Chlorinated Water Induced Aging of Pipe Grade Polypropylene Random Copolymers
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Specimens
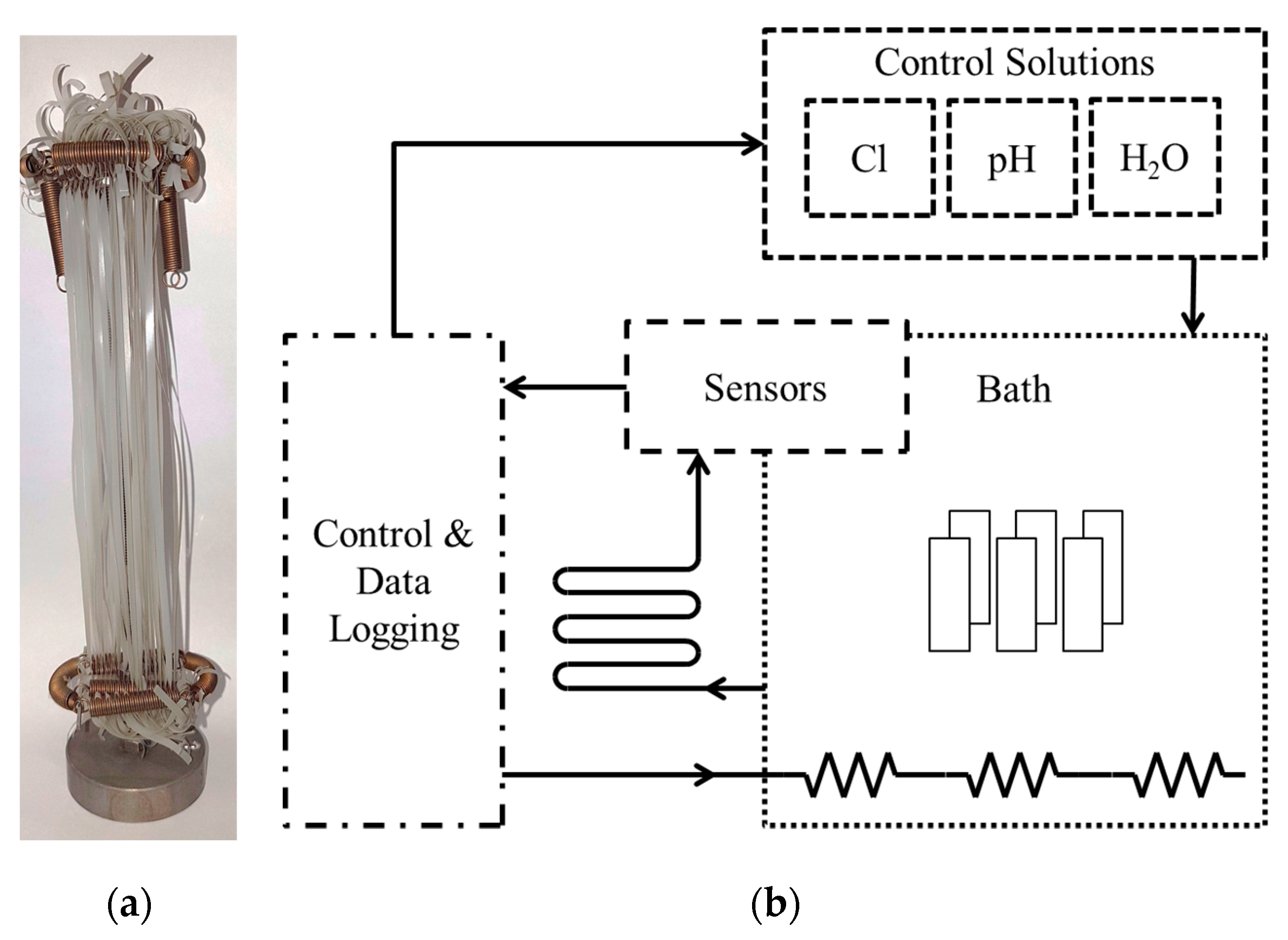
2.3. Pre-Conditioning of Micro-Sized PP-R Specimens for Monitoring of the Aging Behavior
2.4. Test Methods for Monitoring the Aging Behavior of Micro-Sized PP-R Specimens
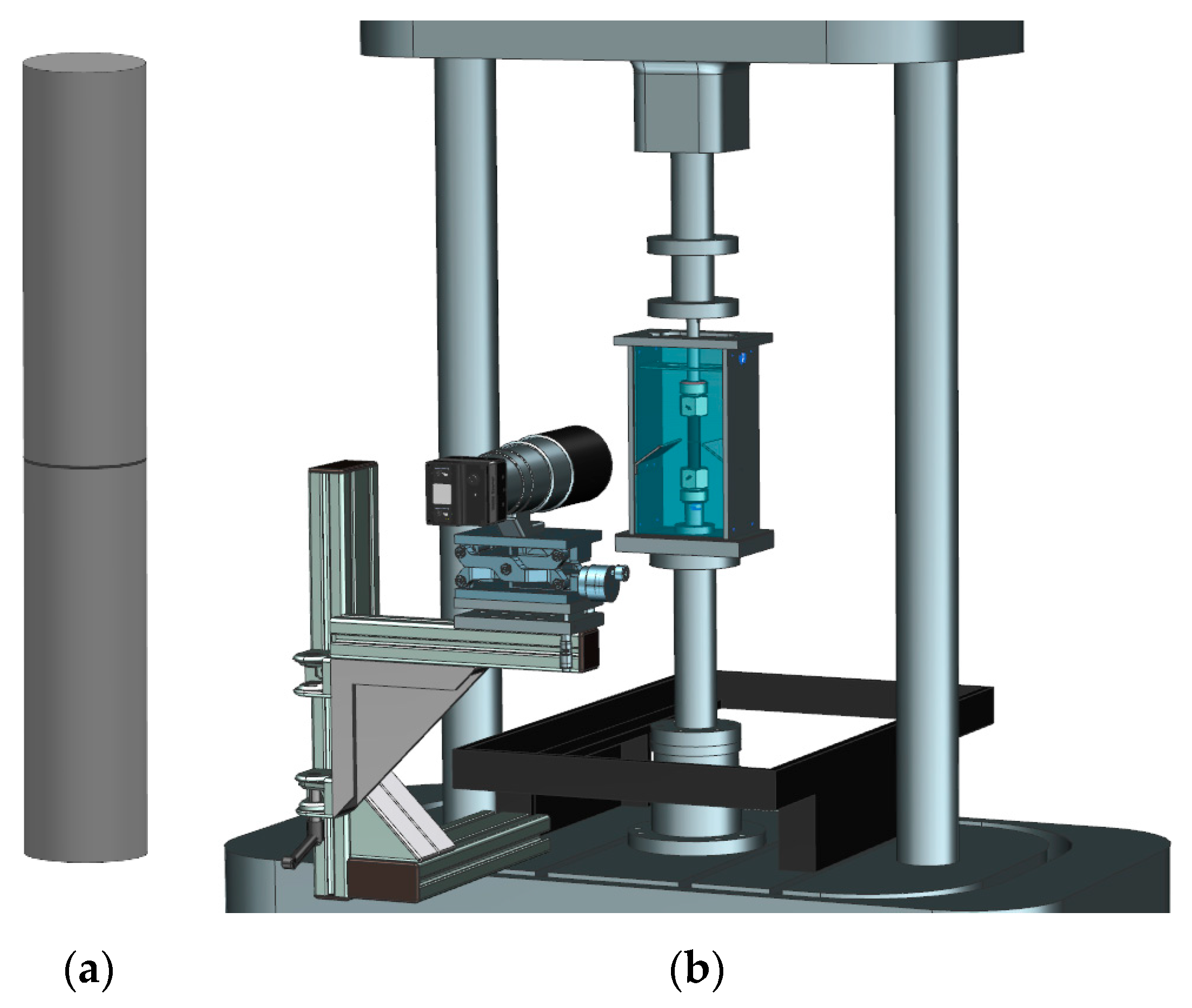
2.5. Superimposed Mechanical-Environmental Fatigue Tests
3. Results
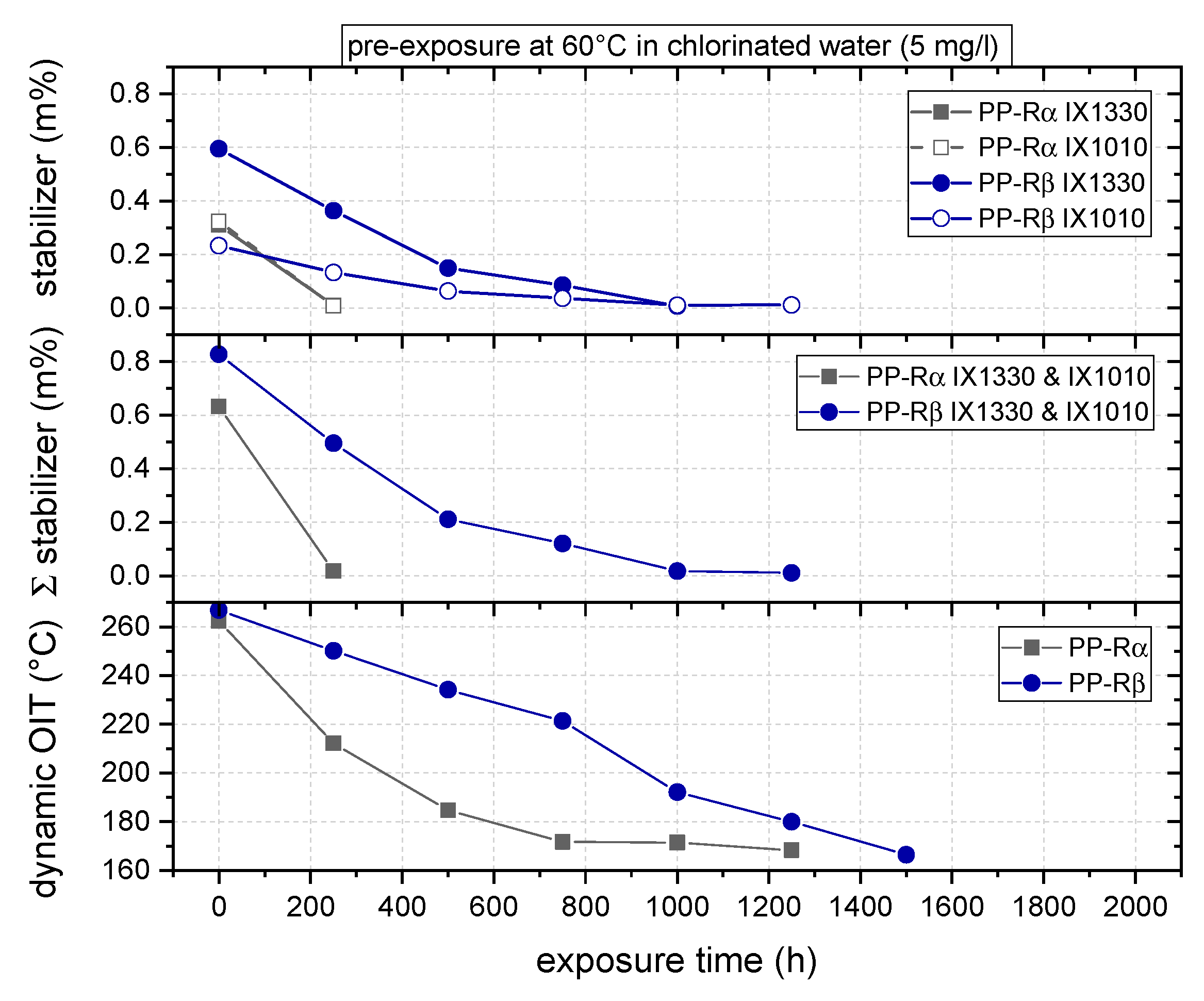
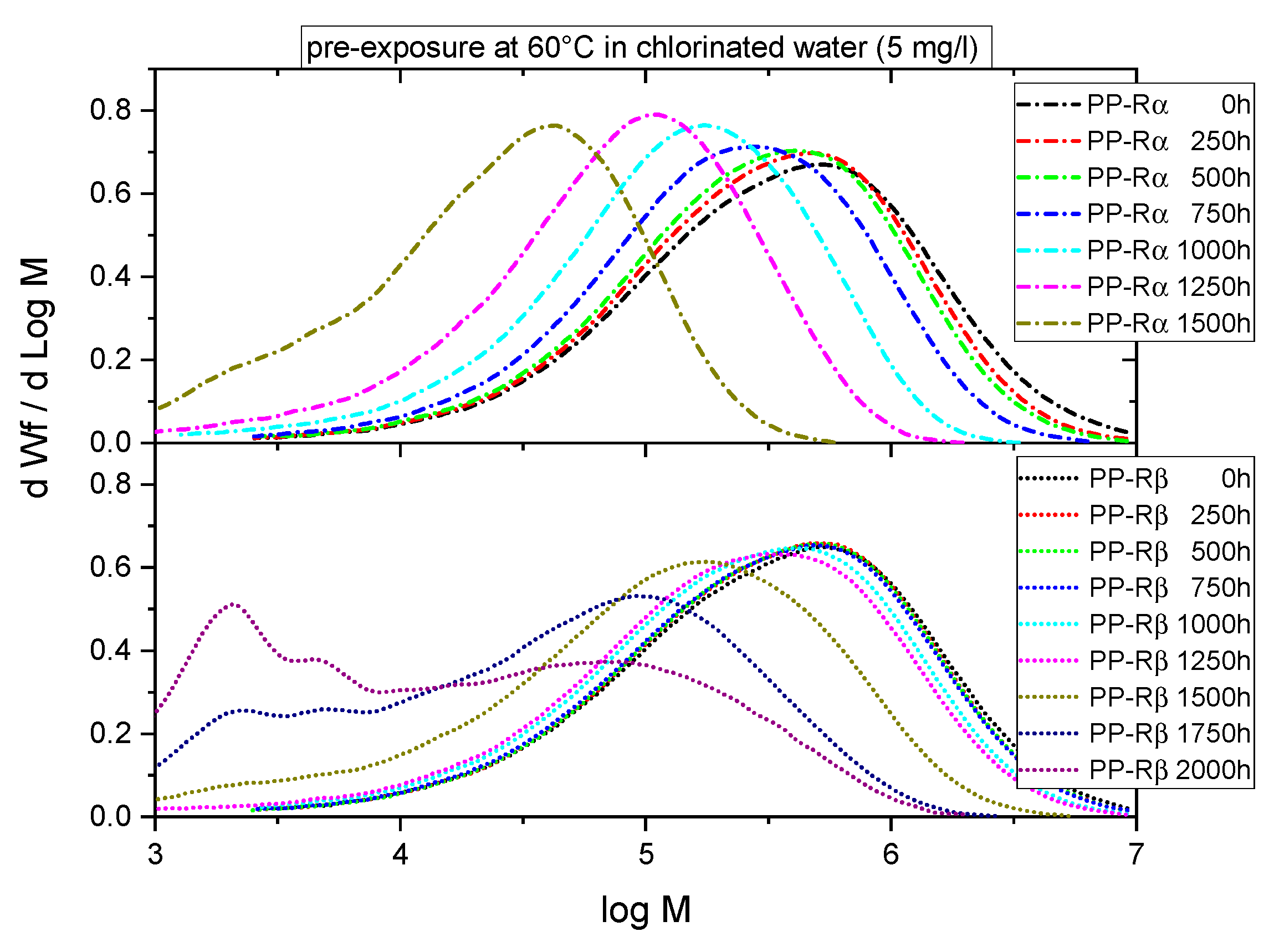
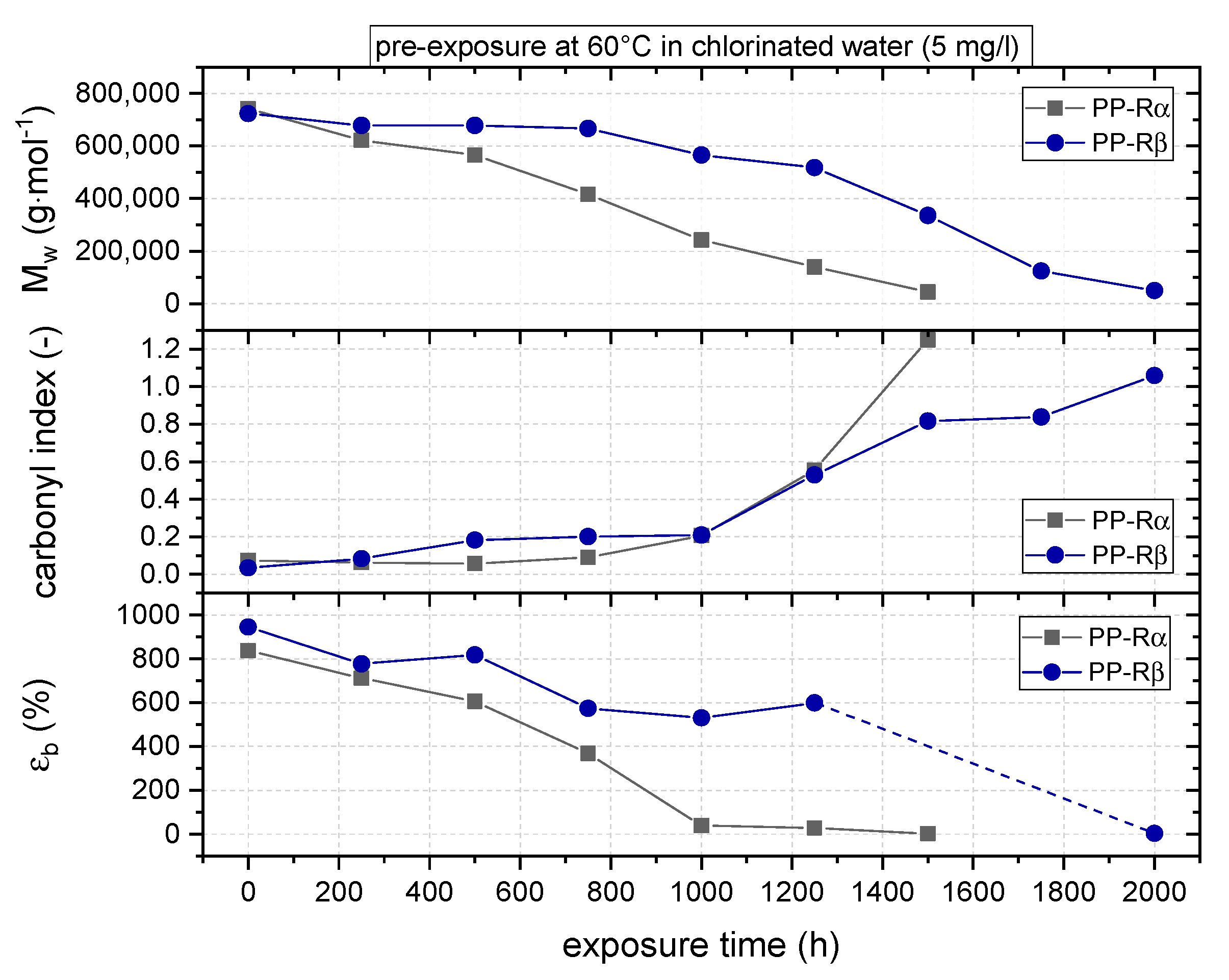
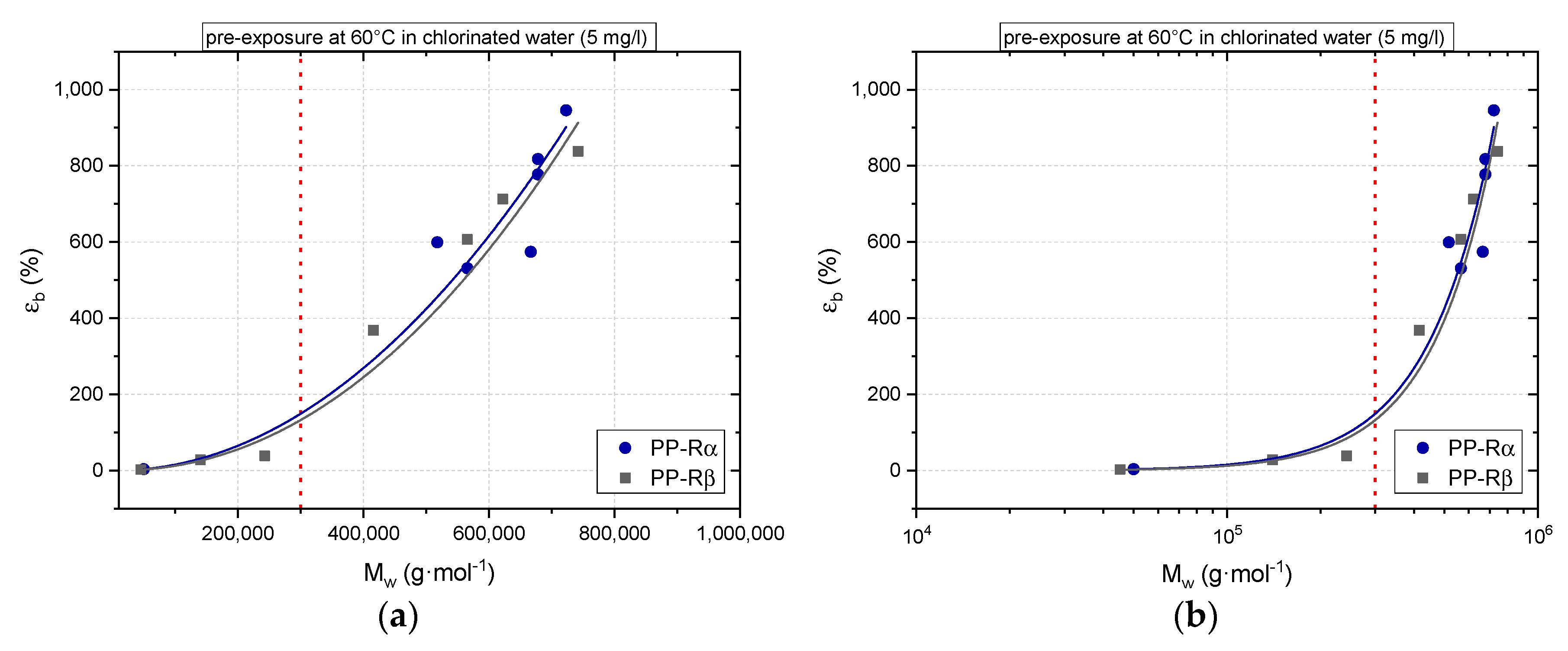
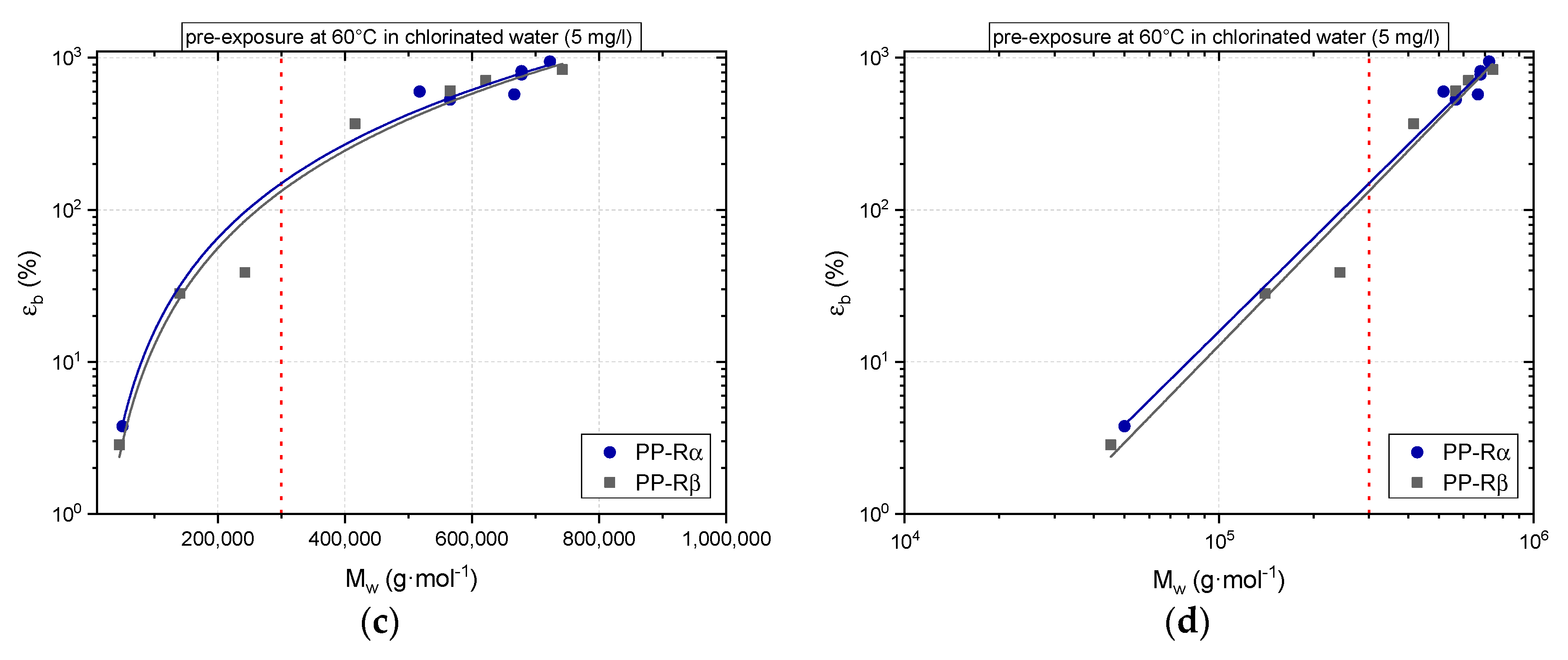
3.1. Effect of Chlorinated Water on the Aging Behavior of Micro-Sized PP-R Specimens
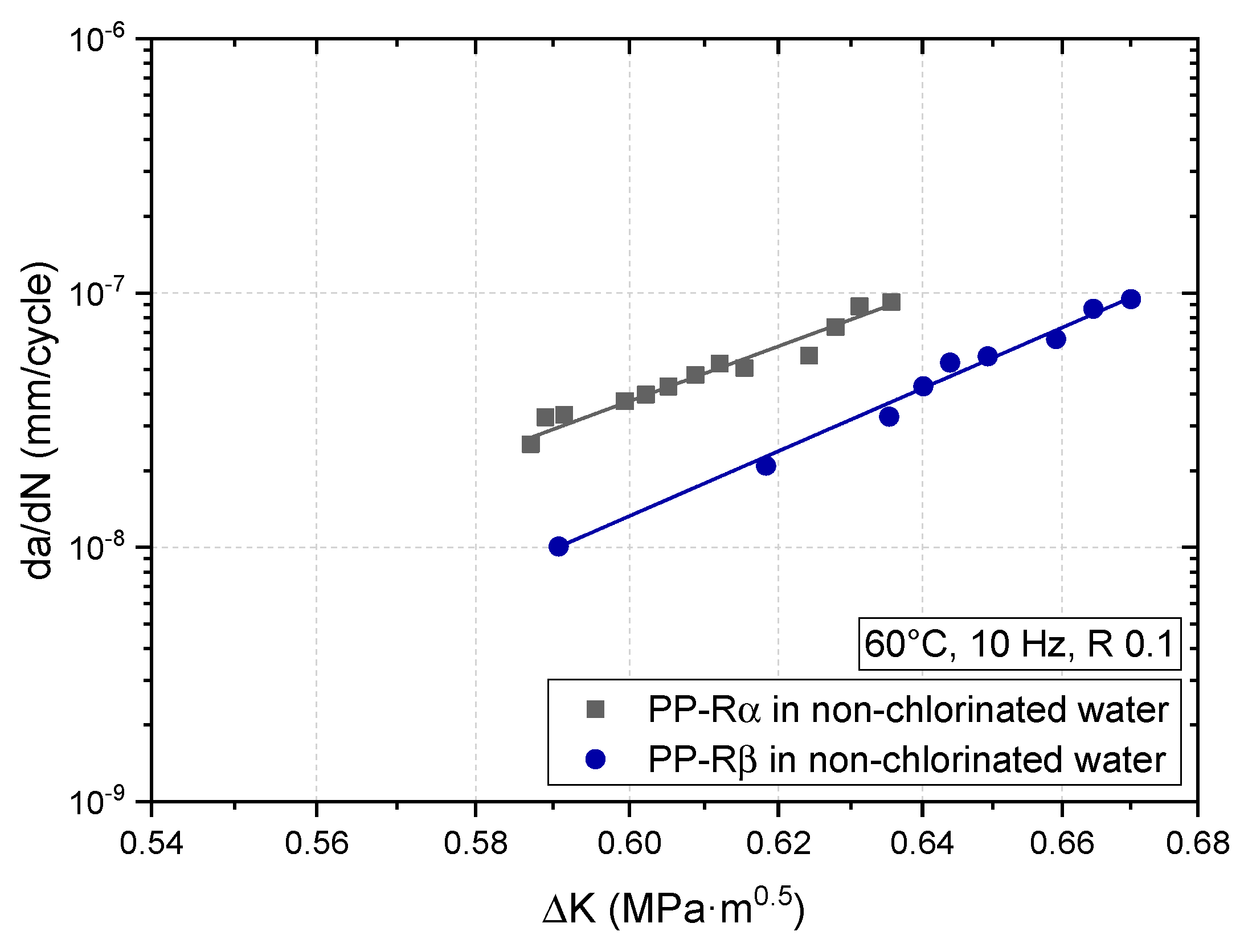
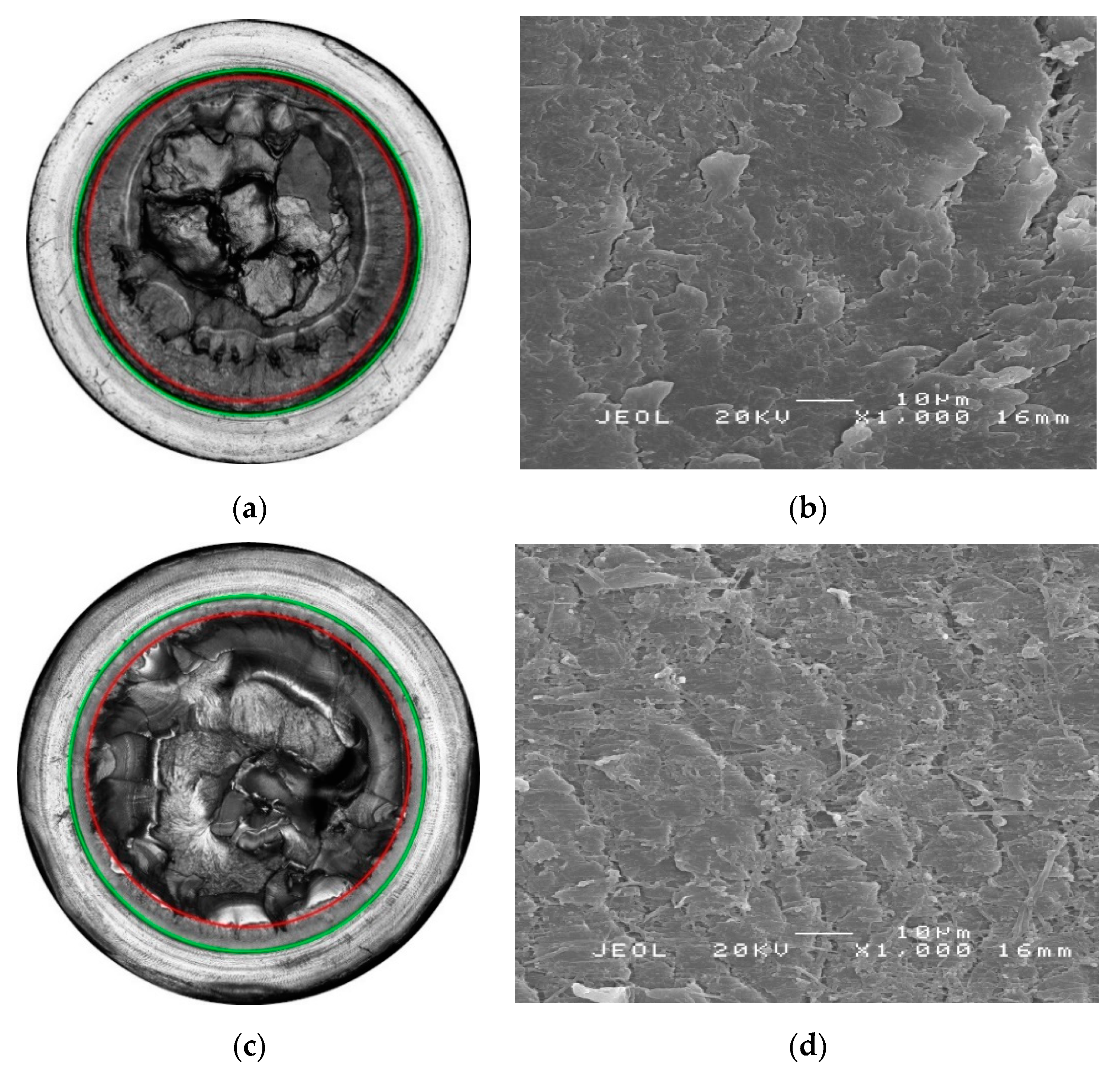
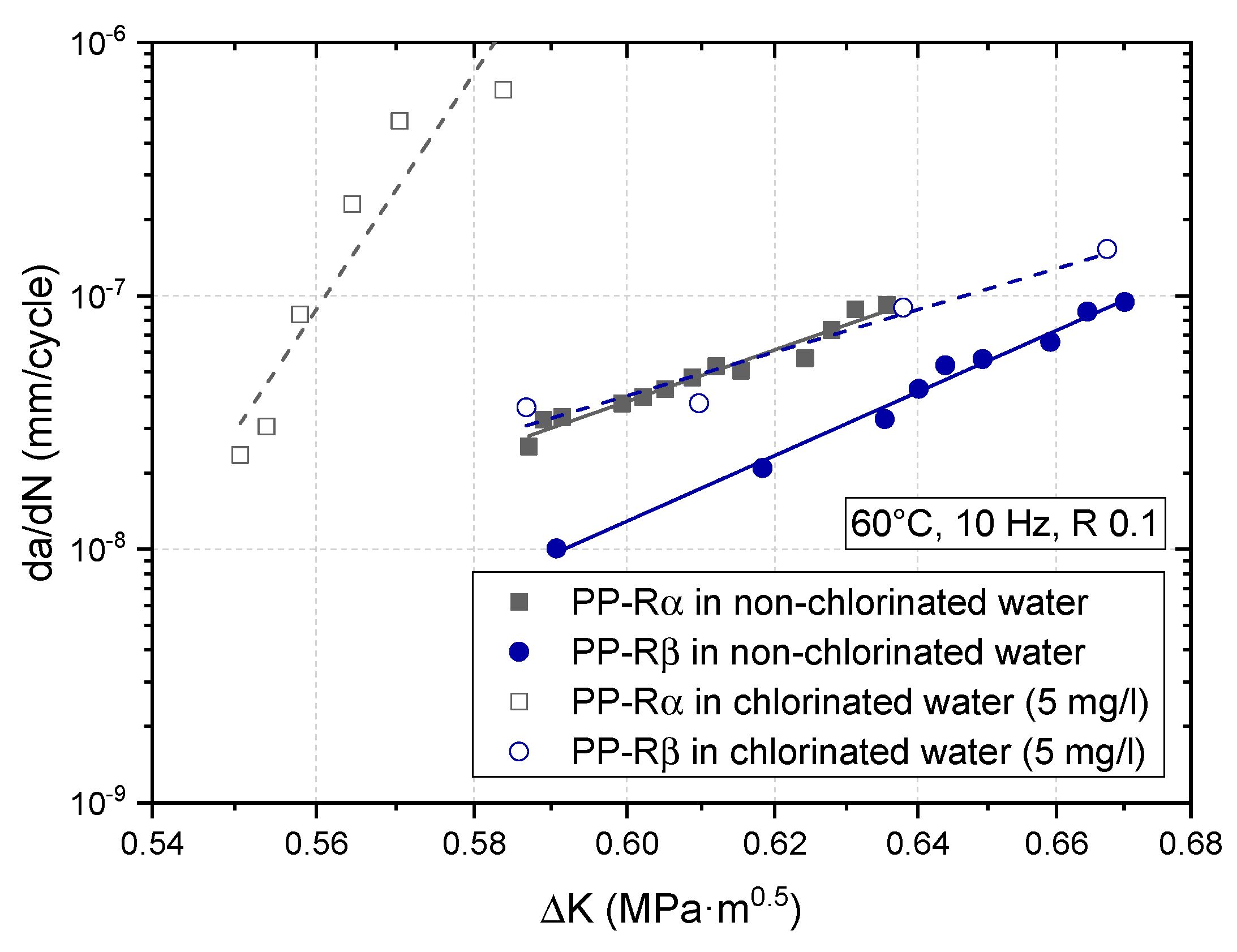
3.2. Effect of Chlorinated Water on the Fatigue Crack Growth Resistance
4. Discussion
5. Summary and Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Gahleitner, M.; Paulik, C. Polypropylene and Other Polyolefins Brydson’s Plastics Materials; Elsevier: Amsterdam, The Nederland, 2017; pp. 279–309. ISBN 9780323358248. [Google Scholar]
- ISO/TC 138/SC 2 Comitee. Plastics Piping Systems for Hot and Cold Water Installations-Polypropylene (PP)-Part 1: General; ISO: Geneva, Switzerland, 2013. [Google Scholar]
- F17 Committee. Specification for Pressure-Rated Polypropylene (PP) Piping Systems; ASTM International: West Conshohocken, PA, USA, 2007. [Google Scholar]
- Grein, C. Toughness of Neat, Rubber Modified and Filled β-nucleated Polypropylene: From Fundamentals to Applications. In Intrinsic Molecular Mobility and Toughness of Polymers II; Kausch, H.-H., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 43–104. ISBN 3-540-26162-1. [Google Scholar]
- Yu, L.; Wu, T.; Chen, T.; Yang, F.; Xiang, M. Polypropylene random copolymer in pipe application: Performance improvement with controlled molecular weight distribution. Thermochim. Acta 2014, 578, 43–52. [Google Scholar] [CrossRef]
- Kurzböck, M.; Wallner, G.M.; Lang, R.W. Black pigmented polypropylene materials for solar absorbers. Energy Proced. 2012, 30, 438–445. [Google Scholar] [CrossRef]
- Grabmann, M.K.; Wallner, G.M.; Maringer, L.; Buchberger, W.; Nitsche, D. Hot air aging behavior of polypropylene random copolymers. J. Appl. Polym. Sci. 2019, 136, 47350. [Google Scholar] [CrossRef]
- Policianová, O.; Hodan, J.; Brus, J.; Kotek, J. Origin of toughness in β-polypropylene: The effect of molecular mobility in the amorphous phase. Polymer 2015, 60, 107–114. [Google Scholar] [CrossRef]
- Raab, M.; Kotek, J.; Baldrian, J.; Grellmann, W. Toughness profile in injection-molded polypropylene: The effect of the β-modification. J. Appl. Polym. Sci. 1998, 69, 2255–2259. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011; ISBN 9241548150. [Google Scholar]
- World Chlorine Council. Drinking Water Chlorination: World Chlorine Council Position Paper; World Chlorine Council: Athens, Greece, 2008. [Google Scholar]
- Bartram, J.; Chartier, Y.; Lee, J.V.; Pond, K.; Surman-Lee, S. Legionella and the Prevention of Legionellosis; World Health Organization: Geneva, Switzerland, 2007; ISBN 92 4 156297 8. [Google Scholar]
- Fischer, J.; Bradler, P.R.; Lang, R.W.; Wallner, G.M. Fatigue crack growth resistance of polypropylene in chlorinated water at different temperatures. In Proceedings of the Plastic Pipes 18th Conference Proceedings, Berlin, Germany, 14 September 2016. [Google Scholar]
- Fischer, J.; Bradler, P.R.; Lang, R.W. Test equipment for fatigue crack growth testing of polymeric materials in chlorinated water at different temperatures. Eng. Fract. Mech. 2018. [Google Scholar] [CrossRef]
- Fischer, J.; Eckerstorfer, M.; Bradler, P.R.; Wallner, G.M.; Lang, R.W. Investigation of the effect of stabilizer system, medium and temperature on the fatigue crack growth resistance of polypropylene for a proper material selection. ANTEC Conf. Proc. 2018. [Google Scholar]
- Fischer, J.; Bradler, P.R.; Lang, R.W. Fatigue crack growth testing in chlorinated water at elevated temperatures-test equipment. In Proceedings of the Plastic Pipes 19th Conference Proceedings, 24–26 September 2018. [Google Scholar]
- Hassinen, J.; Lundbaeck, M.; Ifwarson, M.; Gedde, U. Deterioration of polyethylene pipes exposed to chlorinated water. Polym. Degrad. Stab. 2004, 84, 261–267. [Google Scholar] [CrossRef]
- Majewski, K.; Cosgriff, E.; Mantell, S.; Bhattacharya, M. Fracture Properties of HDPE Exposed to Chlorinated Water. ANTEC, Conf. Proc. 2018. [Google Scholar]
- F17 Committee. Test Method for Evaluating the Oxidative Resistance of Polyethylene (PE) Pipe to Chlorinated Water; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- F17 Committee. Test Method for Evaluating the Oxidative Resistance of Crosslinked Polyethylene (PEX) Tubing and Systems to Hot Chlorinated Water; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Yu, W.; Reitberger, T.; Hjertberg, T.; Oderkerk, J.; Costa, F.R.; Gedde, U.W. Antioxidant consumption in squalane and polyethylene exposed to chlorinated aqueous media. Polym. Degrad. Stab. 2012, 97, 2370–2377. [Google Scholar] [CrossRef]
- Fischer, J.; Mantell, S.C.; Bradler, P.R.; Wallner, G.M.; Lang, R.W. Effect of Aging in Hot Chlorinated Water on the Mechanical Behavior of Polypropylene for Solar-Thermal Applications. In Proceedings of SWC2017/SHC2017. ISES Solar World Conference 2017 and the IEA SHC Solar Heating and Cooling Conference for Buildings and Industry 2017, Abu Dhabi, 29 October–02 November 2017; Romero, M., Mugnier, D., Renné, D., Guthrie, K., Griffiths, S., Eds.; International Solar Energy Society: Freiburg, Germany, 2017; pp. 1–6. ISBN 978-3-9814659-7-6. [Google Scholar]
- Fischer, J.; Mantell, S.C.; Bradler, P.R.; Wallner, G.M.; Lang, R.W. Effect of aging in hot chlorinated water on the mechanical behavior of polypropylene grades differing in their stabilizer systems. Mater. Today Proc. 2019, 10, 385–392. [Google Scholar] [CrossRef]
- Seidler, D. Aus Schaden klug werden. Kunststoffe 2012, 102, 70–71. [Google Scholar]
- Castillo Montes, J.; Cadoux, D.; Creus, J.; Touzain, S.; Gaudichet-Maurin, E.; Correc, O. Ageing of polyethylene at raised temperature in contact with chlorinated sanitary hot water. Part I-Chemical aspects. Polym. Degrad. Stab. 2012, 97, 149–157. [Google Scholar] [CrossRef]
- Vibien, P.; Couch, J.; Oliphant, K.; Zhou, W.; Zhang, B.; Chudnovsky, A. Assessing material perfomance in chlorinated potable water applications. In Proceedings of the 11th Plastic Pipes, Munich, Germany, 6–10 October 2003. [Google Scholar]
- Colin, X.; Audouin, L.; Verdu, J.; Rozental-Evesque, M.; Rabaud, B.; Martin, F.; Bourgine, F. Aging of polyethylene pipes transporting drinking water disinfected by chlorine dioxide. I. Chemical aspects. Polym. Eng. Sci. 2009, 49, 1429–1437. [Google Scholar] [CrossRef]
- Colin, X.; Audouin, L.; Verdu, J.; Rozental-Evesque, M.; Rabaud, B.; Martin, F.; Bourgine, F. Aging of polyethylene pipes transporting drinking water disinfected by chlorine dioxide. Part II-Lifetime prediction. Polym. Eng. Sci. 2009, 49, 1642–1652. [Google Scholar] [CrossRef]
- Damodaran, S.; Schuster, T.; Rode, K.; Sanoria, A.; Brüll, R.; Wenzel, M.; Bastian, M. Monitoring the effect of chlorine on the ageing of polypropylene pipes by infrared microscopy. Polym. Degrad. Stab. 2015, 111, 7–19. [Google Scholar] [CrossRef]
- Lang, R.W. Polymerphysikalische Ansätze zur Beschreibung des Deformations- und Versagensverhaltens von PE-Rohren: Application of polymer physics conceps to deformation and failure behaviour of PE pipes. 3R Int. 1997, 36, 40–44. [Google Scholar]
- Robeson, L.M. Environmental stress cracking: A review. Polym. Eng. Sci. 2013, 53, 453–467. [Google Scholar] [CrossRef]
- Pinter, G.; Lang, R.W. Effect of stabilization on creep crack growth in high-density polyethylene. J. Appl. Polym. Sci. 2003, 90, 3191–3207. [Google Scholar] [CrossRef]
- Pinter, G.; Haager, M.; Wolf, C.; Lang, R.W. Thermo-Oxidative Degradation during Creep Crack Growth of PE-HD Grades as Assessed by FT-IR Spectroscopy. Macromol. Symp. 2004, 217, 307–316. [Google Scholar] [CrossRef]
- Grabmayer, K. Polyolefin-Based Lining Materials for Hot Water Heat Storages Development of Accelerated Aging Characterization Methods and Screening of Novel Compounds. Dissertation; Johannes Kepler University: Linz, Austria, 2014. [Google Scholar]
- ISO/TC 138/SC 5-General properties of pipes, fittings and valves of plastic materials and their accessories-Test methods and basic specifications. Polyethylene (PE) Materials for Piping Systems-Determination of Resistance to Slow Crack Growth under Cyclic Loading-Cracked Round Bar Test Method; ISO: Geneva, Switzerland, 2015; p. 18489. [Google Scholar]
- Cosgriff, E.; Mantell, S. Method for degrading polyethylene sheet samples in an oxidative environment. ANTEC Conf. Proc. 2017, 6, 1228–1233. [Google Scholar]
- Beißmann, S.; Stiftinger, M.; Grabmayer, K.; Wallner, G.; Nitsche, D.; Buchberger, W. Monitoring the degradation of stabilization systems in polypropylene during accelerated aging tests by liquid chromatography combined with atmospheric pressure chemical ionization mass spectrometry. Polym. Degrad. Stab. 2013, 98, 1655–1661. [Google Scholar] [CrossRef]
- NA 054-01-03 AA. Plastics-Differential Scanning Calorimetry (DSC)-Part 6: Determination of Oxidation Induction Time (Isothermal OIT) and Oxidation Induction Temperature (Dynamic OIT); ISO: Geneva, Switzerland, 2018; Chapter 6; p. 11357. [Google Scholar]
- ISO/TC 61/SC 2. ISO 527-1: Plastics-Determination of Tensile Properties-Part 1: General Principles; ISO: Geneva, Switzerland, 2012. [Google Scholar]
- Gross, D.; Seelig, T. Bruchmechanik. Mit Einer Einführung in die Mikromechanik; Springer: Berli/Heidelberg, Germany, 2011; ISBN 3642101968. [Google Scholar]
- ISO/TC 61/SC 2 Mechanical behavior. ISO 15850:2014 Plastics-Determination of Tension-Tension Fatigue Crack Propagation-Linear Elastic Fracture Mechanics (LEFM) Approach; ISO: Geneva, Switzerland, 2014. [Google Scholar]
- Lang, R.W. Applicability of linear elastic fracture mechanics to fatigue in poymers and short-fiber composites. Diss. Abstr. Int. Part B Sci. Eng. 1980. [Google Scholar] [CrossRef]
- Hertzberg, R.W.; Manson, J.A. Fatigue of Engineering Plastics; Academic Press: New York, NY, USA, 1980; ISBN 9780123435507. [Google Scholar]
- Lang, R.W.; Pinter, G.; Balika, W. Konzept zur Nachweisführung für Nutzungsdauer und Sicherheit von PE-Druckrohren bei beliebiger Einbausituation. 3R Int. 2005, 44, 32–41. [Google Scholar]
- Maringer, L.; Grabmann, M.; Muik, M.; Nitsche, D.; Romanin, C.; Wallner, G.; Buchberger, W. Investigations on the distribution of polymer additives in polypropylene using confocal fluorescence microscopy. Int. J. Polym. Anal. Charact. 2017, 22, 692–698. [Google Scholar] [CrossRef]
- Pinter, G.; Haager, M.; Balika, W.; Lang, R.W. Cyclic crack growth tests with CRB specimens for the evaluation of the long-term performance of PE pipe grades. Polym. Test. 2007, 26, 180–188. [Google Scholar] [CrossRef]
- Balika, W.; Pinter, G.; Lang, R.W. Systematic investigations of fatigue crack growth behavior of a PE-HD pipe grade in through-thickness direction. J. Appl. Polym. Sci. 2007, 103, 1745–1758. [Google Scholar] [CrossRef]
- Lang, R.W.; Stern, A.; Doerner, G. Applicability and limitations of current lifetime prediction models for thermoplastics pipes under internal pressure. Angew. Makromol. Chem. 1997, 247, 131–145. [Google Scholar] [CrossRef]
- Grabmann, M.K.; Wallner, G.M.; Grabmayer, K.; Nitsche, D.; Lang, R.W. Aging behavior and lifetime assessment of polyolefin liner materials for seasonal heat storage using micro-specimen. Sol. Energy 2018, 170, 988–990. [Google Scholar] [CrossRef]
- Grabmayer, K.; Beißmann, S.; Wallner, G.M.; Nitsche, D.; Schnetzinger, K.; Buchberger, W.; Schobermayr, H.; Lang, R.W. Characterization of the influence of specimen thickness on the aging behavior of a polypropylene based model compound. Polym. Degrad. Stab. 2015, 111, 185–193. [Google Scholar] [CrossRef]
- Povacz, M.; Wallner, G.M.; Lang, R.W. Black-pigmented polypropylene materials for solar thermal absorbers-Effect of carbon black concentration on morphology and performance properties. Sol. Energy 2014, 110, 420–426. [Google Scholar] [CrossRef]
- Grabmann, M.; Wallner, G.; Grabmayer, K.; Buchberger, W.; Nitsche, D. Effect of thickness and temperature on the global aging behavior of polypropylene random copolymers for seasonal thermal energy storages. Sol. Energy 2018, 172, 152–157. [Google Scholar] [CrossRef]
- Elias, H.-G. Macromolecules. Volume 3: Physical Structures and Properties; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; ISBN 978-3-527-31174-3. [Google Scholar]
- Nguyen, T.Q.; Kausch, H.H. Molecular Weight Distribution and Mechanical Properties. In Mechanical Properties and Testing of Polymers; Brewis, D., Briggs, D., Swallowe, G.M., Eds.; Springer: Dordrecht, The Nederlands, 1999; pp. 143–150. ISBN 978-90-481-4024-4. [Google Scholar]
- Elias, H.-G. Macromolecules. Volume 4: Applications of Polymers; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009; ISBN 978-3-527-31157-0. [Google Scholar]
- Wallner, G.M.; Grabmann, M.K.; Klocker, C.; Buchberger, W.; Nitsche, D. Effect of carbon nanotubes on the global aging behavior of β-nucleated polypropylene random copolymers for absorbers of solar-thermal collectors. Sol. Energy 2018, 172, 141–145. [Google Scholar] [CrossRef]
- Fayolle, B.; Audouin, L.; Verdu, J. A critical molar mass separating the ductile and brittle regimes as revealed by thermal oxidation in polypropylene. Polymer 2004, 45, 4323–4330. [Google Scholar] [CrossRef]
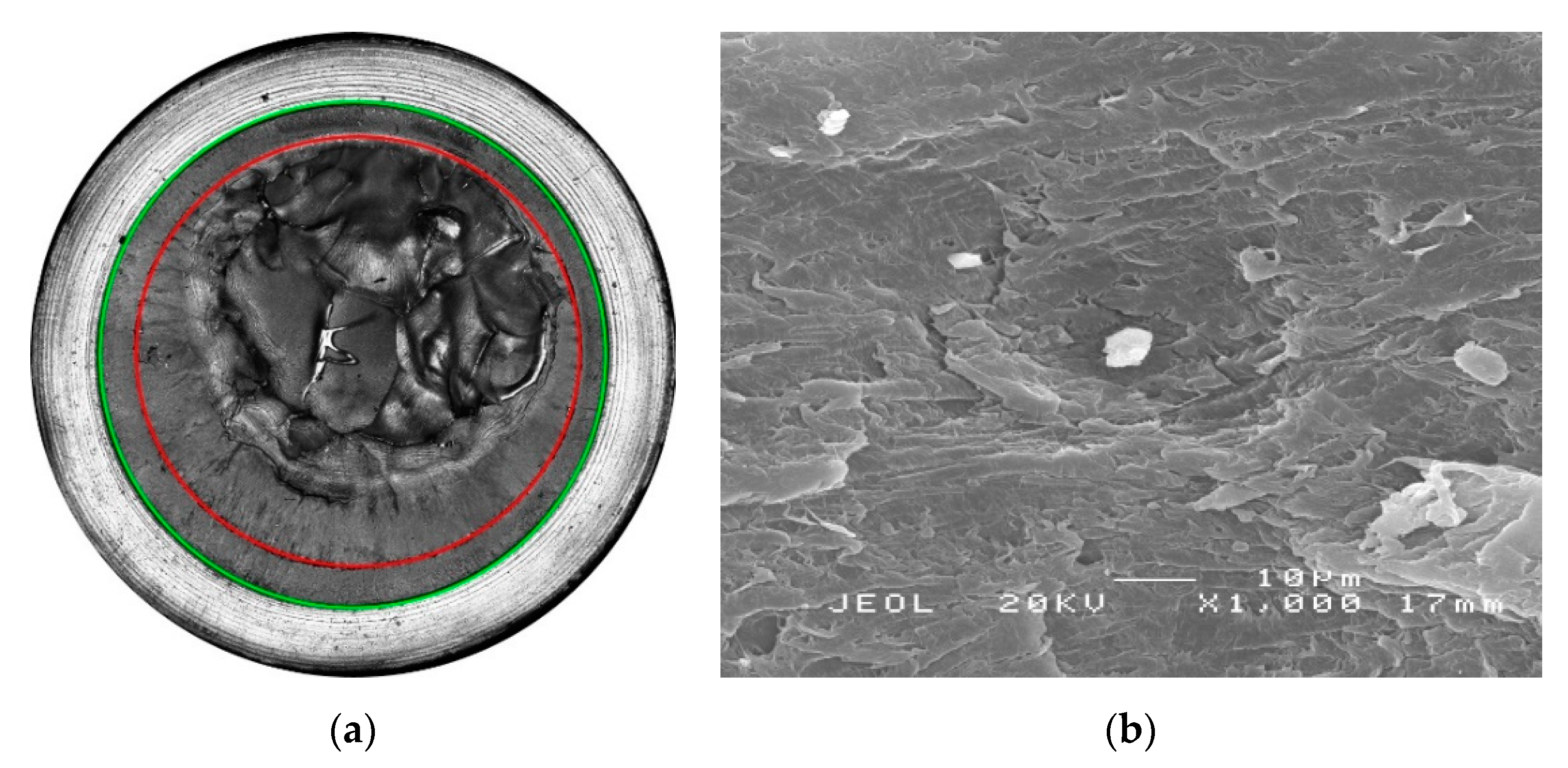
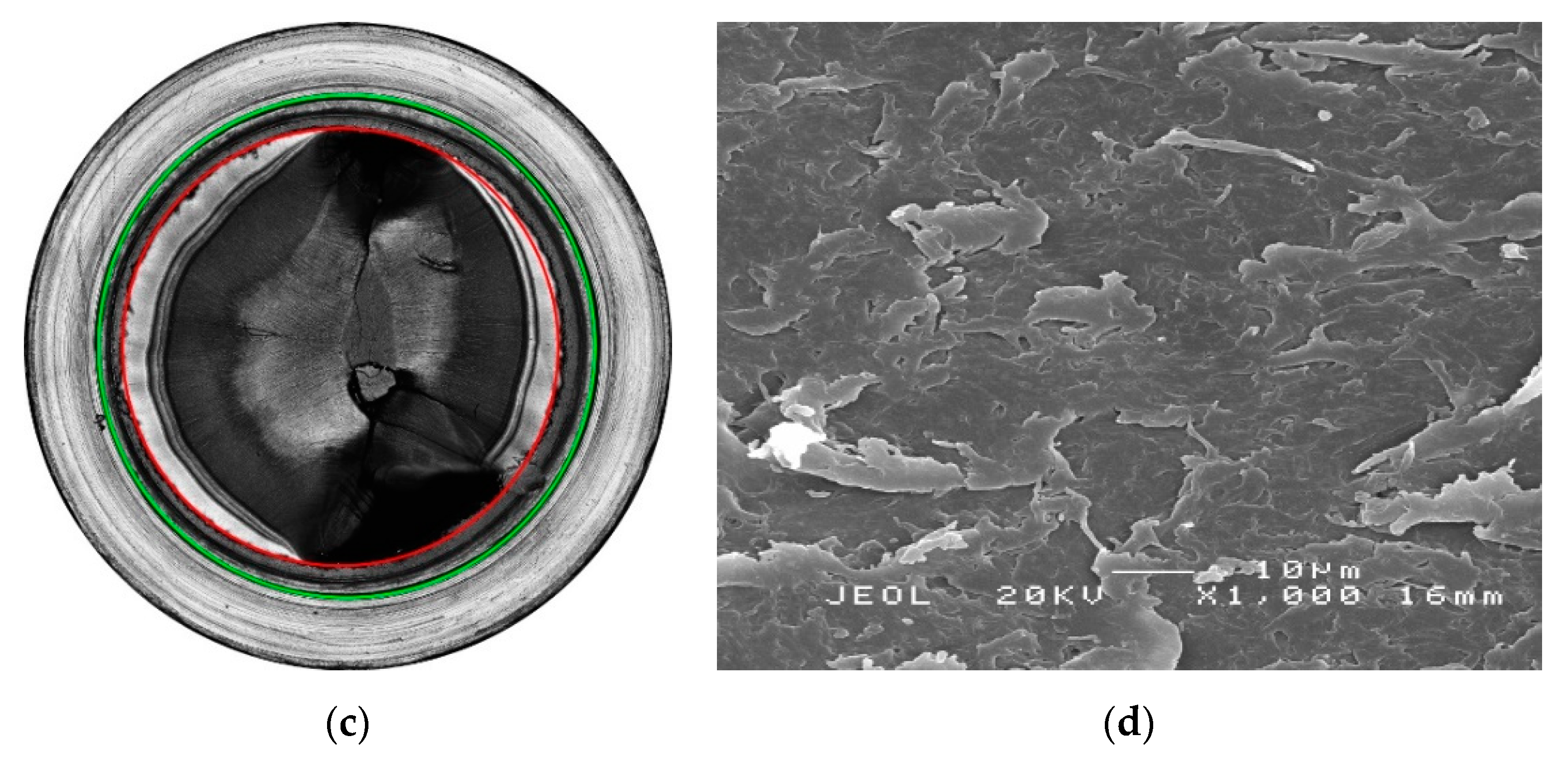
| Material Designation | Crystal Phase | Pipe Material Classification |
|---|---|---|
| PP-Rα | Monoclinic (α) | PP-R: PP random copolymer |
| PP-Rβ | Trigonal (β) | PP-RCT: PP random copolymer with a special crystalline morphology and improved pressure and temperature resistance |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, J.; Freudenthaler, P.J.; Lang, R.W.; Buchberger, W.; Mantell, S.C. Chlorinated Water Induced Aging of Pipe Grade Polypropylene Random Copolymers. Polymers 2019, 11, 996. https://doi.org/10.3390/polym11060996
Fischer J, Freudenthaler PJ, Lang RW, Buchberger W, Mantell SC. Chlorinated Water Induced Aging of Pipe Grade Polypropylene Random Copolymers. Polymers. 2019; 11(6):996. https://doi.org/10.3390/polym11060996
Chicago/Turabian StyleFischer, Joerg, Paul J. Freudenthaler, Reinhold W. Lang, Wolfgang Buchberger, and Susan C. Mantell. 2019. "Chlorinated Water Induced Aging of Pipe Grade Polypropylene Random Copolymers" Polymers 11, no. 6: 996. https://doi.org/10.3390/polym11060996
APA StyleFischer, J., Freudenthaler, P. J., Lang, R. W., Buchberger, W., & Mantell, S. C. (2019). Chlorinated Water Induced Aging of Pipe Grade Polypropylene Random Copolymers. Polymers, 11(6), 996. https://doi.org/10.3390/polym11060996





