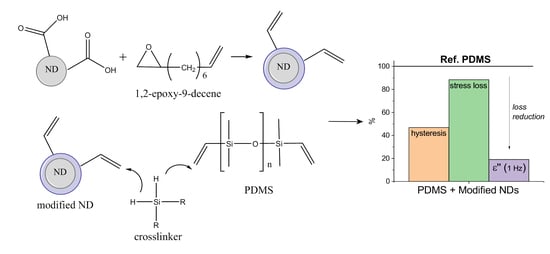
Influence of Surface Modified Nanodiamonds on Dielectric and Mechanical Properties of Silicone Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanodiamond Treatment
2.3. Composite Preparation
2.4. Characterization
3. Results and Discussion
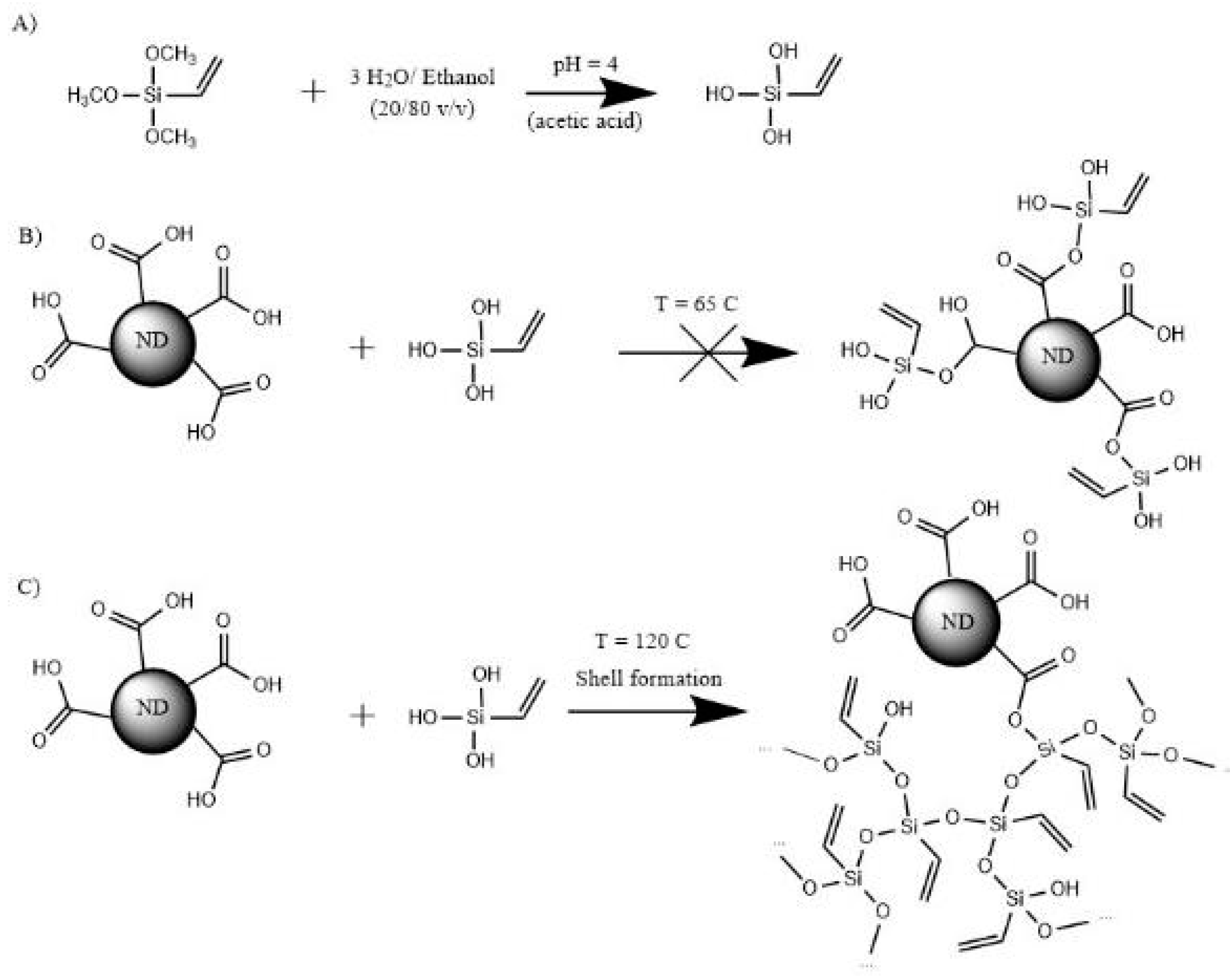
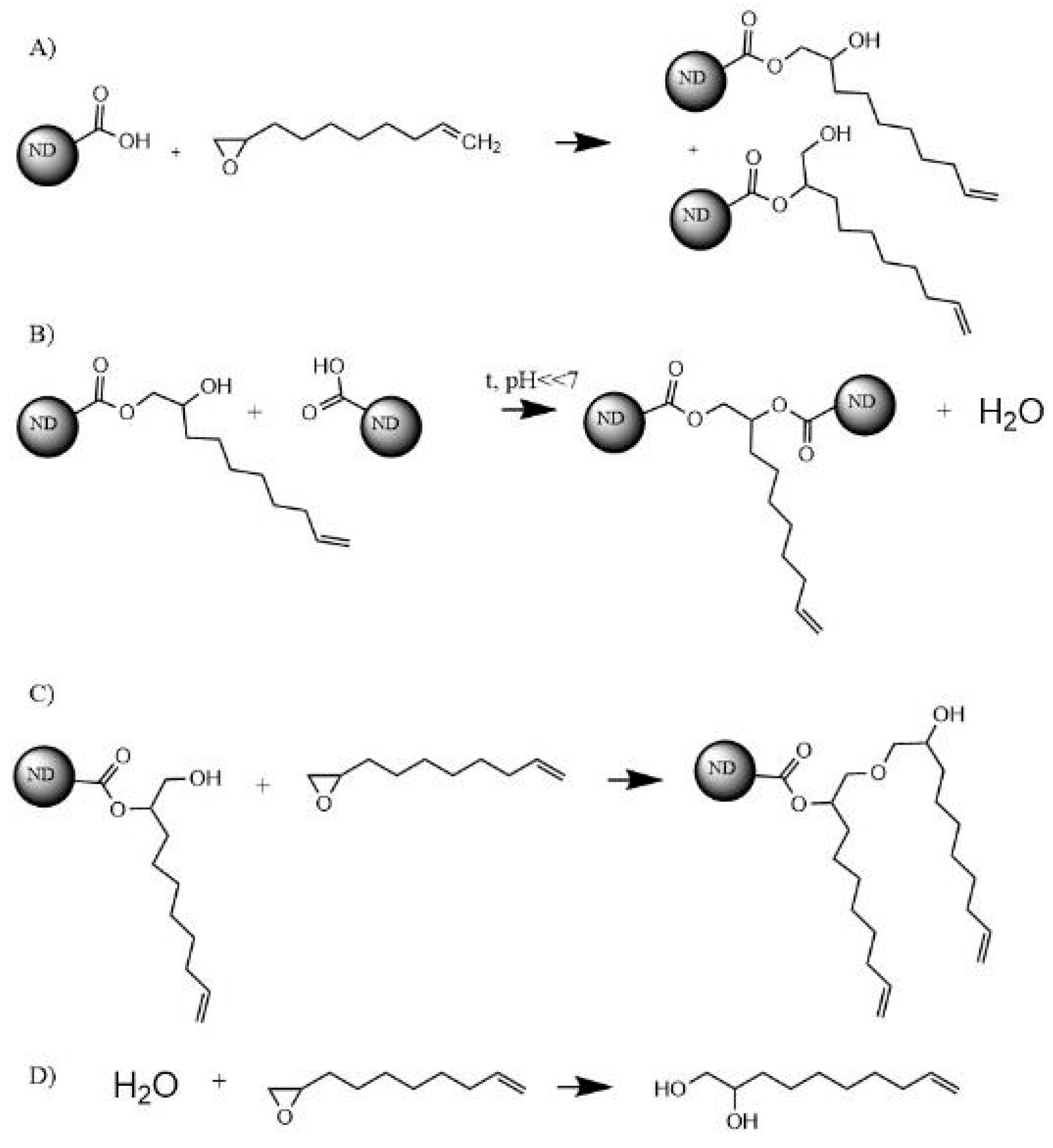
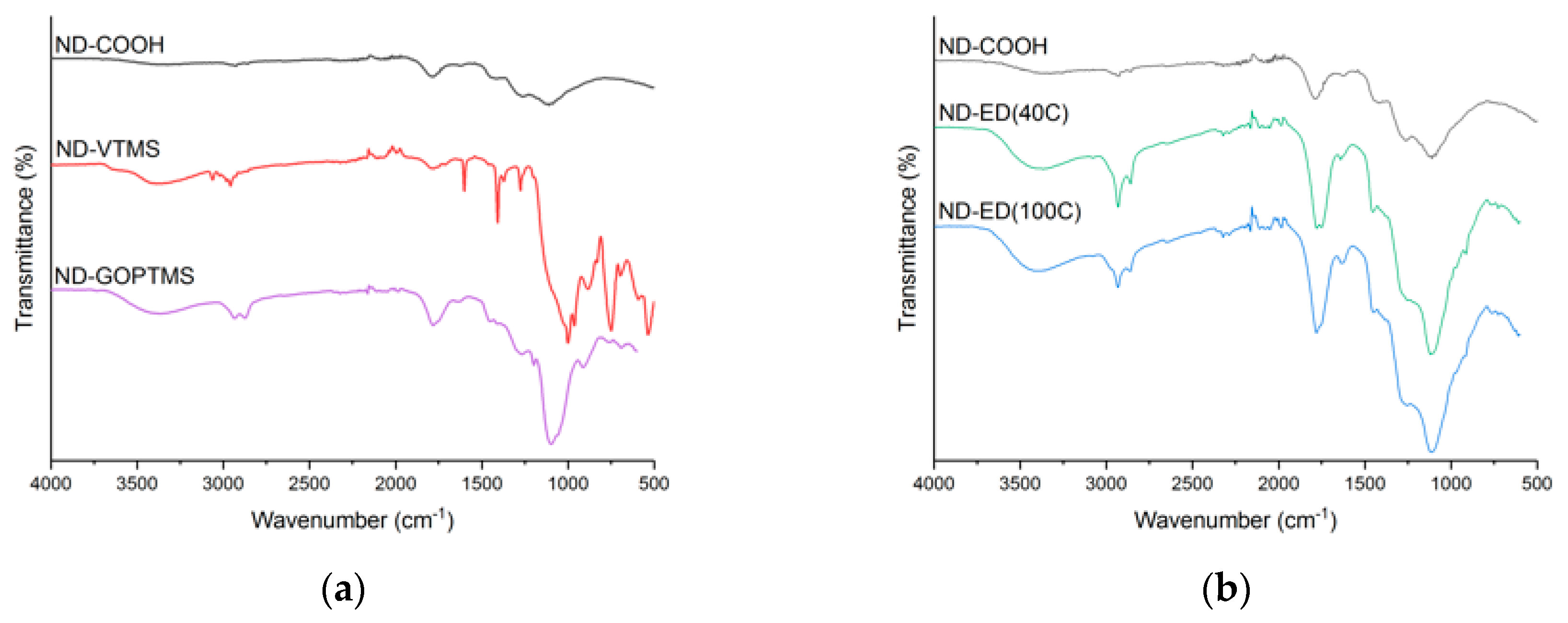
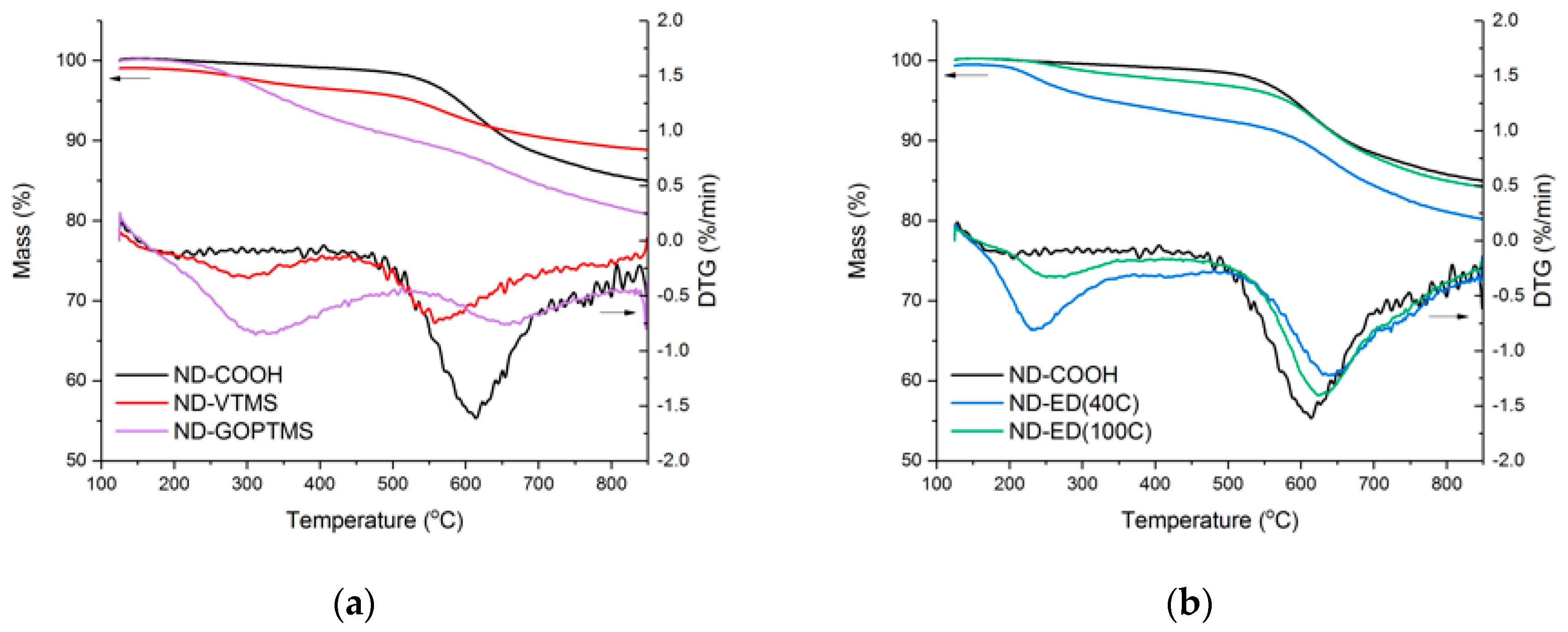
3.1. Surface Modification of Nanodiamonds
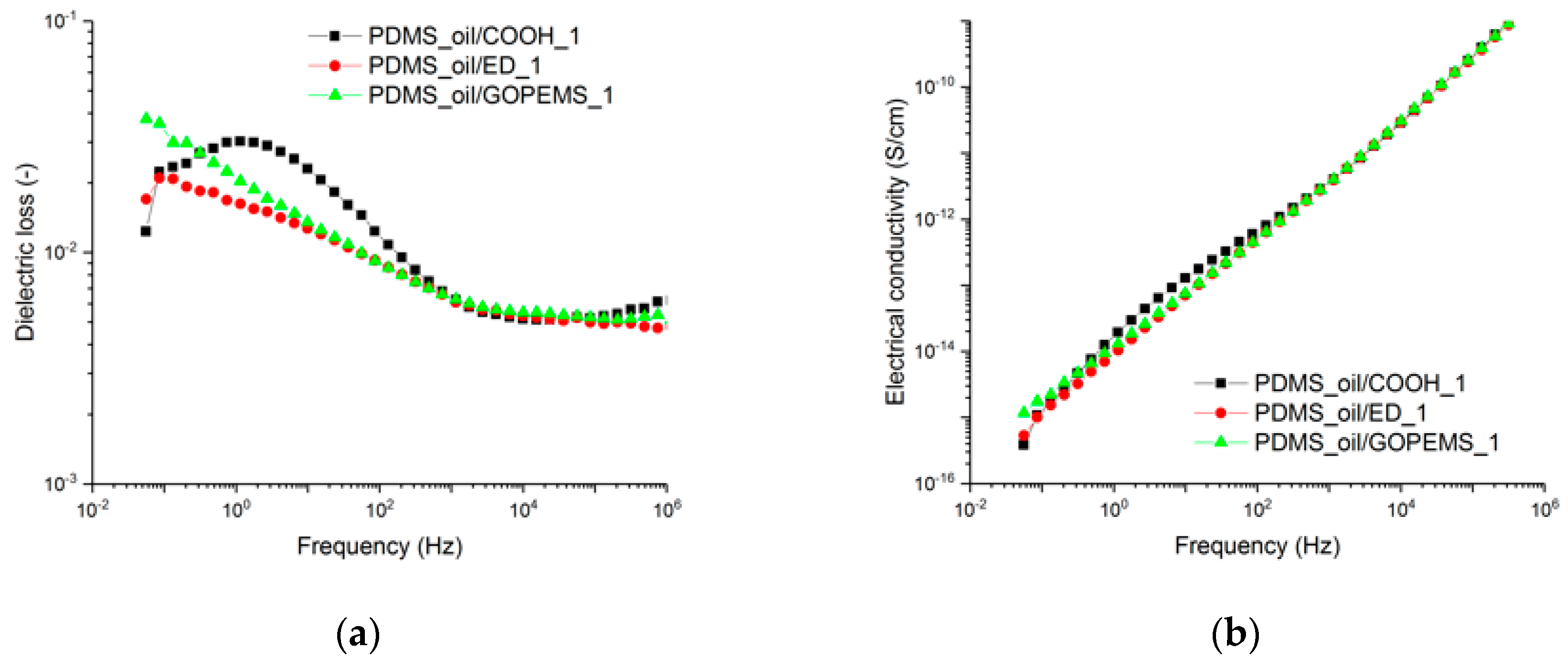
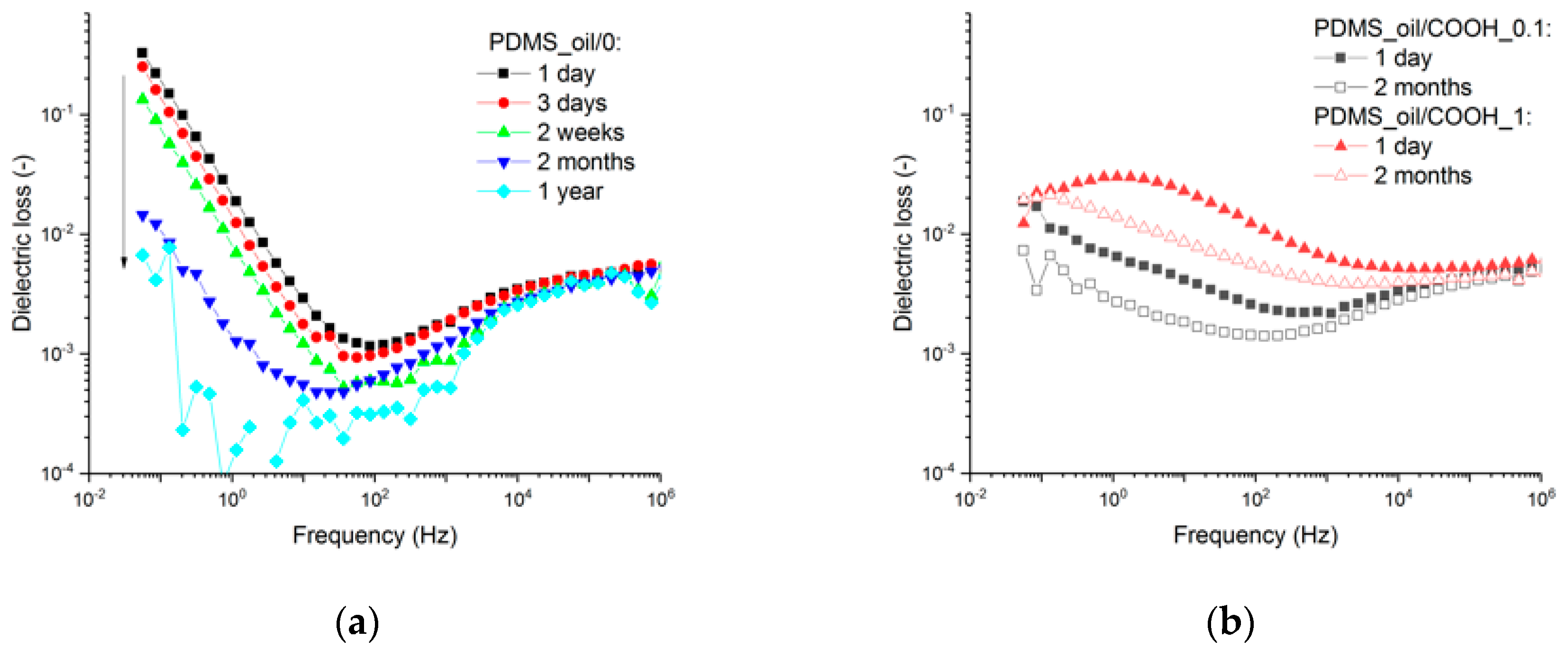
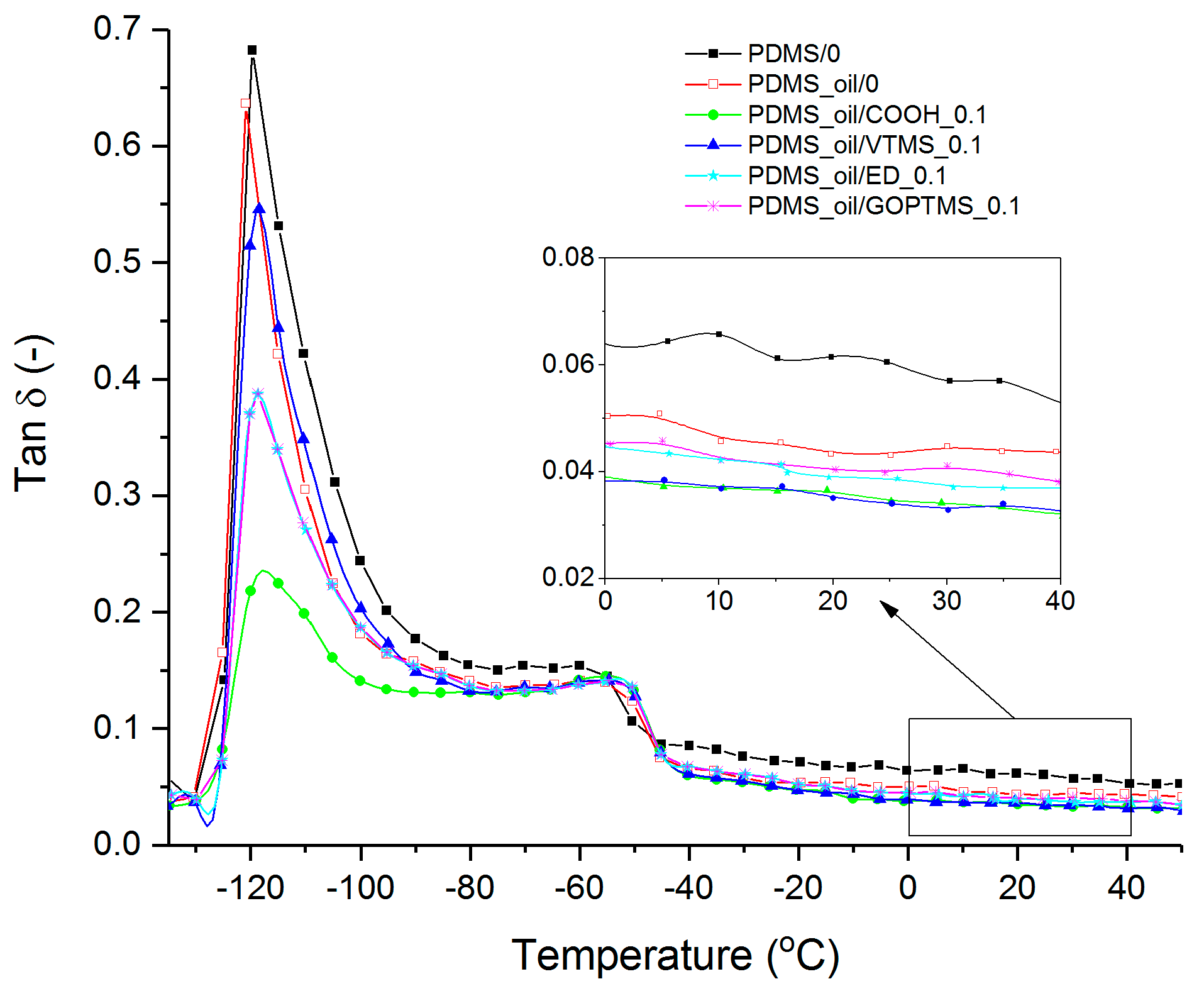
3.2. Nanodiamond-Silicone Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dolmatov, V.; Fujimura, T. Physical and Chemical Problems of Modification of Detonation Nanodiamond Surface Properties. Synth. Prop. Appl. Ultrananocrystalline Diam. 2005, 192, 217–230. [Google Scholar]
- Krueger, A. The structure and reactivity of nanoscale diamond. J. Mater. Chem. 2008, 18, 1485–1492. [Google Scholar] [CrossRef]
- Zhang, Y.; Rhee, K.Y.; Hui, D.; Park, S.-J. A critical review of nanodiamond based nanocomposites: Synthesis, properties and applications. Compos. Part B Eng. 2018, 143, 19–27. [Google Scholar] [CrossRef]
- Shakun, A.; Vuorinen, J.; Hoikkanen, M.; Poikelispää, M.; Das, A. Hard nanodiamonds in soft rubbers: Past, present and future – A review. Compos. Part A Appl. Sci. Manuf. 2014, 64, 49–69. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Gogotsi, Y. Nanodiamond–polymer composites. Diamond Related Mater. 2015, 58, 161–171. [Google Scholar] [CrossRef]
- Shakun, A.; Poikelispää, M.; Das, A.; Vuorinen, J. Improved electromechanical response in acrylic rubber by different carbon-based fillers. Polym. Eng. Sci. 2018, 58, 395–404. [Google Scholar] [CrossRef]
- Shakun, A.; Sarlin, E.; Vuorinen, J. Natural rubber-nanodiamond films for the minimisation of losses in dielectric energy harvesters. Int. J. Exergy 2018, 26, 170. [Google Scholar] [CrossRef]
- Hoffstadt, T.; Graf, C.; Maas, J. Optimization of the energy harvesting control for dielectric elastomer generators. Smart Mater. Struct. 2013, 22, 94028. [Google Scholar] [CrossRef]
- Chiba, S.; Waki, M.; Wada, T.; Hirakawa, Y.; Masuda, K.; Ikoma, T. Consistent ocean wave energy harvesting using electroactive polymer (dielectric elastomer) artificial muscle generators. Appl. Energy 2013, 104, 497–502. [Google Scholar] [CrossRef]
- Kornbluh, R.D.; Pelrine, R.; Prahlad, H.; Wong-Foy, A.; McCoy, B.; Kim, S.; Eckerle, J.; Low, T. From Boots to Buoys: Promises and Challenges of Dielectric Elastomer Energy Harvesting; Springer Science and Business Media LLC: Berlin, Germany, 2012; pp. 67–93. [Google Scholar]
- Kaltseis, R.; Keplinger, C.; Koh, S.J.A.; Baumgartner, R.; Goh, Y.F.; Ng, W.H.; Kogler, A.; Tröls, A.; Foo, C.C.; Suo, Z.; et al. Natural rubber for sustainable high-power electrical energy generation. RSC Adv. 2014, 4, 27905–27913. [Google Scholar] [CrossRef]
- Ahnert, K.; Abel, M.; Kollosche, M.; Jørgensen, P.J.; Kofod, G. Soft capacitors for wave energy harvesting. J. Mater. Chem. 2011, 21, 14492–14497. [Google Scholar] [CrossRef]
- Jean-Mistral, C.; Basrour, S. Scavenging Energy from Human Motion with Tubular Dielectric Polymer. Available online: http://dx.doi.org/10.1117/12 (accessed on 29 June 2019).
- Kang, G.; Kim, K.-S.; Kim, S. Note: Analysis of the efficiency of a dielectric elastomer generator for energy harvesting. Rev. Sci. Instruments 2011, 82, 46101. [Google Scholar] [CrossRef] [PubMed]
- Madsen, F.B.; Yu, L.; Daugaard, A.E.; Hvilsted, S.; Skov, A.L. Silicone elastomers with high dielectric permittivity and high dielectric breakdown strength based on dipolar copolymers. Polymer 2014, 55, 6212–6219. [Google Scholar] [CrossRef]
- Madsen, P.J.; Yu, L.; Boucher, S.; Skov, A.L. Enhancing the electro-mechanical properties of polydimethylsiloxane elastomers through blending with poly(dimethylsiloxane-co-methylphenylsiloxane) copolymers. RSC Adv. 2018, 8, 23077–23088. [Google Scholar] [CrossRef]
- Graf, C.; Hitzbleck, J.; Feller, T. Dielectric elastomer–based energy harvesting: Material, generator design, and optimization. J. Intelligent Mater. System Struct. 2014, 25, 951–966. [Google Scholar] [CrossRef]
- Baidakova, M.; Vul’, A. New prospects and frontiers of nanodiamond clusters. J. Phys. D Appl. Phys. 2007, 40, 6300–6311. [Google Scholar] [CrossRef]
- Shenderova, O.; Jones, C.; Borjanovic, V.; Hens, S.; Cunningham, G.; Moseenkov, S.; Kuznetsov, V.; McGuire, G. Detonation nanodiamond and onion-like carbon: applications in composites. Phys. Status Solidi (a) 2008, 205, 2245–2251. [Google Scholar] [CrossRef]
- Voznyakovski, A.P.; Kudoyarov, M.F.; Pozdnyakov, O.F. Self-organization and sedimentation stability of detonation nanodiamond suspensions. Tech. Phys. Lett. 2007, 33, 29–36. [Google Scholar] [CrossRef]
- Kong, S.; Mariatti, M.; Busfield, J. Effects of types of fillers and filler loading on the properties of silicone rubber composites. J. Reinf. Plast. Compos. 2011, 30, 1087–1096. [Google Scholar] [CrossRef]
- Neverovskaya, A.Y.; Voznyakovskii, A.P.; Dolmatov, V.Y. Structure of the dispersive medium and sedimentation resistance of suspensions of detonation nanodiamonds. Phys. Solid State 2004, 46, 662–664. [Google Scholar] [CrossRef]
- Schrand, A.M.; Hens, S.A.C.; Shenderova, O.A. Nanodiamond Particles: Properties and Perspectives for Bioapplications. Crit. Rev. Solid State Mater. Sci. 2009, 34, 18–74. [Google Scholar] [CrossRef]
- Krueger, A. Chapter 8 - current issues and challenges in surface chemistry of nanodiamonds. In Nanodiamonds; Arnault, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 183–242. [Google Scholar]
- Brochier Salon, M.; Bayle, P.; Abdelmouleh, M.; Boufi, S.; Belgacem, M.N. Kinetics of hydrolysis and self condensation reactions of silanes by NMR spectroscopy. Colloids Surf. A Physicochem. Eng. Asp. 2008, 312, 83–91. [Google Scholar] [CrossRef]
- Altmann, S.; Pfeiffer, J. The Hydrolysis/Condensation Behaviour of Methacryloyloxyalkylfunctional Alkoxysilanes: Structure-Reactivity Relations. Monatshefte Chemie-Chemical Monthly 2003, 134, 1081–1092. [Google Scholar] [CrossRef]
- Krueger, A.; Williams, O.A. (Eds.) The Chemistry of Nanodiamond. Royal Society of Chemistry; No. 2014-January; RSC Nanoscience and Nanotechnology: Cambridge, UK, 2014. [Google Scholar]
- Krüger, A.; Liang, Y.; Jarre, G.; Stegk, J.; Kr?ger, A. Surface functionalisation of detonation diamond suitable for biological applications. J. Mater. Chem. 2006, 16, 2322–2328. [Google Scholar] [CrossRef]
- Edgington, R.; Spillane, K.M.; Papageorgiou, G.; Wray, W.; Ishiwata, H.; Labarca, M.; Leal-Ortiz, S.; Reid, G.; Webb, M.; Foord, J.; et al. Functionalisation of Detonation Nanodiamond for Monodispersed, Soluble DNA-Nanodiamond Conjugates Using Mixed Silane Bead-Assisted Sonication Disintegration. Sci. Rep. 2018, 8, 728. [Google Scholar] [CrossRef] [PubMed]
- Hajiali, F.; Shojaei, A. Silane functionalization of nanodiamond for polymer nanocomposites-effect of degree of silanization. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 506, 254–263. [Google Scholar] [CrossRef]
- Neburkova, J.; Vavra, J.; Cigler, P. Coating nanodiamonds with biocompatible shells for applications in biology and medicine. Curr. Opin. Solid State Mater. Sci. 2017, 21, 43–53. [Google Scholar] [CrossRef]
- Li, Z.; Tatsuya, T.; Masaaki, I.; Naoko, K.; Takahide, K.; Naoki, K. Chromatographic Separation of Highly Soluble Diamond Nanoparticles Prepared by Polyglycerol Grafting. Angew. Chem. Int. Ed. 2011, 50, 1388–1392. [Google Scholar]
- Huang, Z.; Ji, H.; Mays, J.W.; Dadmun, M.D. Understanding the Grafting of Telechelic Polymers on a Solid Substrate to Form Loops. Macromolecules 2008, 41, 1009–1018. [Google Scholar] [CrossRef]
- Blank, W.J.; He, Z.A.; Picci, M. Catalysis of the epoxy-carboxyl reaction. J. Coat. Technol. 2002, 74, 33–41. [Google Scholar] [CrossRef]
- Schönherr, J.; Buchheim, J.R.; Scholz, P.; Adelhelm, P. Boehm Titration Revisited (Part I): Practical Aspects for Achieving a High Precision in Quantifying Oxygen-Containing Surface Groups on Carbon Materials. C 2018, 4, 21. [Google Scholar] [CrossRef]
- Tiwari, M.; Datta, R.N.; Talma, A.G.; Noordermeer, J.W.M.; Dierkes, W.K.; Van Ooij, W.J. Comparative Study of Plasma-Thiophene and -Acetylene Coated Silica in SBR and EPDM Reinforcement. Rubber Chem. Technol. 2009, 82, 473–491. [Google Scholar] [CrossRef]
- Osswald, S.; Yushin, G.; Mochalin, V.; Kucheyev, S.O.; Gogotsi, Y. Control of sp2/sp3Carbon Ratio and Surface Chemistry of Nanodiamond Powders by Selective Oxidation in Air. J. Am. Chem. Soc. 2006, 128, 11635–11642. [Google Scholar] [CrossRef] [PubMed]
- Bradac, C.; Gaebel, T.; Pakes, C.I.; Say, J.M.; Zvyagin, A.V.; Rabeau, J.R. Effect of the Nanodiamond Host on a Nitrogen-Vacancy Color-Centre Emission State. Small 2013, 9, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Stehlik, S.; Glatzel, T.; Pichot, V.; Pawlak, R.; Meyer, E.; Spitzer, D.; Rezek, B. Water interaction with hydrogenated and oxidized detonation nanodiamonds — Microscopic and spectroscopic analyses. Diam. Relat. Mater. 2016, 63, 97–102. [Google Scholar] [CrossRef]
- Ji, S.; Jiang, T.; Xu, K.; Li, S. FTIR study of the adsorption of water on ultradispersed diamond powder surface. Appl. Surf. Sci. 1998, 133, 231–238. [Google Scholar] [CrossRef]
- Efremov, V.P.; Zakatilova, E.I.; Maklashova, I.V.; Shevchenko, N.V. Thermal stability of detonation-produced micro and nanodiamonds. J. Phys. Conf. Ser. 2018, 946, 012107. [Google Scholar]
- Casalini, R.; Roland, C. Aging of a low molecular weight poly(methyl methacrylate). J. Non-Crystalline Solids 2011, 357, 282–285. [Google Scholar] [CrossRef]
- Roggero, A.; Dantras, E.; Paulmier, T.; Tonon, C.; Lewandowski, S.; Dagras, S.; Payan, D. Dynamic glass transition of filled polysiloxane upon electron irradiation. J. Non-Crystalline Solids 2017, 455, 17–23. [Google Scholar] [CrossRef]
- Glatz-Reichenbach, J.K.W.; Sorriero, L.; Fitzgerald, J.J. Influence of Crosslinking on the Molecular Relaxation of an Amorphous Copolymer Near Its Glass-Transition Temperature. Macromolecules 1994, 27, 1338–1343. [Google Scholar] [CrossRef]
- Dolmatov, V.Y. Polymer—Diamond composites based on detonation nanodiamonds. Part 3. J. Superhard Mater. 2007, 29, 199–205. [Google Scholar] [CrossRef]
- Maus, A.; Saalwächter, K. Crystallization Kinetics of Poly(dimethylsiloxane) Molecular-Weight Blends—Correlation with Local Chain Order in the Melt? Macromol. Chem. Phys. 2007, 208, 2066–2075. [Google Scholar] [CrossRef]


| Surface Groups, mmol/g | ||||
|---|---|---|---|---|
| Carboxyl | Lactone | Phenolic | All Surface Groups | |
| ND reference | 0.131 | 0.101 | 0.219 | 0.451 |
| ND oxidized | 0.329 | 0.018 | 0.127 | 0.475 |
| Sample | Element Content, % | |||
|---|---|---|---|---|
| C | O | Si | Others | |
| ND-COOH | 96.0 | 3.2 | - | <1 |
| ND-VTMS | 54.6 | 18.0 | 27.3 | <1 |
| ND-ED(40C) | 96.0 | 4.0 | - | - |
| ND-GOPTMS | 90.8 | 6.6 | 2.6 | - |
| Sample | Modulus, MPa | Apparent Crosslink Density 1/Q, - | ||
|---|---|---|---|---|
| 10% | 50% | 100% | ||
| PDMS/0 | 0.12 ± 0.01 | 0.49 ± 0.03 | 1.34 ± 0.11 | 0.689 ± 0.005 |
| PDMS_oil/0 | 0.12 ± 0.01 | 0.45 ± 0.01 | 1.38 ± 0.09 | 0.614 ± 0.003 |
| PDMS_oil/COOH_0.1 | 0.11 ± 0.01 | 0.49 ± <0.01 | 1.13 ± 0.22 | 0.683 ± 0.003 |
| PDMS_oil/VTMS_0.1 | 0.10 ± 0.01 | 0.47 ± 0.01 | 1.39 ± 0.02 | 0.674 ± 0.004 |
| PDMS_oil/ED_0.1 | 0.10 ± 0.02 | 0.44 ± 0.01 | 1.38 ± 0.10 | 0.679 ± 0.003 |
| PDMS_oil/GOPTMS_0.1 | 0.11 ± 0.01 | 0.45 ± 0.01 | 1.39 ± 0.03 | 0.554 ± 0.047 |
| Sample | Tg, °C | Enthalpy, J/g | Degree of Crystallinity*, % | |
|---|---|---|---|---|
| Cold Crystallization | Melting | |||
| PDMS/0 | −127.0 | 3.26 | 3.84 | 0.9 |
| PDMS_oil/0 | −127.3 | 0.78 | 0.92 | 0.2 |
| PDMS_oil/COOH_0.1 | −124.8 | 1.45 | 15.37 | 22.7 |
| PDMS_oil/VTMS_0.1 | −120.6 | - | 15.47 | 25.2 |
| PDMS_oil/ED_0.1 | −121.6 | - | 14.90 | 24.3 |
| PDMS_oil/GOPTMS_0.1 | −126.6 | 9.57 | 13.58 | 6.5 |
| Sample | Hysteresis Loss, % | Stress Loss, % | |||
|---|---|---|---|---|---|
| 1st Cycle | 10th Cycle | after 1 min | after 5 min | after 1 h | |
| PDMS/0 | 1.49 ± 0.17 | 0.63 ± 0.20 | 1.08 ± 0.04 | 1.77 ± 0.11 | 2.83 ± 0.47 |
| PDMS_oil/0 | 2.21 ± 0.27 | 0.64 ± 0.15 | 0.78 ± 0.13 | 1.30 ± 0.28 | 2.26 ± 0.38 |
| PDMS_oil/COOH_0.1 | 1.70 ± 0.25 | 0.64 ± 0.37 | 0.61 ± 0.17 | 0.96 ± 0.23 | 1.98 ± 0.38 |
| PDMS_oil/VTMS_0.1 | 1.59 ± 0.66 | 0.46 ± 0.23 | 0.56 ± 0.09 | 0.86 ± 0.06 | 1.47 ± 0.16 |
| PDMS_oil/ED_0.1 | 1.89 ± 0.71 | 0.30 ± 0.10 | 0.69 ± 0.04 | 1.22 ± 0.28 | 2.50 ± 0.41 |
| PDMS_oil/GOPTMS_0.1 | 1.48 ± 0.37 | 0.37 ± 0.18 | 0.62 ± 0.12 | 1.07 ± 0.21 | 2.41 ± 0.37 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakun, A.; Anyszka, R.; Sarlin, E.; Blume, A.; Vuorinen, J. Influence of Surface Modified Nanodiamonds on Dielectric and Mechanical Properties of Silicone Composites. Polymers 2019, 11, 1104. https://doi.org/10.3390/polym11071104
Shakun A, Anyszka R, Sarlin E, Blume A, Vuorinen J. Influence of Surface Modified Nanodiamonds on Dielectric and Mechanical Properties of Silicone Composites. Polymers. 2019; 11(7):1104. https://doi.org/10.3390/polym11071104
Chicago/Turabian StyleShakun, Alexandra, Rafal Anyszka, Essi Sarlin, Anke Blume, and Jyrki Vuorinen. 2019. "Influence of Surface Modified Nanodiamonds on Dielectric and Mechanical Properties of Silicone Composites" Polymers 11, no. 7: 1104. https://doi.org/10.3390/polym11071104
APA StyleShakun, A., Anyszka, R., Sarlin, E., Blume, A., & Vuorinen, J. (2019). Influence of Surface Modified Nanodiamonds on Dielectric and Mechanical Properties of Silicone Composites. Polymers, 11(7), 1104. https://doi.org/10.3390/polym11071104