Morphology, Crystallinity, and Molecular Weight of Poly(ε-caprolactone)/Graphene Oxide Hybrids
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
2.2.1. Sample Preparation
2.2.2. Morphology: SEM and TEM
2.2.3. FT-IR Analysis
2.2.4. POM
2.2.5. GPC
2.2.6. Atomic Force Microscopy (AFM)
2.2.7. DSC
2.2.8. XRD
2.2.9. TGA
2.2.10. Cell Culture and Statistical Analysis
3. Results and Discussion
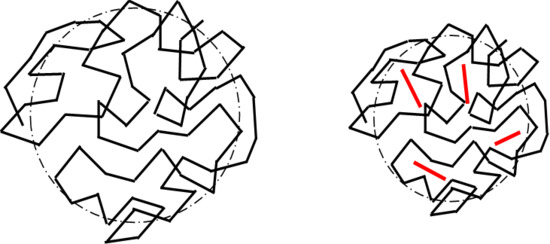
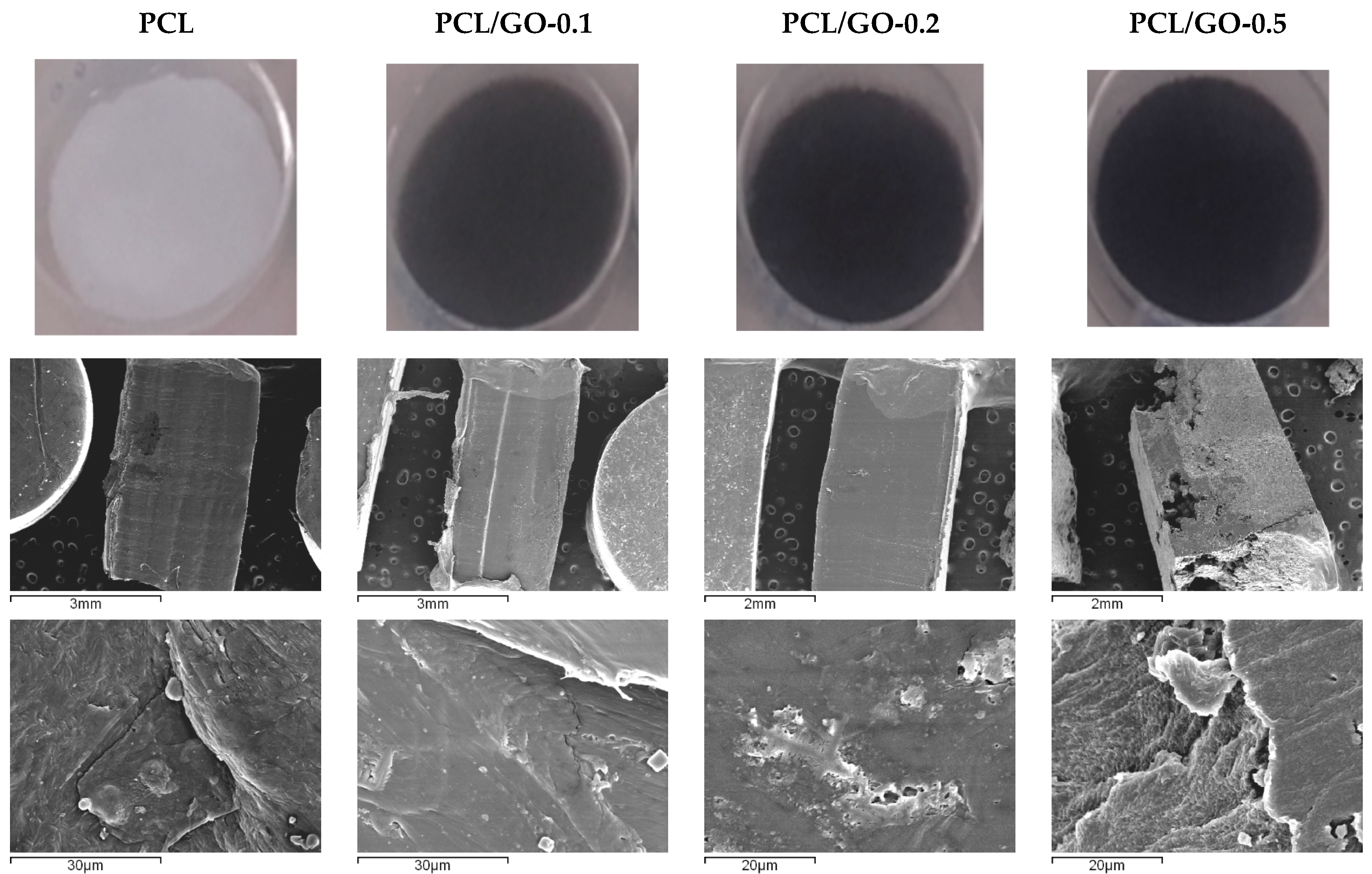
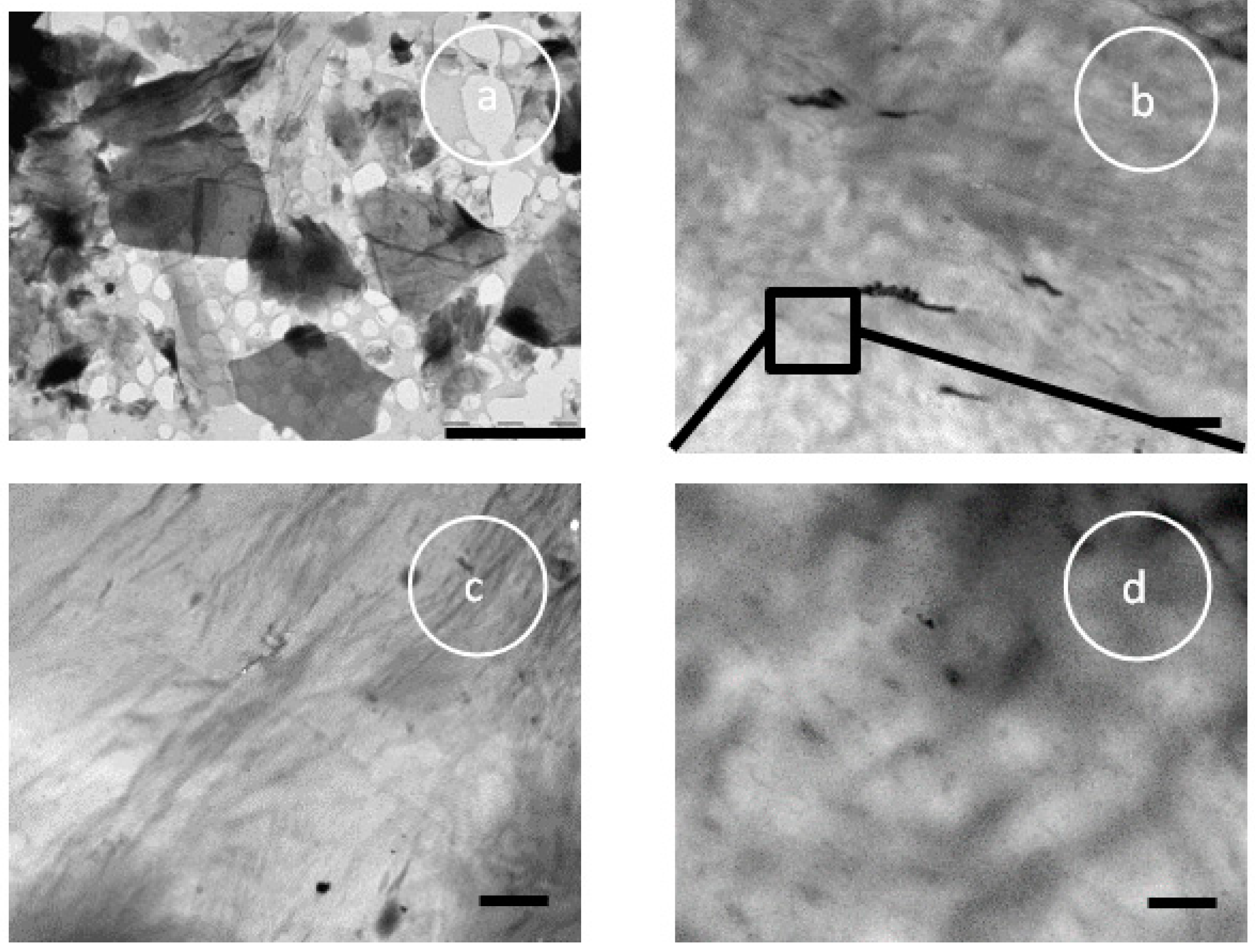
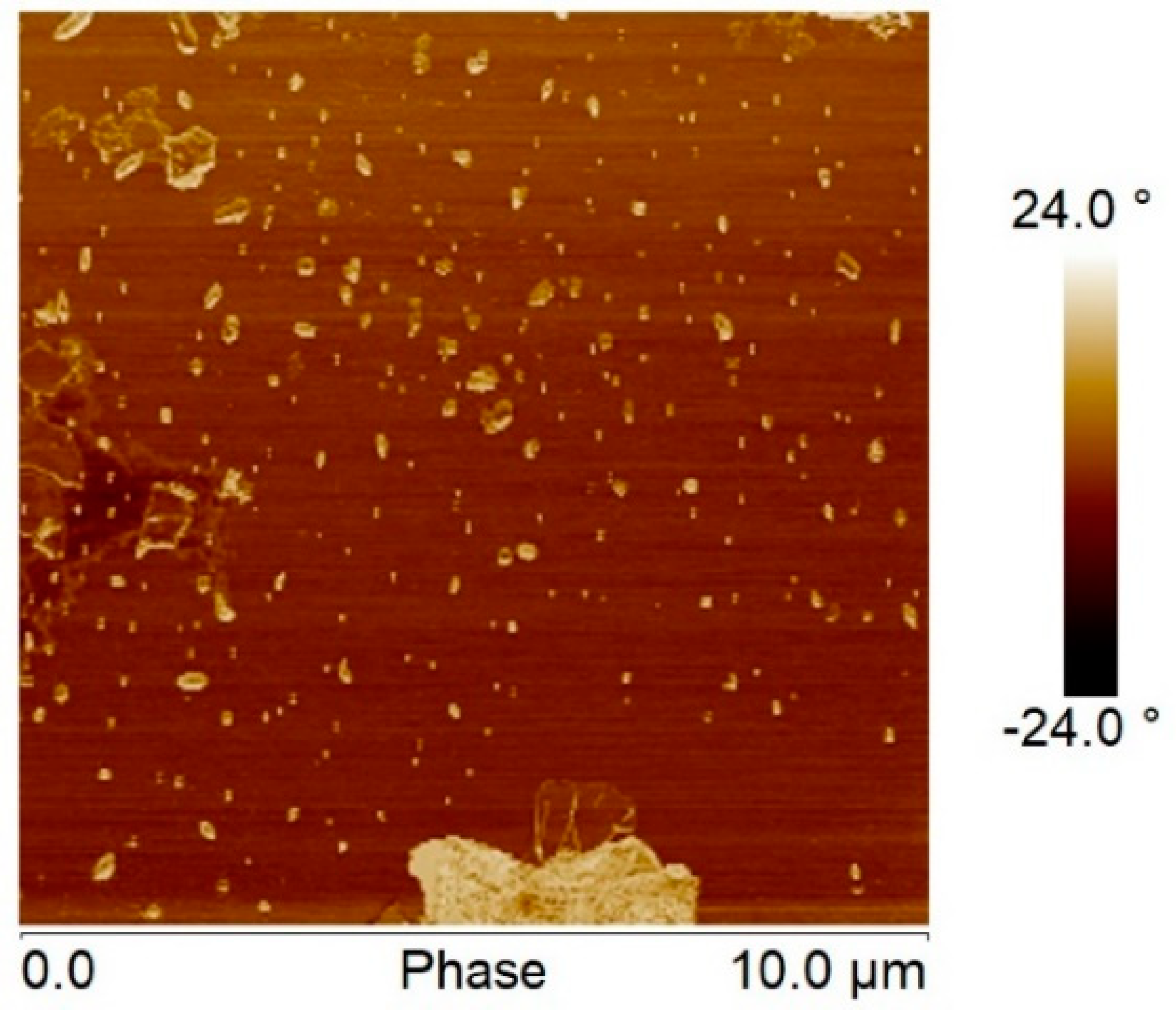
3.1. Visual Examination, SEM, and TEM
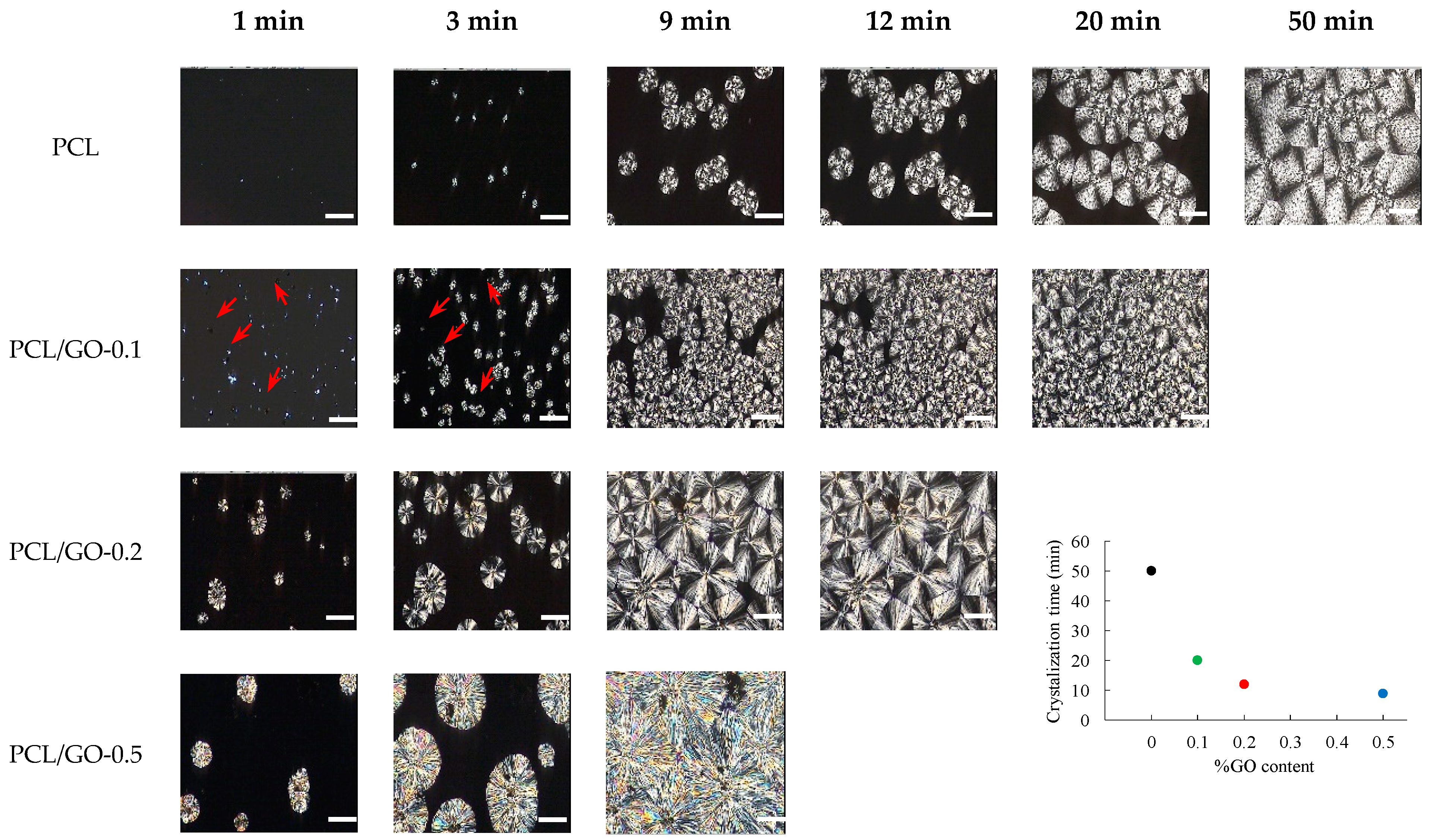
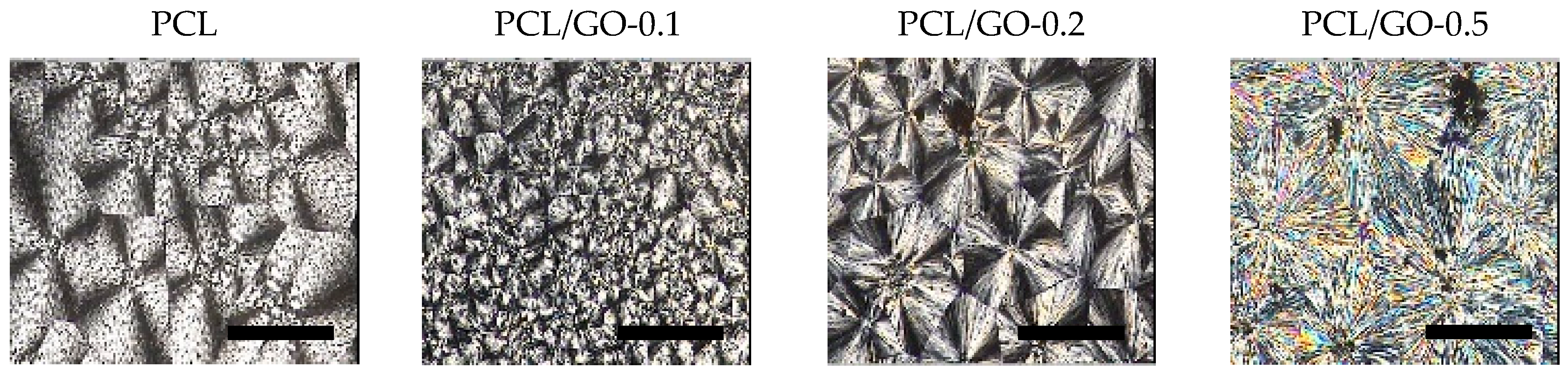
3.2. POM
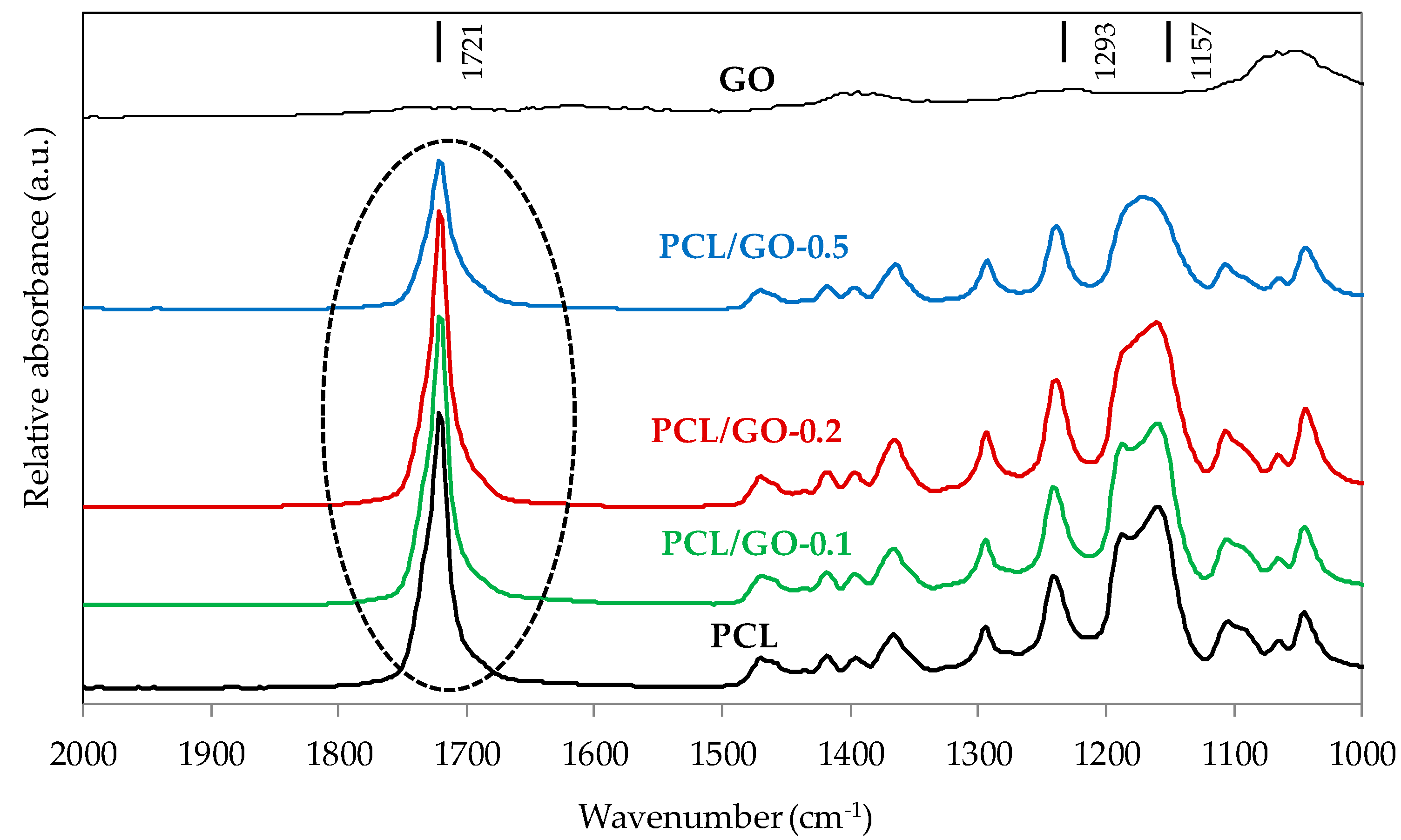
3.3. FT-IR
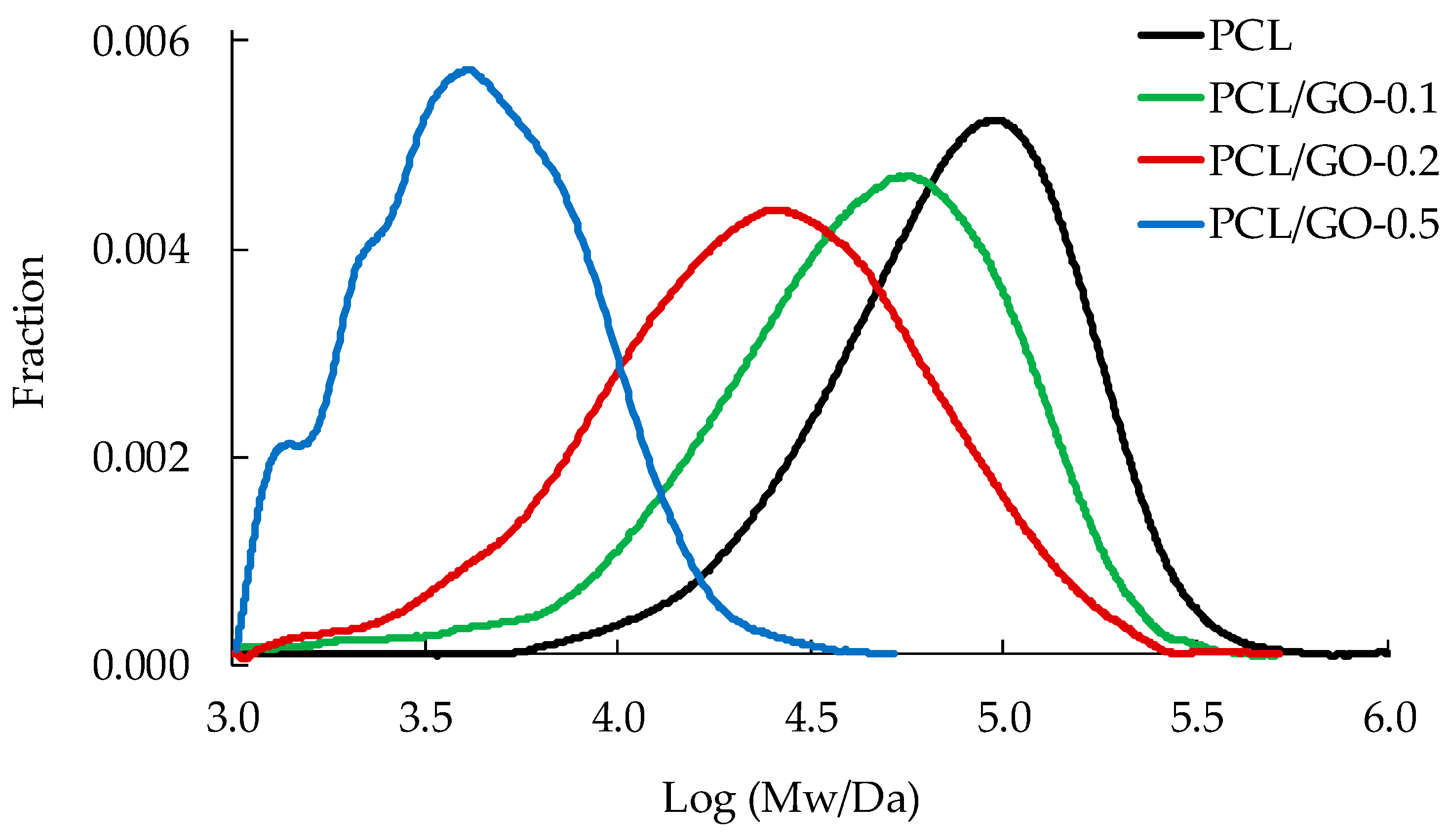
3.4. GPC
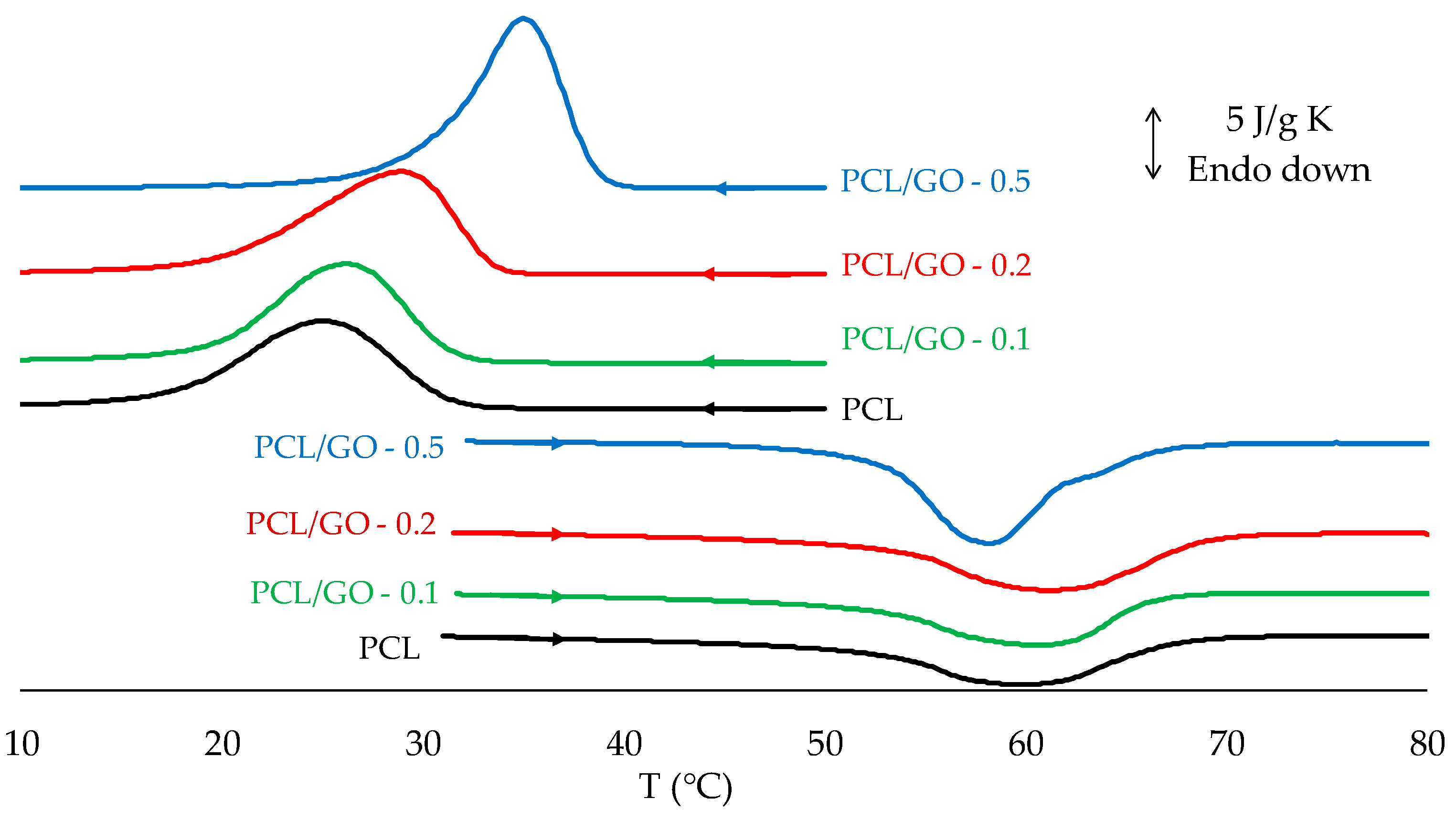
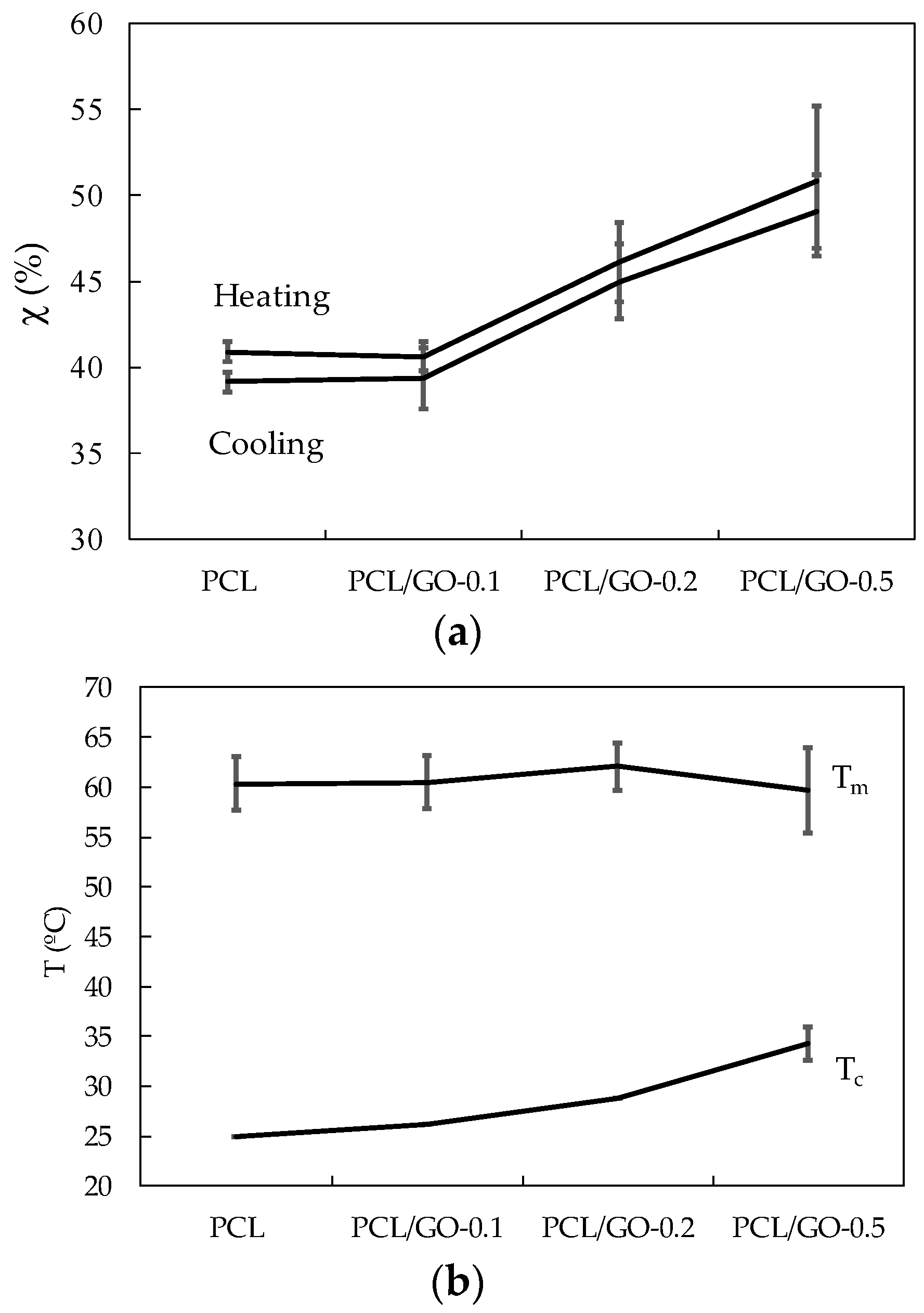
3.5. DSC
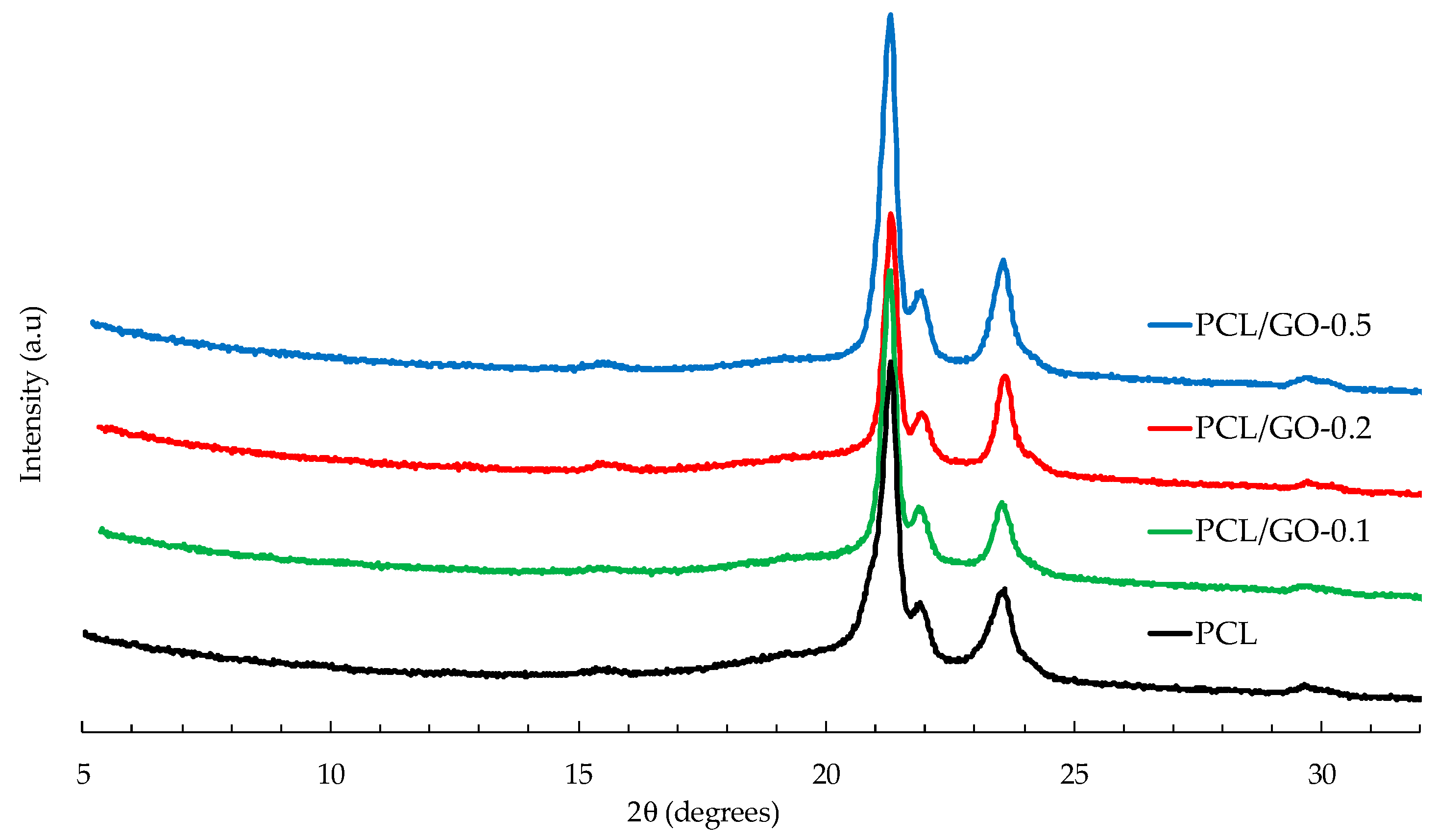
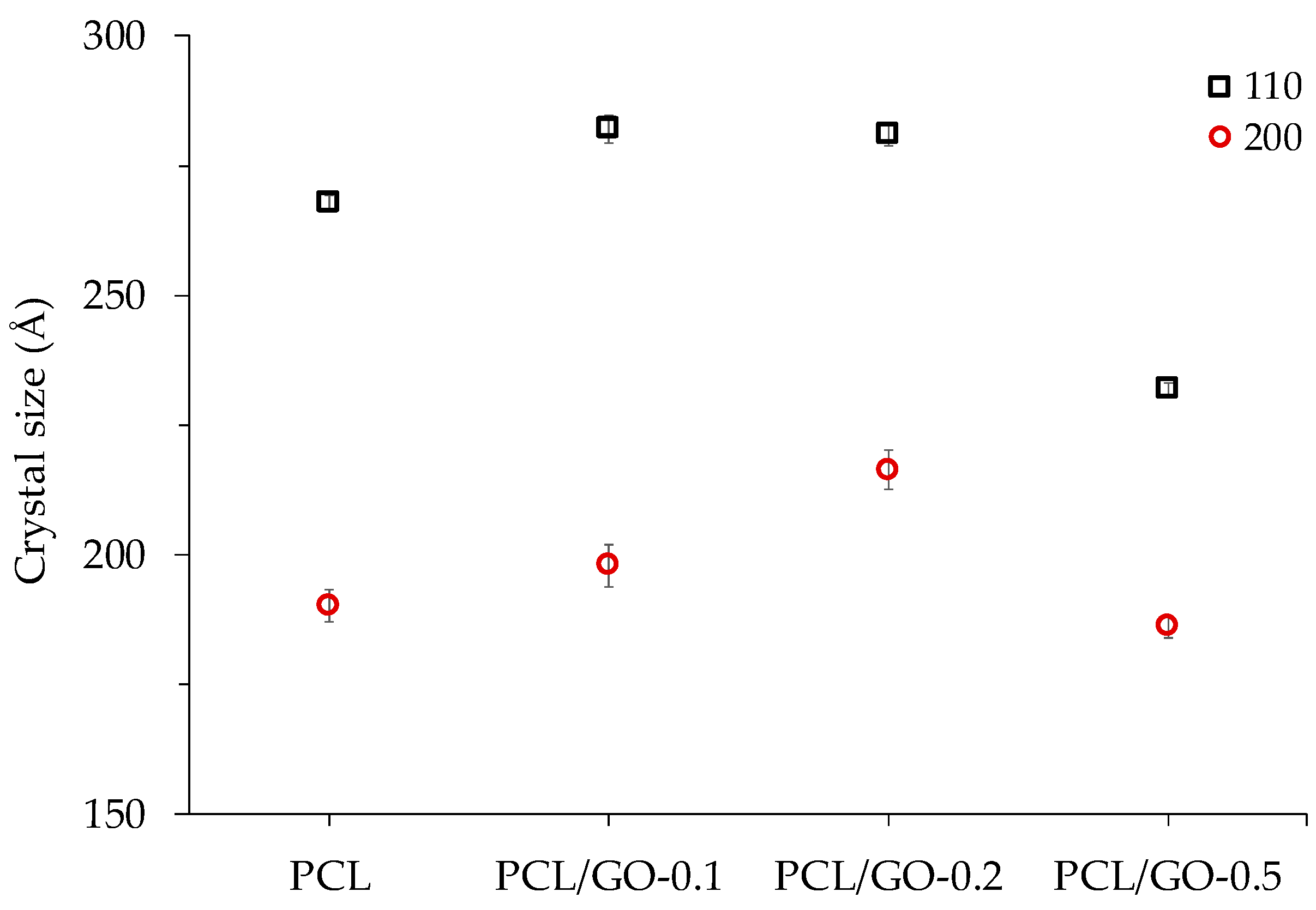
3.6. XRD
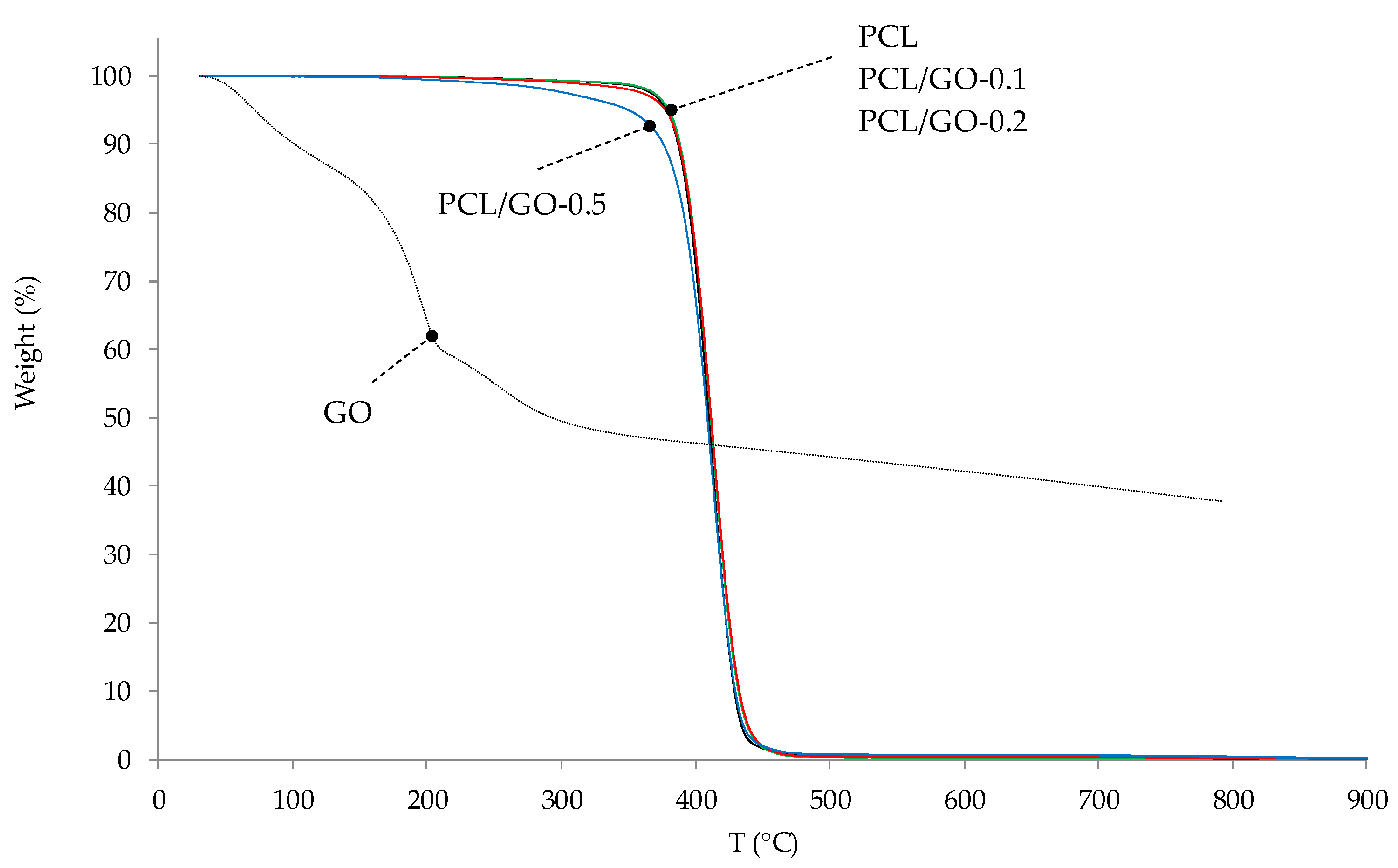
3.7. TGA
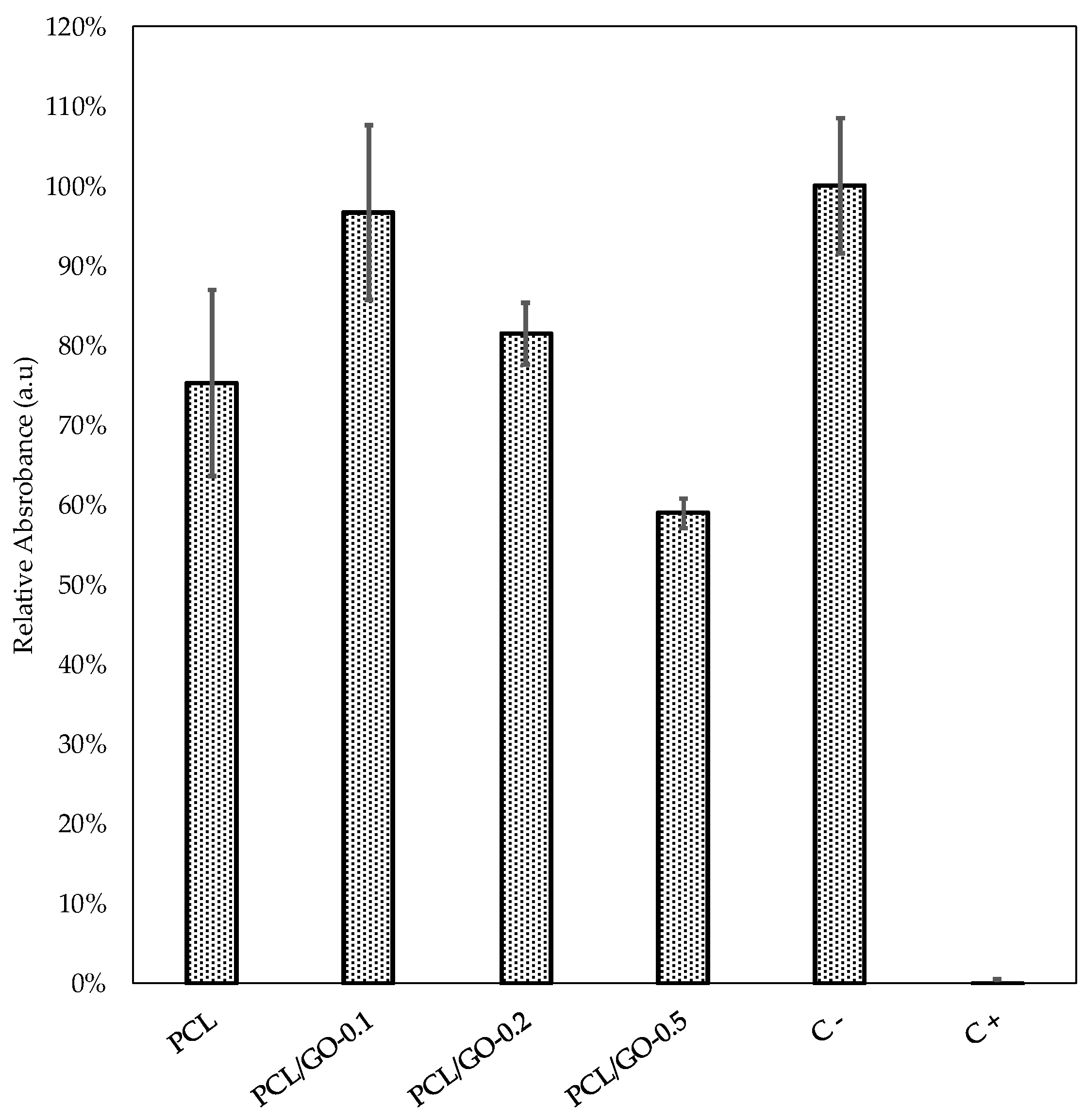
3.8. Cellular Viability
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Brodie, B.C. Sur le poids atomique du graphite. Ann. Chim. Phys. 1860, 59, e472. [Google Scholar]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Nguyen, S.B.T.; Ruoff, R.S. Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon N. Y. 2006, 44, 3342–3347. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. Graphite oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Konios, D.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E. Dispersion behaviour of graphene oxide and reduced graphene oxide. J. Colloid Interface Sci. 2014, 430, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer (Guildf) 2011, 52, 5–25. [Google Scholar] [CrossRef]
- Liang, J.; Huang, Y.; Zhang, L.; Wang, Y.; Ma, Y.; Cuo, T.; Chen, Y. Molecular-level dispersion of graphene into poly(vinyl alcohol) and effective reinforcement of their nanocomposites. Adv. Funct. Mater. 2009, 19, 2297–2302. [Google Scholar] [CrossRef]
- Han, D.; Yan, L.; Chen, W.; Li, W. Preparation of chitosan/graphene oxide composite film with enhanced mechanical strength in the wet state. Carbohydr. Polym. 2011, 83, 653–658. [Google Scholar] [CrossRef]
- Luong, N.D.; Hippi, U.; Korhonen, J.T.; Soininen, A.J.; Ruokolainen, J.; Johansson, L.S.; Do Nam, J.; Sinh, L.H.; Seppälä, J. Enhanced mechanical and electrical properties of polyimide film by graphene sheets via in situ polymerization. Polymer (Guildf) 2011, 52, 5237–5242. [Google Scholar] [CrossRef]
- Yang, X.; Tu, Y.; Li, L.; Shang, S.; Tao, X.M. Well-dispersed chitosan/graphene oxide nanocomposites. ACS Appl. Mater. Interfaces 2010, 2, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Salavagione, H.J.; Gómez, M.A.; Martínez, G. Polymeric modification of graphene through esterification of graphite oxide and poly(vinyl alcohol). Macromolecules 2009, 42, 6331–6334. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, C. In situ polymerization approach to graphene-reinforced nylon-6 composites. Macromolecules 2010, 43, 6716–6723. [Google Scholar] [CrossRef]
- Kulkarni, D.D.; Choi, I.; Singamaneni, S.S.; Tsukruk, V.V. Graphene oxide - Polyelectrolyte nanomembranes. ACS Nano 2010, 4, 4667–4676. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Guo, Y.; Song, L.; Hu, Y. Poly(vinyl alcohol) nanocomposites based on graphene and graphite oxide: a comparative investigation of property and mechanism. J. Mater. Chem. 2011, 21, 13942. [Google Scholar] [CrossRef]
- Tang, L.-C.; Wan, Y.-J.; Yan, D.; Pei, Y.-B.; Zhao, L.; Li, Y.-B.; Wu, L.-B.; Jiang, J.-X.; Lai, G.-Q. The effect of graphene dispersion on the mechanical properties of graphene/epoxy composites. Carbon N. Y. 2013, 60, 16–27. [Google Scholar] [CrossRef]
- Song, Y.S.; Youn, J.R. Influence of dispersion states of carbon nanotubes on physical properties of epoxy nanocomposites. Carbon N. Y. 2005, 43, 1378–1385. [Google Scholar] [CrossRef]
- Kim, H.; Miura, Y.; MacOsko, C.W. Graphene/polyurethane nanocomposites for improved gas barrier and electrical conductivity. Chem. Mater. 2010, 22, 3441–3450. [Google Scholar] [CrossRef]
- Ahmad, H.; Fan, M.; Hui, D. Graphene oxide incorporated functional materials: A review. Compos. Part B Eng. 2018, 145, 270–280. [Google Scholar] [CrossRef]
- Kai, W.; Hirota, Y.; Hua, L.; Yoshio, I. Thermal and Mechanical Properties of a Poly(«-caprolactone)/Graphite Oxide Composite. J. Appl. Polym. Sci. 2008, 107, 1395–1400. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer - Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Wan, C.; Chen, B. Poly(ε-caprolactone)/graphene oxide biocomposites: mechanical properties and bioactivity. Biomed. Mater. 2011, 6, 055010. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Gao, H.; Zhu, G.; Cao, X.; Shi, X.; Wang, Y. The preparation and characterization of polycaprolactone/graphene oxide biocomposite nanofiber scaffolds and their application for directing cell behaviors. Carbon N. Y. 2015, 95, 1039–1050. [Google Scholar] [CrossRef]
- Hua, L.; Kai, W.H.; Inoue, Y. Crystallization behavior of poly(ε-caprolactone)/graphite oxide composites. J. Appl. Polym. Sci. 2007, 106, 4225–4232. [Google Scholar] [CrossRef]
- Sayyar, S.; Murray, E.; Thompson, B.C.; Gambhir, S.; Officer, D.L.; Wallace, G.G. Covalently linked biocompatible graphene/polycaprolactone composites for tissue engineering. Carbon N. Y. 2013, 52, 296–304. [Google Scholar] [CrossRef]
- Murray, E.; Sayyar, S.; Thompson, B.C.; Gorkin III, R.; Officer, D.L.; Wallace, G.G. A bio-friendly, green route to processable, biocompatible graphene/polymer composites. RSC Adv. 2015, 5, 45284–45290. [Google Scholar] [CrossRef]
- Hassanzadeh, S.; Adolfsson, K.H.; Wu, D.; Hakkarainen, M. Supramolecular Assembly of Biobased Graphene Oxide Quantum Dots Controls the Morphology of and Induces Mineralization on Poly(ε-caprolactone) Films. Biomacromolecules 2016, 17, 256–261. [Google Scholar] [CrossRef]
- Kumar, S.; Azam, D.; Raj, S.; Kolanthai, E.; Vasu, K.S.; Sood, A.K.; Chatterjee, K. 3D scaffold alters cellular response to graphene in a polymer composite for orthopedic applications. J. Biomed. Mater. Res. B 2016, 104, 732–749. [Google Scholar] [CrossRef]
- Shin, S.R.; Li, Y.C.; Jang, H.L.; Khoshakhlagh, P.; Akbari, M.; Nasajpour, A.; Zhang, Y.S.; Tamayol, A.; Khademhosseini, A. Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev. 2016, 105, 255–274. [Google Scholar] [CrossRef]
- Bianco, A. Graphene: Safe or toxic? the two faces of the medal. Angew. Chemie - Int. Ed. 2013, 52, 4986–4997. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, J.; Peng, C.; Hu, W.; Zhu, Z.; Li, W.; Fan, C.; Huang, Q. Distribution and biocompatibility studies of graphene oxide in mice after intravenous administration. Carbon N. Y. 2011, 49, 986–995. [Google Scholar] [CrossRef]
- Jassim, D.A.; Murphy, S.; Newman, L.; Mironov, A.; Prestat, E.; McCaffrey, J.; Menard-Moyon, C.; Rodrigues, A.F.; Bianco, A.; Haigh, S.; et al. The Effects of extensive glomerular filtration of thin graphene oxide sheets on kidney physiology. ACS Nano 2016, 10, 10753–10767. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.M.; Mangadlao, J.; Ahmed, F.; Leon, A.; Advincula, R.C.; Rodrigues, D.F. Graphene nanocomposite for biomedical applications: Fabrication, antimicrobial and cytotoxic investigations. Nanotechnology 2012, 23, 395101. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.N.; Huang, N.M.; Loo, C.H. Facile preparation of graphene-based chitosan films: Enhanced thermal, mechanical and antibacterial properties. J. Non. Cryst. Solids 2012, 358, 525–530. [Google Scholar] [CrossRef]
- Some, S.; Ho, S.M.; Dua, P.; Hwang, E.; Shin, Y.H.; Yoo, H.; Kang, J.S.; Lee, D.K.; Lee, H. Dual functions of highly potent graphene derivative-poly-l-lysine composites to inhibit bacteria and support human cells. ACS Nano 2012, 6, 7151–7161. [Google Scholar] [CrossRef]
- Donlan, R.M.; Wu, P.; Grainger, D.W.; Ratner, B.D. An introduction to materials in medicine; Elsevier Academic Press: San Diego, CA, USA, 2004; ISBN 0125824637. [Google Scholar]
- Sydlik, S.A.; Jhunjhunwala, S.; Webber, M.J. In Vivo Compatibility of Graphene Oxide with Di ff ering Oxidation States. ACS Nano 2015, 9, 3866–3874. [Google Scholar] [CrossRef]
- Crescenzi, V.; Manzini, G.; Calzolari, G.; Borri, C. Thermodynamics of fusion of poly-beta-propiolactone and poly-ε-caprolactone. comparative analysis of the melting of aliphatic polylactone and polyester chains. Eur. Polym. J. 1972, 8, 449–463. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-Ray Diffraction Procedures: For Polycrystalline and Amorphous Materials, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1974; pp. 618–708. ISBN 978-0-471-49369-3. [Google Scholar]
- Guinier, A. X-Ray Diffraction In Crystals, Imperfect Crystals, and Amorphous Bodies; W.H. Freeman and Company: San Francisco, CA, USA; London, UK, 1963; ISBN 978-0-486-68011-8. [Google Scholar]
- Luo, H.; Meng, X.; Cheng, C.; Dong, Z.; Zhang, S.; Li, B. Enzymatic Degradation of Supramolecular Materials Based on Partial Inclusion Complex Formation between r -Cyclodextrin and Poly ( ε-caprolactone ). J. Phys. Chem. B 2010, 114, 4739–4745. [Google Scholar] [CrossRef]
- Vidaurre, A.; Meseguer-Duenas, J.M.; Mas-Estelles, J.; Castilla-Cortazar, I. Influence of enzymatic degradation on physical properties of Poly(ε-caprolactone) films and sponges. Macromol. Symp. 2008, 269, 38–46. [Google Scholar] [CrossRef]
- Honma, T.; Senda, T.; Inoue, Y. Thermal properties and crystallization behaviour of blends of poly(ε-caprolactone) with chitin and chitosan. Polym. Int. 2003, 52, 1839–1846. [Google Scholar] [CrossRef]
- Ramazani, S.; Karimi, M. Aligned poly(ε-caprolactone)/graphene oxide and reduced graphene oxide nanocomposite nanofibers: Morphological, mechanical and structural properties. Mater. Sci. Eng. C 2015, 56, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.M.; Zarian, J. Fourier-Transform Infrared Studies of Polymer Blends. II. Poly(ε-Caprolactone)-Poly(vinyl Chloride) System. J. Polym. Sci. B 1979, 17, 837–850. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, Z.; Huang, Y.; Ma, D.; Yang, J.; Mays, J.W. Characterization of Poly(ε-Caprolactone) via Size Exclusion Chromatography with Online Right-Angle Laser-Light Scattering and Viscometric Detectors. Int. J. Polym. Anal. Charact. 2003, 8, 383–394. [Google Scholar] [CrossRef]
- Sharaf, M.A.; Kloczkowski, A.; Sen, T.Z.; Jacob, K.I.; Mark, J.E. Filler-induced deformations of amorphous polyethylene chains. the effects of the deformations on elastomeric properties, and some comparisons with experiments. Eur. Polym. J. 2006, 42, 796–806. [Google Scholar] [CrossRef]
- Nusser, K.; Neueder, S.; Schneider, G.J.; Meyer, M.; Pyckhout-Hintzen, W.; Willner, L.; Radulescu, A.; Richter, D. Conformations of Silica−Poly(ethylene−propylene) Nanocomposites. Macromolecules 2010, 43, 9837–9847. [Google Scholar] [CrossRef]
- Vacatello, M. Chain Dimensions in Filled Polymers_ An Intriguing Problem. Macromolecules 2002, 35, 8191–8193. [Google Scholar] [CrossRef]
- Duan, T.; Lv, Y.; Xu, H.; Jin, J.; Wang, Z. Structural Effects of Residual Groups of Graphene Oxide on Poly(ε-Caprolactone)/Graphene Oxide Nanocomposite. Crystals 2018, 8, 270. [Google Scholar] [CrossRef]
- Wang, G.S.; Wei, Z.Y.; Sang, L.; Chen, G.Y.; Zhang, W.X.; Dong, X.F.; Qi, M. Morphology, crystallization and mechanical properties of poly(ε-caprolactone)/graphene oxide nanocomposites. Chinese J. Polym. Sci. (English Ed.) 2013, 31, 1148–1160. [Google Scholar]
- Balkova, R.; Hermanova, S.; Voberkova, S.; Damborsky, P.; Richtera, L.; Omelkova, J.; Jancar, J. Structure and Morphology of Microbial Degraded Poly(ε-caprolactone)/Graphite Oxide Composite. J. Polym. Environ. 2014, 22, 190–199. [Google Scholar] [CrossRef]
- Yıldırım, S.; Demirtaş, T.T.; Dinçer, C.A.; Yıldız, N.; Karakeçili, A. Preparation of polycaprolactone/graphene oxide scaffolds: A green route combining supercritial CO2 technology and porogen leaching. J. Supercrit. Fluids 2018, 133, 156–162. [Google Scholar] [CrossRef]
- Peng, H.; Han, Y.; Liu, T.; Tjiu, W.C.; He, C. Morphology and thermal degradation behavior of highly exfoliated CoAl-layered double hydroxide/polycaprolactone nanocomposites prepared by simple solution intercalation. Thermochim. Acta 2010, 502, 1–7. [Google Scholar] [CrossRef]
- Michailidis, M.; Verros, G.D.; Deliyanni, E.A.; Andriotis, E.G.; Achilias, D.S. An experimental and theoretical study of butyl methacrylate in situ radical polymerization kinetics in the presence of graphene oxide nanoadditive. J. Polym. Sci. A 2017, 55, 1433–1441. [Google Scholar] [CrossRef]
- Tsagkalias, I.S.; Manios, T.K.; Achilias, D.S. Effect of graphene oxide on the reaction kinetics of methyl methacrylate in situ radical polymerization via the bulk or solution technique. Polymers (Basel) 2017, 9, 432. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.-H.; Peng, X.-F.; Jing, X.; Li, L.-W.; Huang, A.; Xu, B.-P.; Chen, B.-Y.; Mi, H.-Y. Investigation of poly(l-lactic acid)/graphene oxide composites crystallization and nanopore foaming behaviors via supercritical carbon dioxide low temperature foaming. J. Mater. Res. 2016, 31, 348–359. [Google Scholar] [CrossRef]
- Song, P.; Cao, Z.; Cai, Y.; Zhao, L.; Fang, Z.; Fu, S. Fabrication of exfoliated graphene-based polypropylene nanocomposites with enhanced mechanical and thermal properties. Polymer (Guildf) 2011, 52, 4001–4010. [Google Scholar] [CrossRef]
- Bao, C.; Guo, Y.; Song, L.; Kan, Y.; Qian, X.; Hu, Y. In situ preparation of functionalized graphene oxide/epoxy nanocomposites with effective reinforcements. J. Mater. Chem. 2011, 21, 13290. [Google Scholar] [CrossRef]
- Sánchez-Correa, F.; Vidaurre-Agut, C.; Serrano-Aroca, A.; Campillo-Fernández, A.J. Poly(2-hydroxyethyl acrylate) hydrogels reinforced with graphene oxide: Remarkable improvement of water diffusion and mechanical properties. J. Appl. Polym. Sci. 2017, 135, 46158. [Google Scholar] [CrossRef]
- Liao, K.H.; Lin, Y.S.; MacOsko, C.W.; Haynes, C.L. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef]


| Sample | Crystalline | Amorphous | H-Bonding |
|---|---|---|---|
| PCL | 76.3% | 19.3% | 4.4% |
| PCL/GO-0.1 | 77.1% | 18.0% | 4.8% |
| PCL/GO-0.2 | 77.4% | 14.0% | 8.5% |
| PCL/GO-0.5 | 77.4% | 11.1% | 11.5% |
| Sample | 1293 cm−1 Crystalline | 1157 cm−1 Amorphous | Crystalline/Amorphous Ratio |
|---|---|---|---|
| PCL | 0.19667 | 0.53359 | 37% |
| PCL/GO-0.1 | 0.22069 | 0.56913 | 39% |
| PCL/GO-0.2 | 0.25354 | 0.57757 | 44% |
| PCL/GO-0.5 | 0.22497 | 0.49367 | 46% |
| Sample | Mw | PDI |
|---|---|---|
| PCL | 97095 | 1.63 |
| PCL/GO-0.1 | 61472 | 1.77 |
| PCL/GO-0.2 | 37878 | 2.66 |
| PCL/GO-0.5 | 4436 | 1.59 |
| Sample | IDT (°C) | Tmax (°C) | Residue at 800 °C (%) | Theoretical Residue at 800 °C (%) |
|---|---|---|---|---|
| PCL | 378.3 | 412.2 | 0.10 | |
| PCL/GO-0.1 | 379.9 | 413.3 | 0.17 | 0.14 |
| PCL/GO-0.2 | 377.5 | 414.4 | 0.27 | 0.18 |
| PCL/GO-0.5 | 350.2 | 412.6 | 0.45 | 0.29 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castilla-Cortázar, I.; Vidaurre, A.; Marí, B.; Campillo-Fernández, A.J. Morphology, Crystallinity, and Molecular Weight of Poly(ε-caprolactone)/Graphene Oxide Hybrids. Polymers 2019, 11, 1099. https://doi.org/10.3390/polym11071099
Castilla-Cortázar I, Vidaurre A, Marí B, Campillo-Fernández AJ. Morphology, Crystallinity, and Molecular Weight of Poly(ε-caprolactone)/Graphene Oxide Hybrids. Polymers. 2019; 11(7):1099. https://doi.org/10.3390/polym11071099
Chicago/Turabian StyleCastilla-Cortázar, Isabel, Ana Vidaurre, Bernabé Marí, and Alberto J. Campillo-Fernández. 2019. "Morphology, Crystallinity, and Molecular Weight of Poly(ε-caprolactone)/Graphene Oxide Hybrids" Polymers 11, no. 7: 1099. https://doi.org/10.3390/polym11071099
APA StyleCastilla-Cortázar, I., Vidaurre, A., Marí, B., & Campillo-Fernández, A. J. (2019). Morphology, Crystallinity, and Molecular Weight of Poly(ε-caprolactone)/Graphene Oxide Hybrids. Polymers, 11(7), 1099. https://doi.org/10.3390/polym11071099