Self-Regeneration Performance and Characterization of Silver-Containing Activated Carbon Fibers Coated by Titanium Dioxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Ag-Containing WACF Coated by TiO2 Film
2.2. Characterization
2.3. Self-Regeneration Measurements
3. Results and Discussion
3.1. Surface Morphology
3.2. Crystallite Structure
3.3. Surface Chemical Structure
3.4. Pore Structure
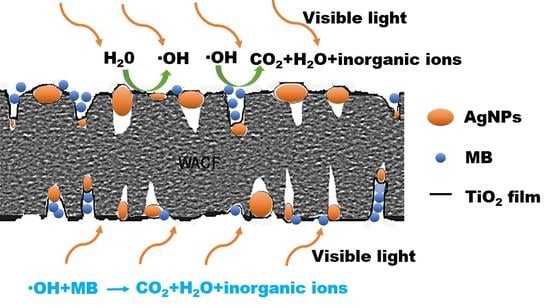
3.5. Self-Regeneration Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ledesma, B.; Román, S.; Sabio, E.; Álvarez-Murillo, A. Improvement of spent activated carbon regeneration by wet oxidation processe. J. Supercrit. Fluid. 2015, 104, 94–103. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, G. Effect of temperature and time on microstructure and surface functional groups of activated carbon fibers prepared from liquefied wood. BioResources 2012, 7, 5552–5567. [Google Scholar] [CrossRef]
- Zanella, O.; Tessaro, I.C.; Féris, L.A. Desorption- and Decomposition-Based Techniques for the Regeneration of Activated Carbon. Chem. Eng. Technol. 2014, 37, 1447–1459. [Google Scholar] [CrossRef]
- Yap, P.; Lim, T. Solar regeneration of powdered activated carbon impregnated with visible-light responsive photocatalyst: Factors affecting performances and predictive model. Water Res. 2012, 46, 3054–3064. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Deng, L.; Sun, C.; Li, J.; Zhu, Z. Titanium dioxide encapsulation of supported Ag nanoparticles on the porous silica bead for increased photocatalytic activity. Appl. Surf. Sci. 2015, 326, 82–90. [Google Scholar] [CrossRef]
- Ma, X.; Chen, Y. Preparation and Characterization of Mn/N Co-Doped TiO2 Loaded on Wood-Based Activated Carbon Fiber and Its Visible Light Photodegradation. Polymers 2015, 7, 1660–1673. [Google Scholar] [CrossRef]
- Weng, Y.; Wang, Y.; Asbury, J.; Ghosh, H.; Lian, T. Back Electron Transfer from TiO2 Nanoparticles to FeIII(CN)63-: Origin of Non-Single-Exponential and Particle Size Independent Dynamics. J. Phys. Chem. B 2000, 104, 92–104. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Xu, P.; Li, M.; Zeng, M. Controlled synthesis of ordered mesoporous TiO2-supported on activated carbon and pore-pore synergistic photocatalytic performance. Mater. Chem. Phys 2015, 149–150, 69–76. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, G. Effect of TiO2 content on the microstructure and antibacterial activity of TiO2-loaded activated carbon fibers derived from liquefied wood. Surf. Interface Anal. 2015, 47, 931–937. [Google Scholar] [CrossRef]
- Shi, J. Preparation of Fe(III) and Ho(III) co-doped TiO2 films loaded on activated carbon fibers and their photocatalytic activities. Chem. Eng. J. 2009, 151, 241–246. [Google Scholar] [CrossRef]
- Ao, Y.; Xu, J.; Gao, Y.; Wang, P.; Wang, C.; Hou, J.; Qian, J. Preparation of Ag nanoparticles loaded TiO2 nanoplate arrays on activated carbon fibers with enhanced photocatalytic activity. Catal. Commun. 2014, 53, 21–24. [Google Scholar] [CrossRef]
- Li, M.; Lu, B.; Ke, Q.; Guo, Y.; Guo, Y. Synergetic effect between adsorption and photodegradation on nanostructured TiO2/activated carbon fiber felt porous composites for toluene removal. J. Hazard. Mater. 2017, 333, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, X.; Zhang, M. Preparation of highly mesoporous wood-derived activated carbon fiber and the mechanism of its porosity development. Holzforschung 2017, 71, 363–371. [Google Scholar] [CrossRef]
- Yue, Z.; Jiang, W.; Wang, L.; Toghiani, H.; Gardner, S.; Pittman, C.U., Jr. Adsorption of precious metal ions onto electrochemically oxidized carbon fibers. Carbon 1999, 37, 1607–1618. [Google Scholar] [CrossRef]
- Su, C.; Hong, B.; Tseng, C. Sol-gel preparation and photocatalysis of titanium dioxide. Catal. Today 2004, 96, 119–126. [Google Scholar] [CrossRef]
- Shi, J.; Cui, H.; Chen, J.; Fu, M.; Xu, B.; Luo, H.; Ye, Z. TiO2/activated carbon fibers photocatalyst: Effects of coating procedures on the microstructure, adhesion property, and photocatalytic ability. J. Colloid Interf. Sci. 2012, 388, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, R.R.; Pujari, S.R.; Lande, M.K.; Arbad, B.R.; Pawar, S.B.; Gambhire, A.B. Photocatalytic activity and characterization of sol-gel-derived Ni-doped TiO2-coated active carbon composites. Appl. Surf. Sci. 2012, 261, 835–841. [Google Scholar] [CrossRef]
- Jose, M.; Kumari, M.; Karunakaran, R.; Shukla, S. Hydrothermal synthesis of highly crystalline nanotubes/nanoplates of pure and silver-doped anatase-titania using acid-catalyst-modified sol-gel precursors. J. Sol-Gel Sci. Technol. 2015, 73, 38–47. [Google Scholar] [CrossRef]
- Liu, W.; Ma, E.; Zhao, G. Characterization of Silver Particles in Silver-Containing Activated Carbon Fibers Prepared from Liquefied Wood. J. Wood Chem. Technol. 2015, 35, 291–301. [Google Scholar] [CrossRef]
- Liu, W.; Shi, M.; Ma, E.; Zhao, G. Microstructure and properties of liquefied wood-based activated carbon fibers prepared from precursors and carbon fibers. Wood Fiber Sci. 2014, 46, 39–47. [Google Scholar]
- Matos, J.; Hofman, M.; Pietrzak, R. Synergy effect in the photocatalytic degradation of methylene blue on a suspended mixture of TiO2 and N-containing carbons. Carbon 2013, 54, 460–471. [Google Scholar] [CrossRef]
- Jiang, Z.; Wei, W.; Mao, D.; Chen, C.; Shi, Y.; Lv, X. Silver-loaded nitrogen-doped yolk–shell mesoporous TiO2 hollow microspheres with enhanced visible light photocatalytic activity. Nanoscale 2014, 7, 784–797. [Google Scholar] [CrossRef] [PubMed]







| Selected Area | C | O | Ti | Ag | N |
|---|---|---|---|---|---|
| 1 | 26.17 | 10.39 | 2.92 | 56.15 | 4.36 |
| 2 | 73.92 | 21.35 | 3.91 | 0.82 | 0.00 |
| 3 | 91.16 | 5.77 | 1.08 | 1.99 | 0.00 |
| Sample | 2θ (°) | β (°) | D (nm) |
|---|---|---|---|
| WACF/TiO2/Ag-0.05 | 38.18 | 0.35 | 23.81 |
| WACF/TiO2/Ag-0.1 | 38.20 | 0.35 | 23.82 |
| WACF/TiO2/Ag-0.2 | 38.16 | 0.32 | 26.04 |
| WACF/TiO2/Ag-0.4 | 38.20 | 0.30 | 27.78 |
| Sample | Content of the Element (at %) | C1s (%) | O1s (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1s | O1s | Ti2p | Ag3d | N1s | Graphite | C–O | C=O | –COOH, RCOO– | CO32-, CO, CO2 | O–Ti | O–H, O=C | O–C | O2, H2O | |
| WACF | 90.79 | 8.89 | 0 | 0 | 0.32 | 64.11 | 14.89 | 9.88 | 5.91 | 5.21 | 0 | 20.05 | 71.22 | 8.72 |
| WACF/TiO2/Ag-0.05 | 70.96 | 18.61 | 6.38 | 0.70 | 3.36 | 56.83 | 18.49 | 14.55 | 6.64 | 3.49 | 53.34 | 25.09 | 21.57 | 0 |
| WACF/TiO2/Ag-0.1 | 73.21 | 16.23 | 5.91 | 1.61 | 3.04 | 56.31 | 19.21 | 12.88 | 6.72 | 4.87 | 52.61 | 25.26 | 22.13 | 0 |
| WACF/TiO2/Ag-0.2 | 73.11 | 16.01 | 5.20 | 2.26 | 3.42 | 51.82 | 24.02 | 14.04 | 5.58 | 4.52 | 51.97 | 24.69 | 23.33 | 0 |
| WACF/TiO2/Ag-0.4 | 69.46 | 18.18 | 6.52 | 2.30 | 3.54 | 49.92 | 24.68 | 14.13 | 5.37 | 5.89 | 46.36 | 27.55 | 26.09 | 0 |
| Sample | Total Pores | Micropores | Mesopores | |||
|---|---|---|---|---|---|---|
| SBET | Vtotal | Smicro | Vmicro | Smeso | Vmeso | |
| WACF | 1250 | 0.644 | 968 | 0.384 | 186 | 0.207 |
| WACF/TiO2/Ag-0.05 | 411 | 0.285 | 213 | 0.091 | 143 | 0.162 |
| WACF/TiO2/Ag-0.1 | 342 | 0.202 | 205 | 0.087 | 98 | 0.091 |
| WACF/TiO2/Ag-0.2 | 254 | 0.195 | 105 | 0.048 | 111 | 0.122 |
| WACF/TiO2/Ag-0.4 | 245 | 0.185 | 96 | 0.044 | 104 | 0.119 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Han, W.; Zhang, M.; Guo, Z. Self-Regeneration Performance and Characterization of Silver-Containing Activated Carbon Fibers Coated by Titanium Dioxide. Polymers 2019, 11, 983. https://doi.org/10.3390/polym11060983
Liu W, Han W, Zhang M, Guo Z. Self-Regeneration Performance and Characterization of Silver-Containing Activated Carbon Fibers Coated by Titanium Dioxide. Polymers. 2019; 11(6):983. https://doi.org/10.3390/polym11060983
Chicago/Turabian StyleLiu, Wenjing, Wang Han, Minghui Zhang, and Zeyu Guo. 2019. "Self-Regeneration Performance and Characterization of Silver-Containing Activated Carbon Fibers Coated by Titanium Dioxide" Polymers 11, no. 6: 983. https://doi.org/10.3390/polym11060983
APA StyleLiu, W., Han, W., Zhang, M., & Guo, Z. (2019). Self-Regeneration Performance and Characterization of Silver-Containing Activated Carbon Fibers Coated by Titanium Dioxide. Polymers, 11(6), 983. https://doi.org/10.3390/polym11060983