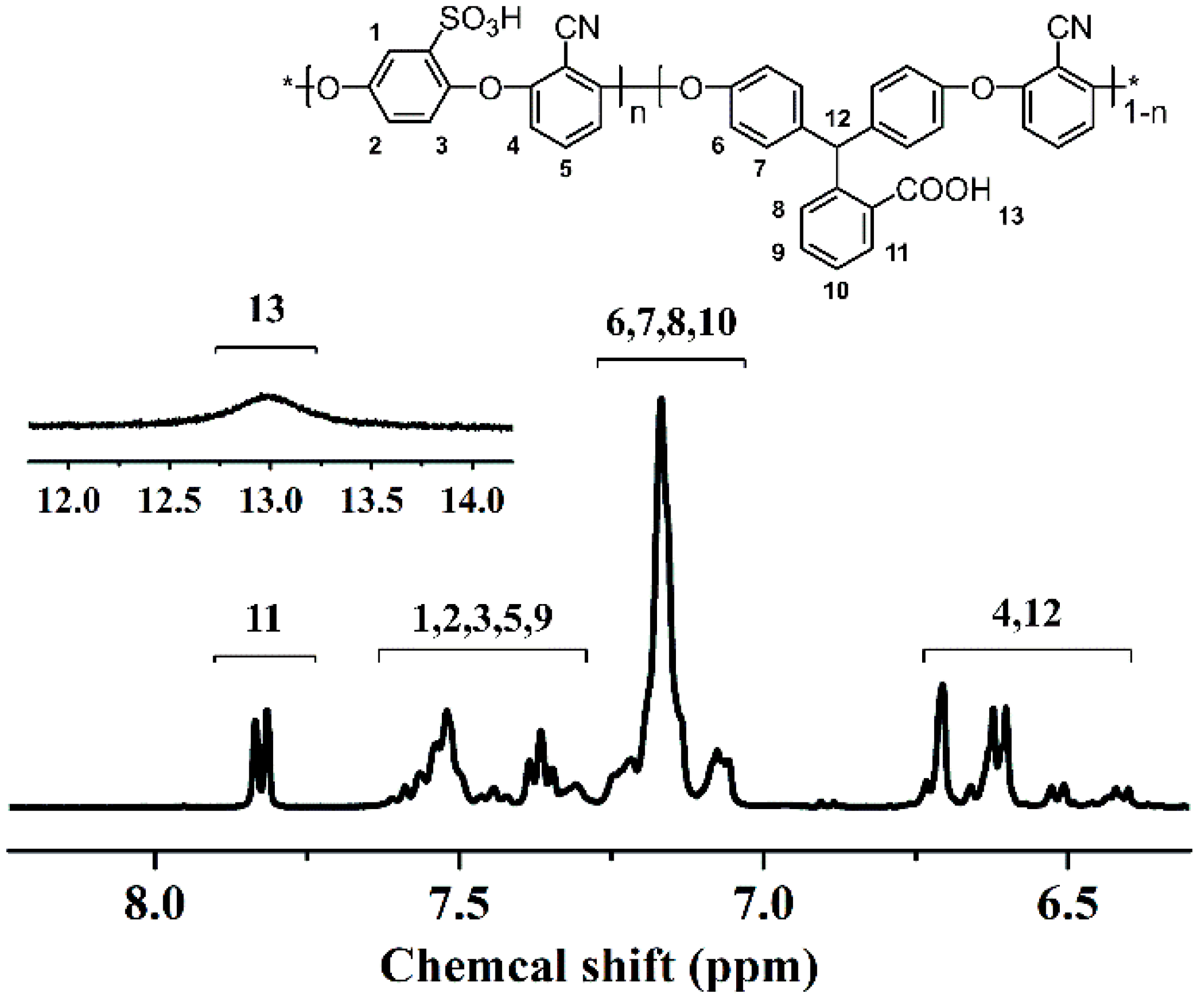
3.1. Synthesis of SCPEN Copolymers
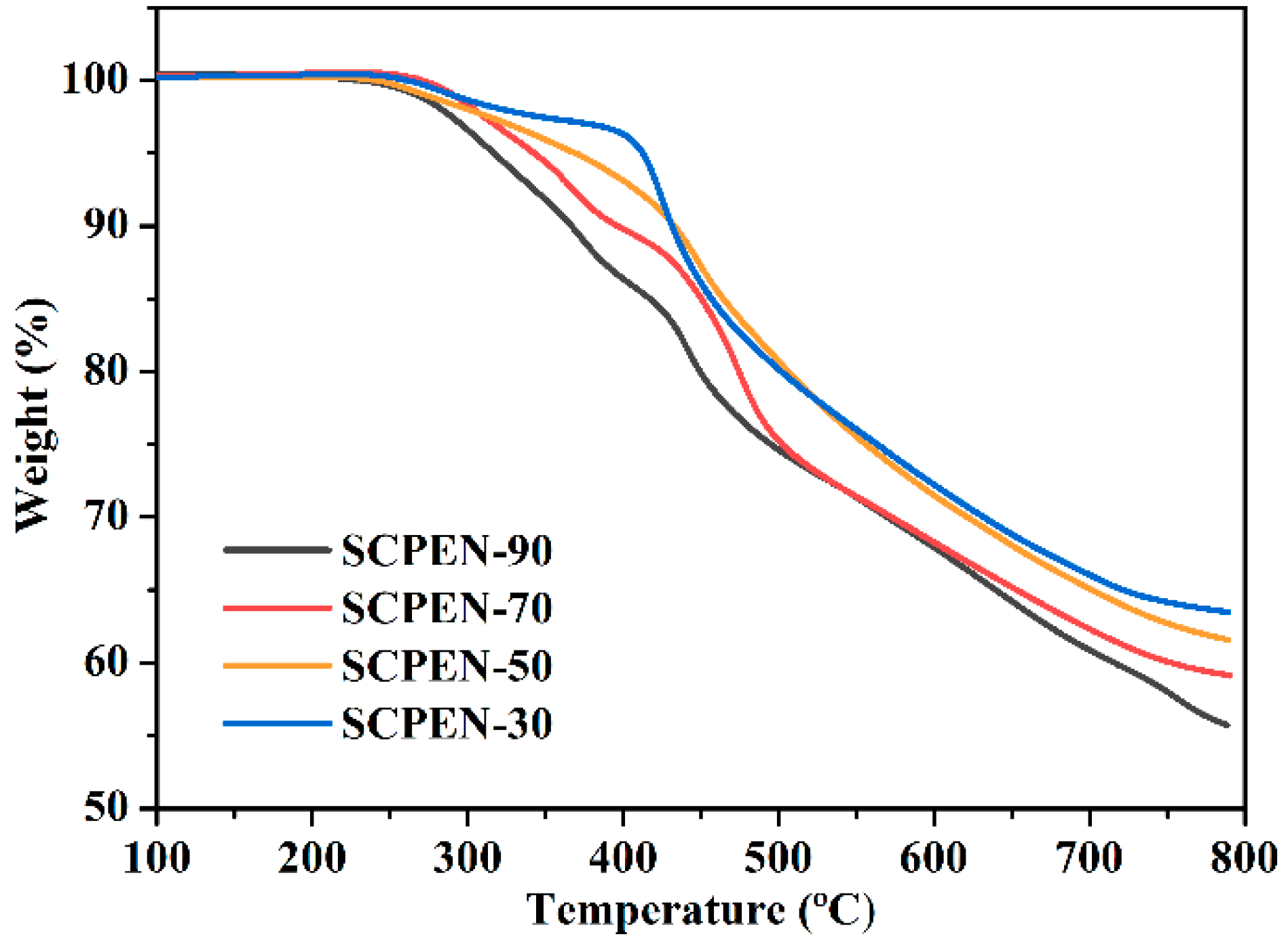
3.2. Thermal properties of SCPEN
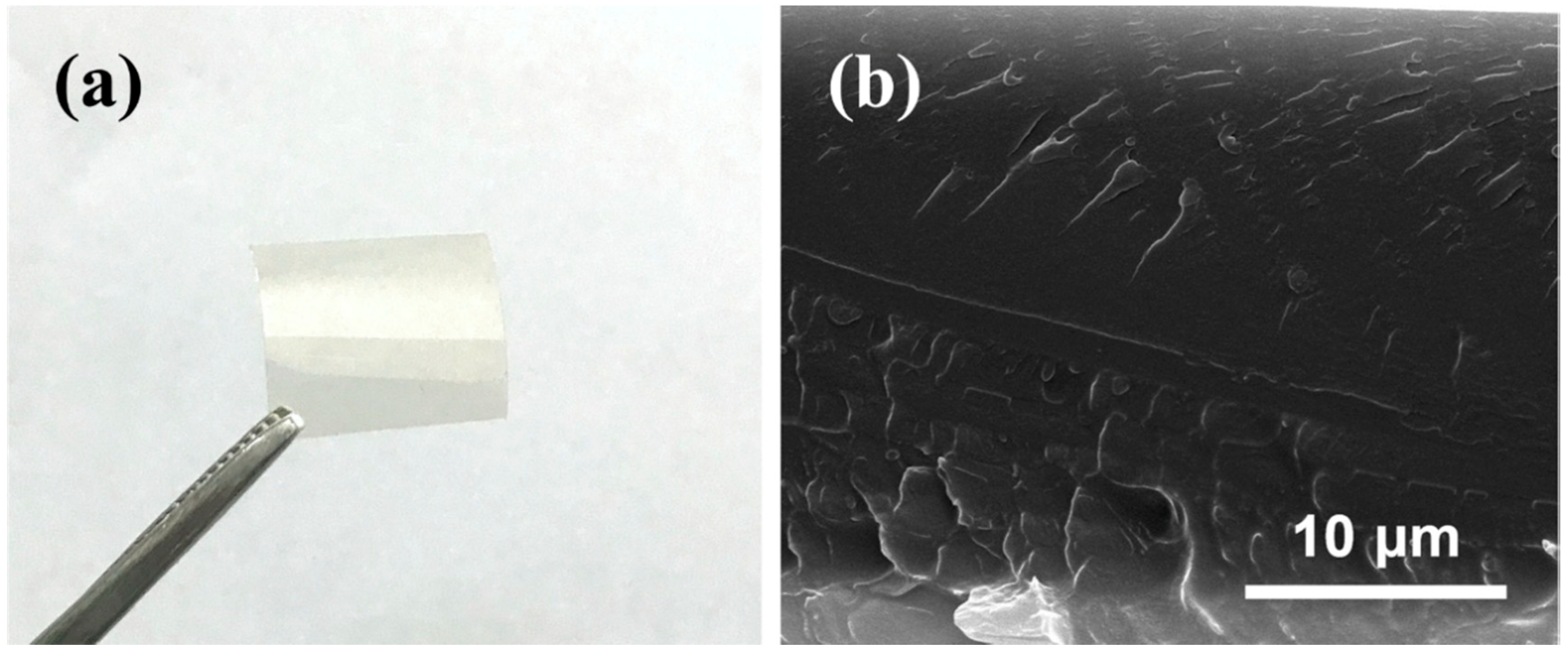
3.3. Morphology of SCPEN Films
3.4. IEC, Water Uptake and Swelling Ratio of SCPEN Films
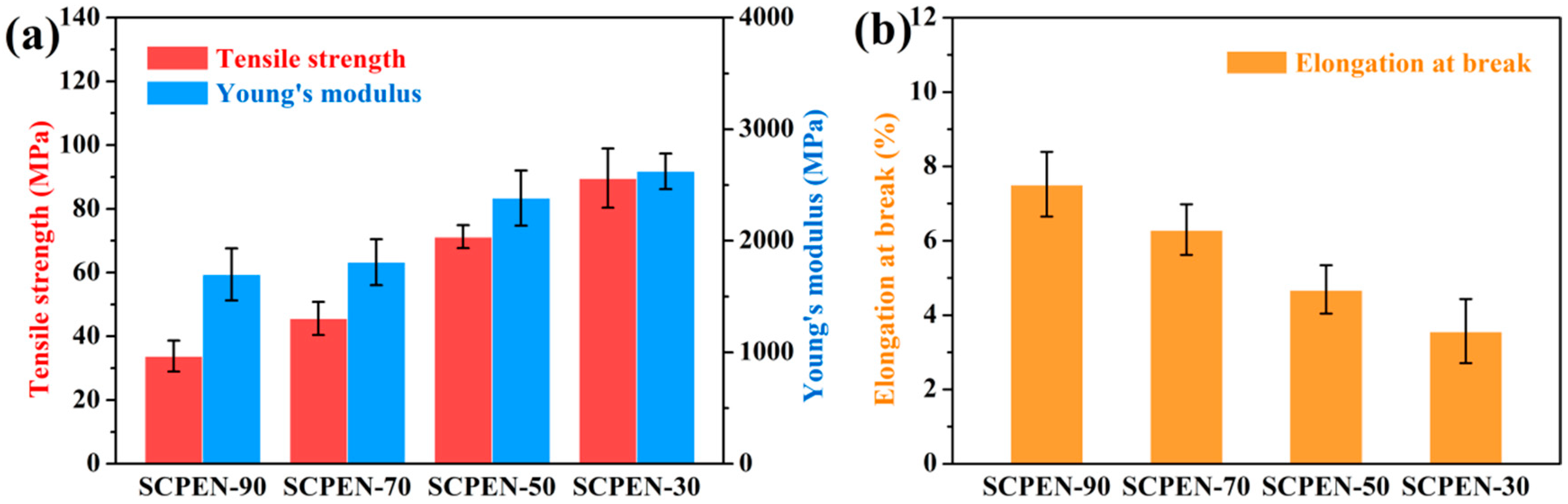
3.5. Mechanical Properties of SCPEN Films
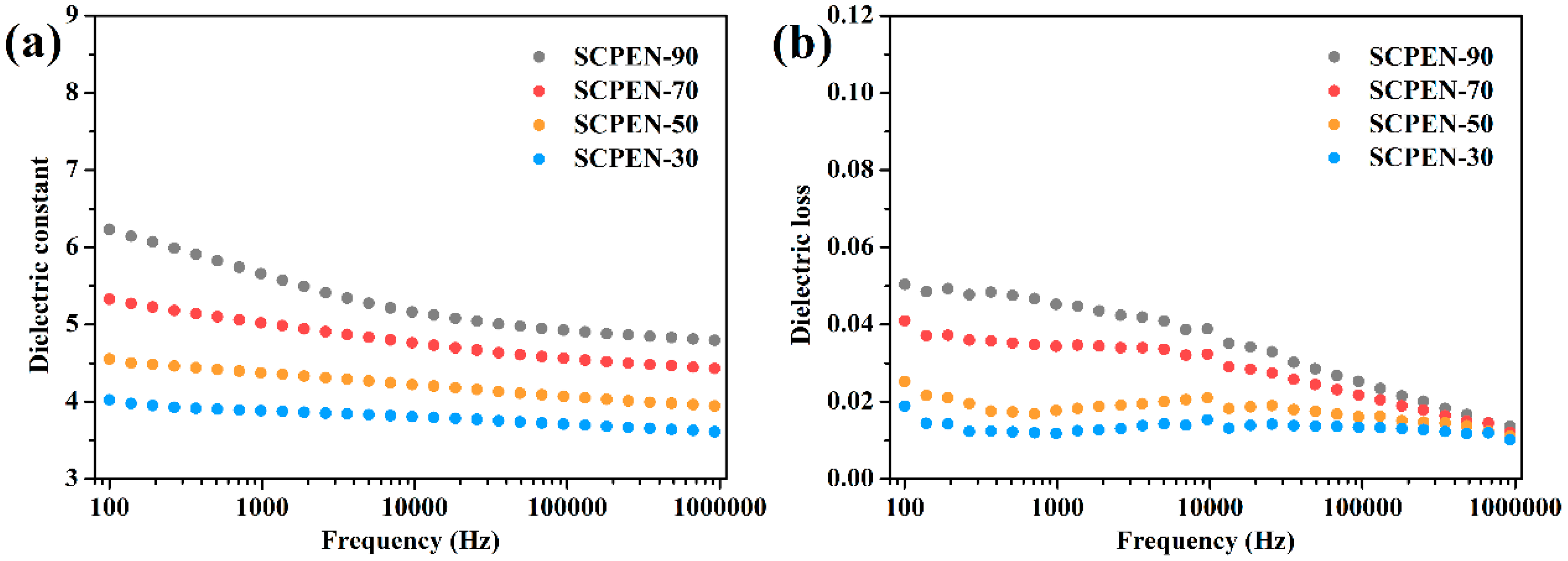
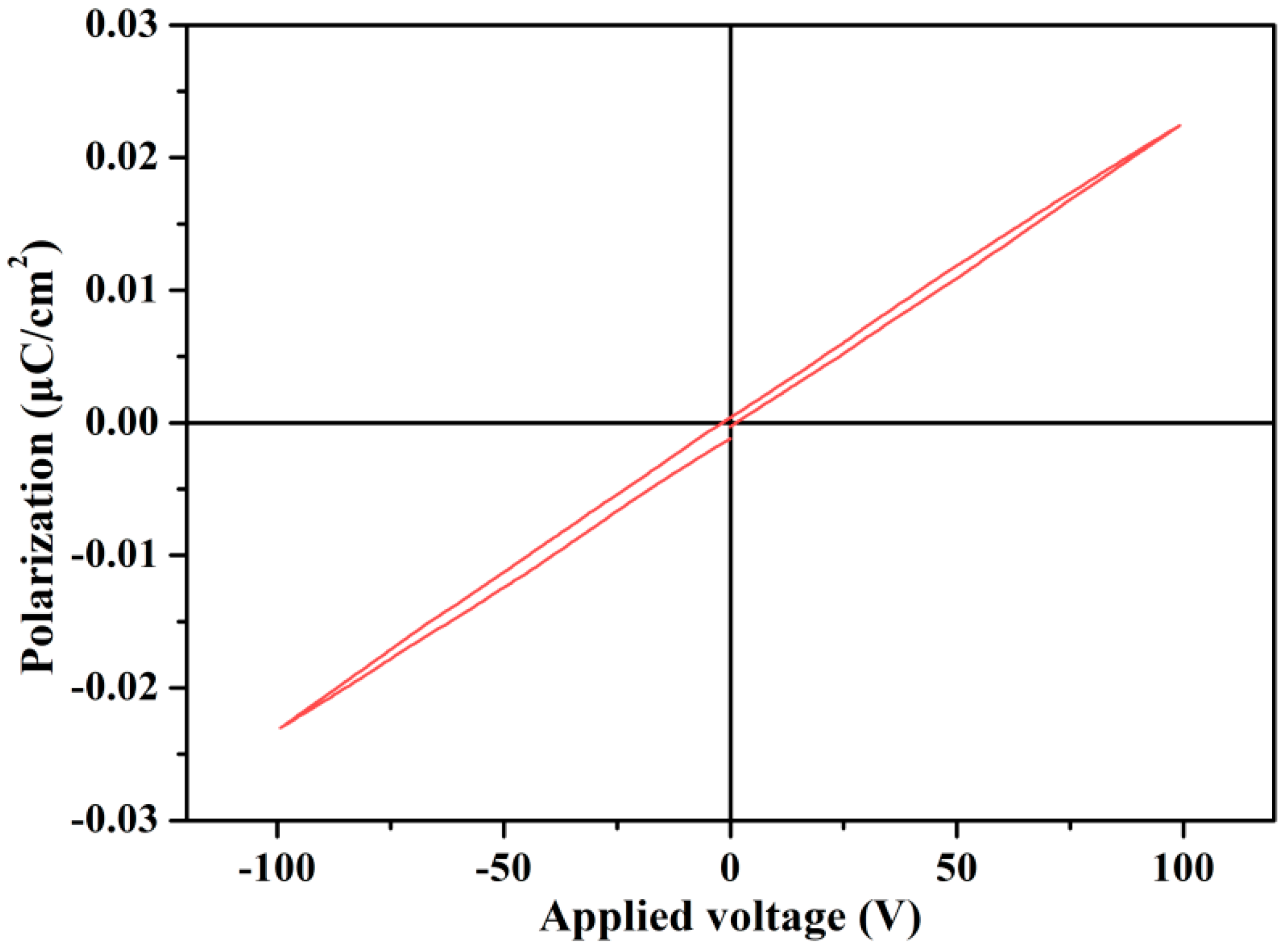
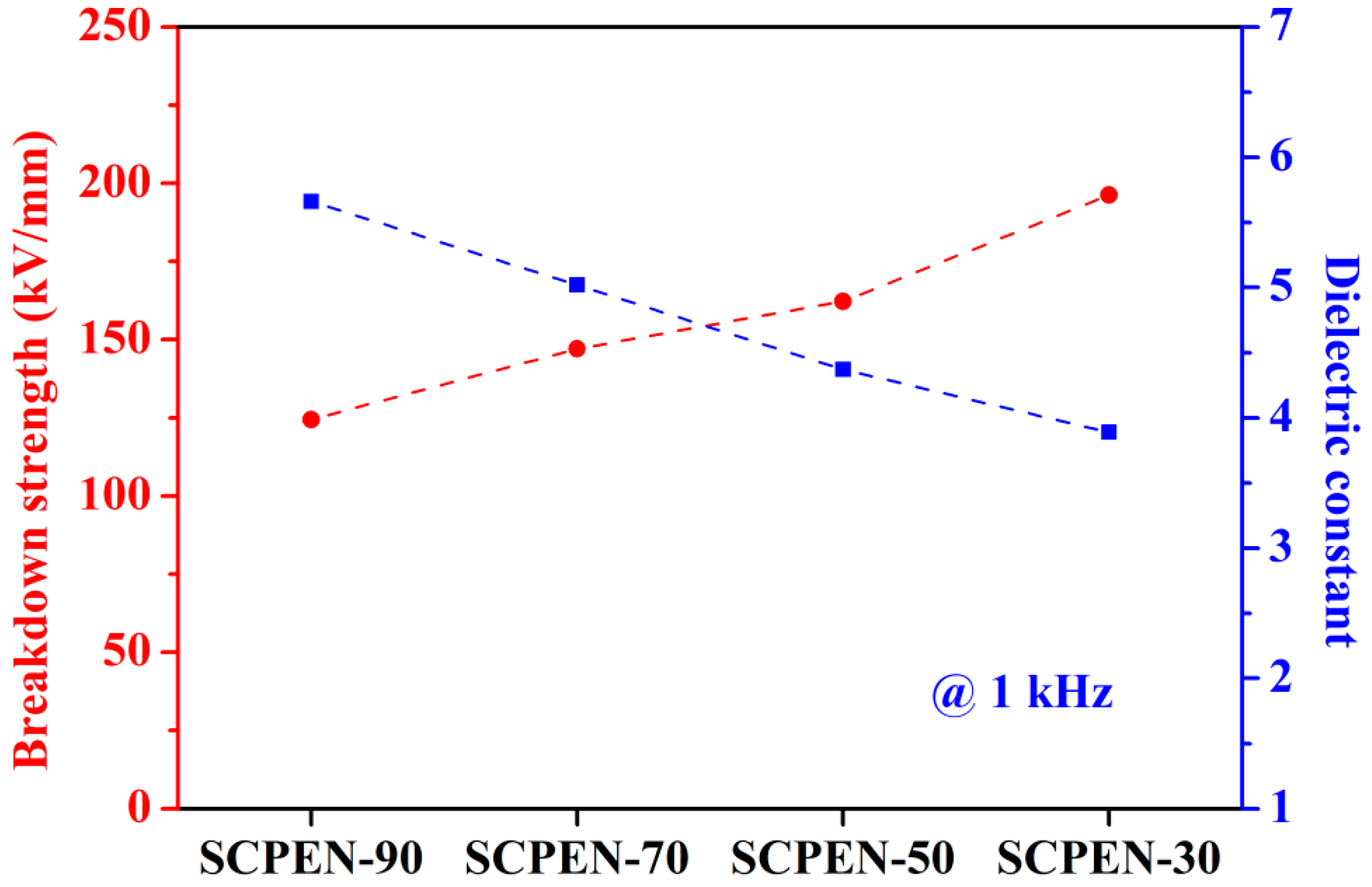
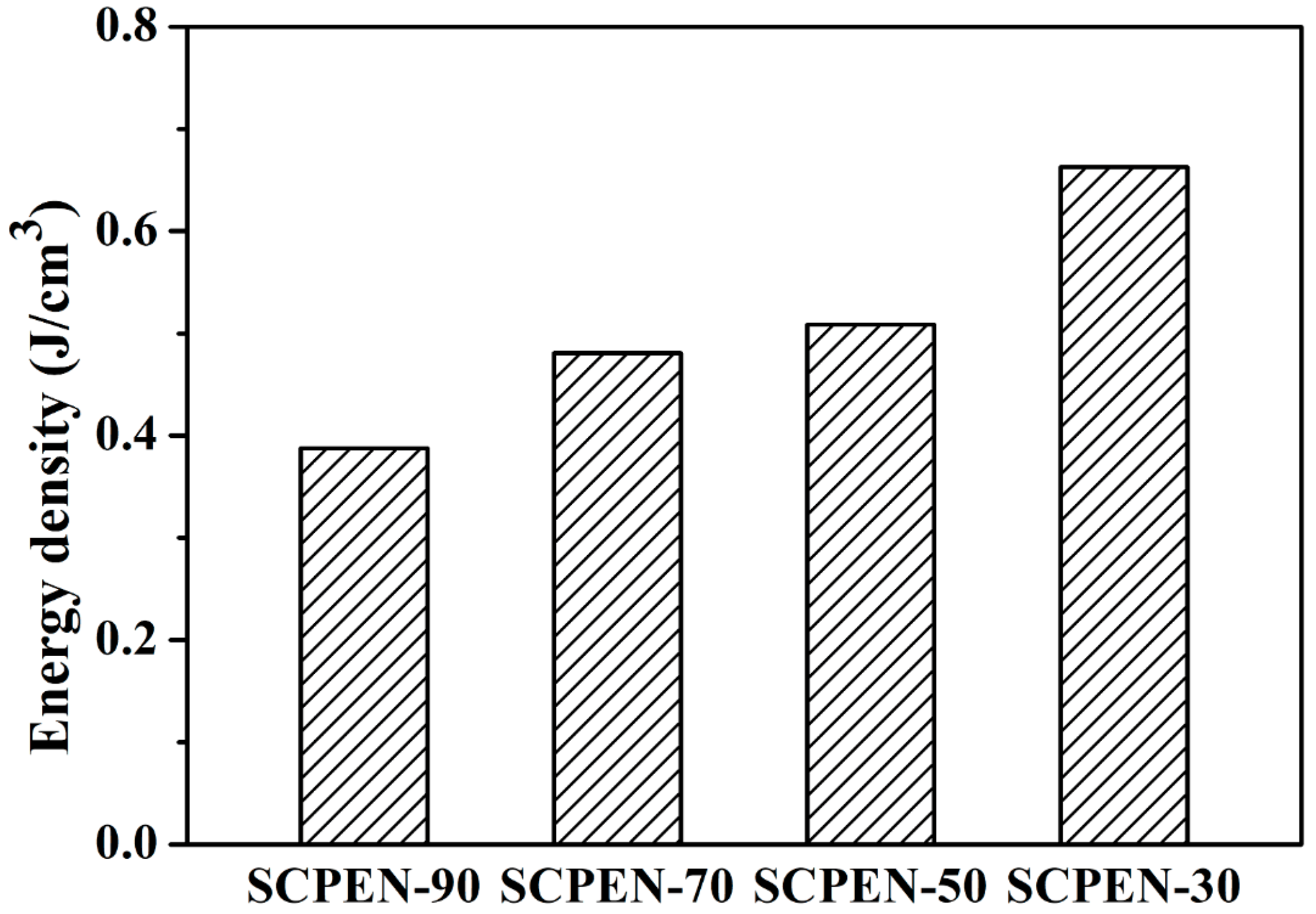
3.6. Dielectric Properties of SCPEN Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dang, Z.-M.; Yuan, J.-K.; Zha, J.-W.; Zhou, T.; Li, S.-T.; Hu, G.-H. Fundamentals, processes and applications of high-permittivity polymer-matrix composites. Prog. Mater. Sci. 2012, 57, 660–723. [Google Scholar] [CrossRef]
- Qiao, Y.; Yin, X.; Zhu, T.; Li, H.; Tang, C. Dielectric polymers with novel chemistry, compositions and architectures. Prog. Polym. Sci. 2018, 80, 153–162. [Google Scholar] [CrossRef]
- Luo, S.; Shen, Y.; Yu, S.; Wan, Y.; Liao, W.-H.; Sun, R.; Wong, C.-P. Construction of a 3D-BaTiO3 network leading to significantly enhanced dielectric permittivity and energy storage density of polymer composites. Energy Environ. Sci. 2017, 10, 137–144. [Google Scholar] [CrossRef]
- Yao, L.; Pan, Z.; Zhai, J.; Zhang, G.; Liu, Z.; Liu, Y. High-energy-density with polymer nanocomposites containing of SrTiO3 nanofibers for capacitor application. Compos. Part A 2018, 109, 48–54. [Google Scholar] [CrossRef]
- Yang, M.; Zhao, H.; He, D.; Bai, J. Constructing a continuous amorphous carbon interlayer to enhance dielectric performance of carbon nanotubes/polyvinylidene fluoride nanocomposites. Carbon 2017, 116, 94–102. [Google Scholar] [CrossRef]
- Feng, H.; Ma, W.; Cui, Z.-K.; Liu, X.; Gu, J.; Lin, S.; Zhuang, Q. Core/shell-structured hyperbranched aromatic polyamide functionalized graphene nanosheets-poly(p-phenylene benzobisoxazole) nanocomposite films with improved dielectric properties and thermostability. J. Mater. Chem. A 2017, 5, 8705–8713. [Google Scholar] [CrossRef]
- Wåhlander, M.; Nilsson, F.; Andersson, R.L.; Sanchez, C.C.; Taylor, N.; Carlmark, A.; Hillborg, H.; Malmström, E. Tailoring dielectric properties using designed polymer-grafted ZnO nanoparticles in silicone rubber. J. Mater. Chem. A 2017, 5, 14241–14258. [Google Scholar] [CrossRef]
- Wang, Y.; Kai, Y.; Tong, L.; You, Y.; Huang, Y.; Liu, X. The frequency independent functionalized MoS2 nanosheet/poly(arylene ether nitrile) composites with improved dielectric and thermal properties via mussel inspired surface chemistry. Appl. Surf. Sci. 2019, 481, 1239–1248. [Google Scholar] [CrossRef]
- Huang, X.; Sun, B.; Zhu, Y.; Li, S.; Jiang, P. High-k polymer nanocomposites with 1D filler for dielectric and energy storage applications. Prog. Mater. Sci. 2019, 100, 187–225. [Google Scholar] [CrossRef]
- Baer, E.; Zhu, L. 50th Anniversary Perspective: Dielectric Phenomena in Polymers and Multilayered Dielectric Films. Macromolecules 2017, 50, 2239–2256. [Google Scholar] [CrossRef]
- Wei, J.; Meng, X.; Chen, X.; Bai, Y.; Song, J.; Yan, N.; Zhu, L.; Shen, A. Facile synthesis of fluorinated poly(arylene ether nitrile) and its dielectric properties. J. Appl. Polym. Sci. 2018, 135, 46837. [Google Scholar] [CrossRef]
- Pu, Z.; Zheng, X.; Tian, Y.; Hu, L.; Zhong, J. Flexible Ultrahigh-Temperature Polymer-Based Dielectrics with High Permittivity for Film Capacitor Applications. Polymers 2017, 9, 596. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Zhan, Y.; Long, Z.; Zeng, G.; Ren, Y.; He, Y. High-performance magnetic poly (arylene ether nitrile) nanocomposites: Co-modification of Fe3O4 via mussel inspired poly(dopamine) and amino functionalized silane KH550. Appl. Surf. Sci. 2017, 425, 905–914. [Google Scholar] [CrossRef]
- Kim, A.R.; Gabunada, J.C.; Yoo, D.J. Amelioration in physicochemical properties and single cell performance of sulfonated poly(ether ether ketone) block copolymer composite membrane using sulfonated carbon nanotubes for intermediate humidity fuel cells. Int. J. Energy. Res. 2019, 43, 2974–2989. [Google Scholar] [CrossRef]
- Lee, K.H.; Chu, J.Y.; Kim, A.R.; Yoo, D.J. Facile Fabrication and Characterization of Improved Proton Conducting Sulfonated Poly(Arylene Biphenylether Sulfone) Blocks Containing Fluorinated Hydrophobic Units for Proton Exchange Membrane Fuel Cell Applications. Polymers 2018, 10, 1367. [Google Scholar] [CrossRef] [PubMed]
- Vinothkannan, M.; Kim, A.R.; Gnana kumar, G.; Yoon, J.-M.; Yoo, D.J. Toward improved mechanical strength, oxidative stability and proton conductivity of an aligned quadratic hybrid (SPEEK/FPAPB/Fe3O4-FGO) membrane for application in high temperature and low humidity fuel cells. RSC Adv. 2017, 7, 39034–39048. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, G.; Xu, D.; Wang, J.; Yang, X.; Jiang, Z. Ternary graphite nanosheet/copper phthalocyanine/sulfonated poly(aryl ether ketone) dielectric percolative composites: Preparation, micromorphologies and dielectric properties. RSC Adv. 2014, 4, 28721–28727. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Huo, P.F.; Wang, J.F.; Liu, X.; Rong, C.R.; Wang, G.B. Dielectric percolative composites with high dielectric constant and low dielectric loss based on sulfonated poly(aryl ether ketone) and a-MWCNTs coated with polyaniline. J. Mater. Chem. C 2013, 1, 4035–4041. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, P.; Wang, L.; Liu, X. Preparation of Sulfonated Poly(arylene ether nitrile)-Based Adsorbent as a Highly Selective and Efficient Adsorbent for Cationic Dyes. Polymers 2019, 11, 32. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Z. Novel polyaryletherketones bearing pendant carboxyl groups and their rare earth complexes, Part I: Synthesis and characterization. Polymer 2008, 49, 4960–4967. [Google Scholar] [CrossRef]
- Li, H.; Cui, Z.; Zhao, C.; Wu, J.; Fu, T.; Zhang, Y.; Shao, K.; Zhang, H.; Na, H.; Xing, W. Synthesis and Property of a Novel Sulfonated Poly(Ether Ether Ketone) with High Selectivity for Direct Methanol Fuel Cell Applications. J. Membr. Sci. 2009, 343, 164–170. [Google Scholar] [CrossRef]
- Geng, Z.; Huo, M.; Mu, J.; Zhang, S.; Lu, Y.; Luan, J.; Huo, P.; Du, Y.; Wang, G. Ultra Low Dielectric Constant Soluble Polyhedral Oligomeric Silsesquioxane (POSS)-Poly(aryl ether ketone) Nanocomposites with Excellent Thermal and Mechanical Properties. J. Mater. Chem. C 2014, 2, 1094–1103. [Google Scholar] [CrossRef]
- Xu, J.; Ni, H.; Wang, S.; Wang, Z.; Zhang, H. Directly Polymerization of a Novel Sulfonated Poly(arylene ether ketone sulfone)/Sulfonated Poly(vinylalcohol) Crosslinked Membrane for Direct Methanol Fuel Cell Applications. J. Membr. Sci. 2015, 492, 505–517. [Google Scholar] [CrossRef]
- Lee, H.; Han, J.; Kim, K.; Kim, J.; Kim, E.; Shin, H.; Lee, J.-C. Highly sulfonated polymer-grafted graphene oxide composite membranes for proton exchange membrane fuel cells. J. Ind. Eng. Chem. 2019, 74, 223–232. [Google Scholar] [CrossRef]
- Tang, H.; Wang, P.; Zheng, P.; Liu, X. Core-shell structured BaTiO3@polymer hybrid nanofiller for poly(arylene ether nitrile) nanocomposites with enhanced dielectric properties and high thermal stability. Compos. Sci. Technol. 2016, 123, 134–142. [Google Scholar] [CrossRef]
- Tang, H.; Pu, Z.; Huang, X.; Wei, J.; Liu, X.; Lin, Z. Novel blue-emitting carboxyl-functionalized poly(arylene ether nitrile)s with excellent thermal and mechanical properties. Polym. Chem. 2014, 5, 3673–3679. [Google Scholar] [CrossRef]
- Kim, A.R.; Yoo, D.J. A Comparative Study on Physiochemical, Thermomechanical, and Electrochemical Properties of Sulfonated Poly(Ether Ether Ketone) Block Copolymer Membranes with and without Fe3O4 Nanoparticles. Polymers 2019, 11, 536. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cheng, T.; Zhang, X.; Zhang, W.; Liu, X. Novel composite proton exchange membrane with long-range proton transfer channels constructed by synergistic effect between acid and base functionalized graphene oxide. Polymer 2018, 149, 305–315. [Google Scholar] [CrossRef]
- Ni, C.; Wang, H.; Zhao, Q.; Liu, B.; Sun, Z.; Zhang, M.; Hu, W.; Liang, L. Crosslinking effect in nanocrystalline cellulose reinforced sulfonated poly(aryl ether ketone) proton exchange membranes. Solid State Ionics 2018, 323, 5–15. [Google Scholar] [CrossRef]
- Butkewitsch, S.; Scheinbeim, J. Dielectric properties of a hydrated sulfonated poly(styrene–ethylene/butylenes–styrene) triblock copolymer. Appl. Surf. Sci. 2006, 252, 8277–8286. [Google Scholar] [CrossRef]
- Kohl, P.A. Low–Dielectric Constant Insulators for Future Integrated Circuits and Packages. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 379–401. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, Q.M. Fully Functionalized High-Dielectric-Constant Nanophase Polymers with High Electromechanical Response. Adv. Mater. 2005, 17, 1153–1158. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Q. Enhanced Dielectric and Electromechanical Responses in High Dielectric Constant All-Polymer Percolative Composites. Adv. Funct. Mater. 2004, 14, 501–506. [Google Scholar] [CrossRef]
- Sato, Y.; Yashiro, T. The effect of polar groups on the dielectric loss of polyethylene. J. Appl. Polym. Sci. 1978, 22, 2141–2153. [Google Scholar] [CrossRef]
- Wang, D.H.; Kurish, B.A.; Treufeld, I.; Zhu, L.; Tan, L.-S. Synthesis and characterization of high nitrile content polyimides as dielectric films for electrical energy storage. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 422–436. [Google Scholar] [CrossRef]
- Yang, J.; Tang, Z.; Yin, H.; Liu, Y.; Wang, L.; Tang, H.; Li, Y. Poly(arylene ether nitrile) Composites with Surface-Hydroxylated Calcium Copper Titanate Particles for High-Temperature-Resistant Dielectric Applications. Polymers 2019, 11, 766. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L. Exploring Strategies for High Dielectric Constant and Low Loss Polymer Dielectrics. J. Phys. Chem. Lett. 2014, 5, 3677–3687. [Google Scholar] [CrossRef]
- Stark, K.H.; Garton, C.G. Electric Strength of Irradiated Polythene. Nature 1955, 176, 1225–1226. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, D.H.; Litt, M.H.; Tan, L.-S.; Zhu, L. High-temperature and high-energy-density dipolar glass polymers based on sulfonylated poly(2,6-dimethyl-1,4-phenylene oxide). Angew. Chem. 2018, 130, 1544–1547. [Google Scholar] [CrossRef]

| Samples | SCPEN-90 | SCPEN-70 | SCPEN-50 | SCPEN-30 |
|---|---|---|---|---|
| SHQ: PPL (molar) | 9:1 | 7:3 | 5:5 | 3:7 |
| SHQ (mol) | 0.09 | 0.07 | 0.05 | 0.03 |
| PPL (mol) | 0.01 | 0.03 | 0.05 | 0.07 |
| DFBN (mol) | 0.1 | 0.1 | 0.1 | 0.1 |
| K2CO3 (mol) | 0.245 | 0.235 | 0.225 | 0.215 |
| Yield (%) | 89.2 | 90.4 | 91.5 | 90.8 |
| ηinha (dL·g−1) | 1.15 | 1.11 | 1.10 | 1.15 |
| Sample | SCPEN-90 | SCPEN-70 | SCPEN-50 | SCPEN-30 |
|---|---|---|---|---|
| T5% (°C) | 317 | 343 | 369 | 412 |
| CY (%) a | 55.7 | 59.1 | 61.6 | 63.5 |
| Films | IEC (mmol/g) | Water Uptake (%) a | Swelling Ratio (%) a | ||
|---|---|---|---|---|---|
| Mass | Length (SL) | Area (SA) | Volume (SV) | ||
| SCPEN-90 | 2.89 | 53.5 | 10.9 | 21.4 | 35.3 |
| SCPEN-70 | 2.07 | 29.1 | 6.5 | 13.0 | 16.5 |
| SCPEN-50 | 1.38 | 19.5 | 4.2 | 9.9 | 13.4 |
| SCPEN-30 | 0.69 | 10.3 | 2.0 | 3.6 | 6.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Liu, S.; Lin, J.; Wang, L.; Huang, Y.; Liu, X. Component Adjustment of Poly(arylene ether nitrile) with Sulfonic and Carboxylic Groups for Dielectric Films. Polymers 2019, 11, 1135. https://doi.org/10.3390/polym11071135
Liu C, Liu S, Lin J, Wang L, Huang Y, Liu X. Component Adjustment of Poly(arylene ether nitrile) with Sulfonic and Carboxylic Groups for Dielectric Films. Polymers. 2019; 11(7):1135. https://doi.org/10.3390/polym11071135
Chicago/Turabian StyleLiu, Chenchen, Shuning Liu, Jian Lin, Lingling Wang, Yumin Huang, and Xiaobo Liu. 2019. "Component Adjustment of Poly(arylene ether nitrile) with Sulfonic and Carboxylic Groups for Dielectric Films" Polymers 11, no. 7: 1135. https://doi.org/10.3390/polym11071135
APA StyleLiu, C., Liu, S., Lin, J., Wang, L., Huang, Y., & Liu, X. (2019). Component Adjustment of Poly(arylene ether nitrile) with Sulfonic and Carboxylic Groups for Dielectric Films. Polymers, 11(7), 1135. https://doi.org/10.3390/polym11071135






