Influence of Epoxidized Canola Oil (eCO) and Cellulose Nanocrystals (CNCs) on the Mechanical and Thermal Properties of Polyhydroxybutyrate (PHB)—Poly(lactic acid) (PLA) Blends
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Morphology
2.4. Mechanical Characterization
2.5. Thermal Characterization
2.6. Statistical Analysis
3. Results
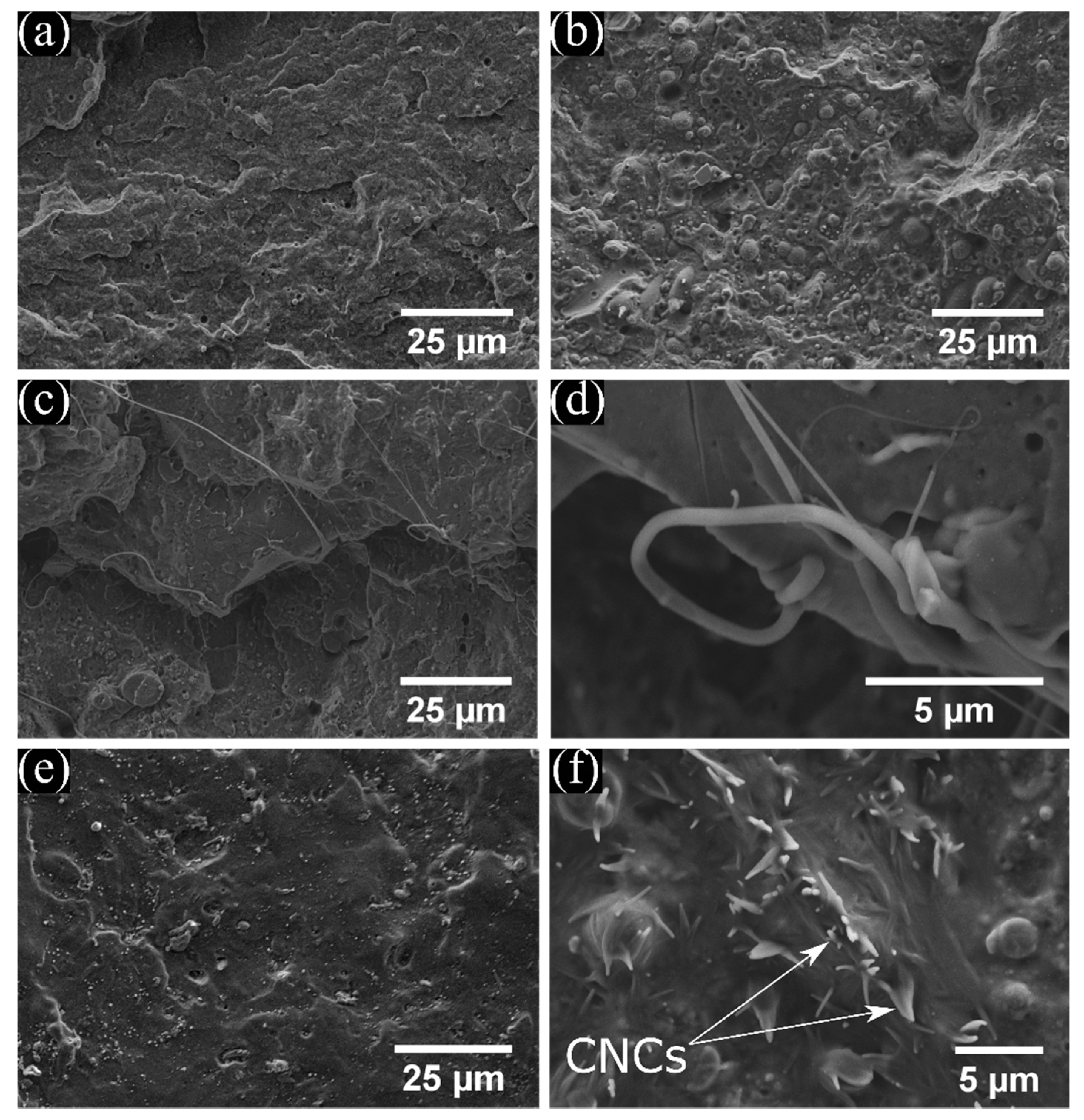
3.1. Morphology
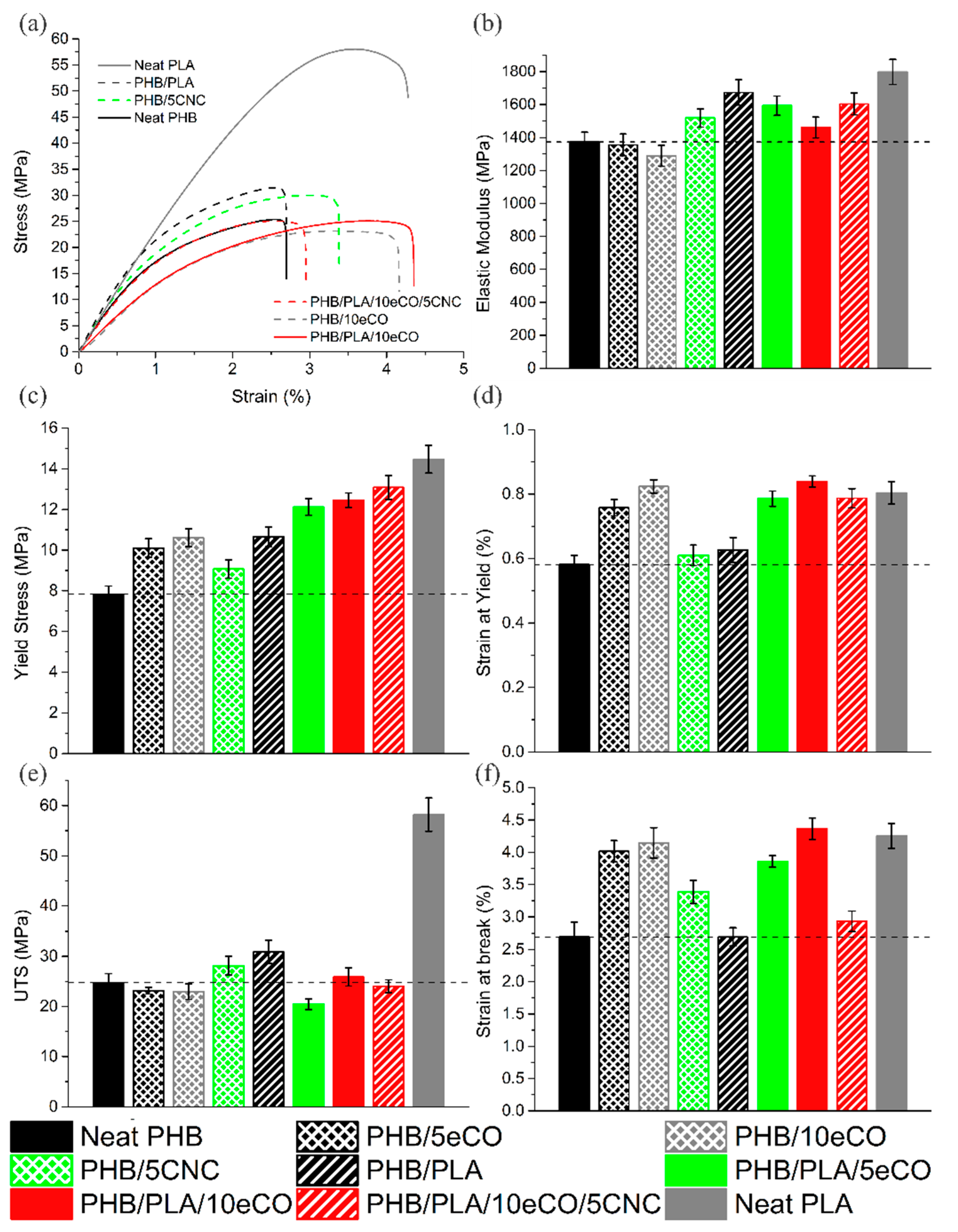
3.2. Mechanical Properties
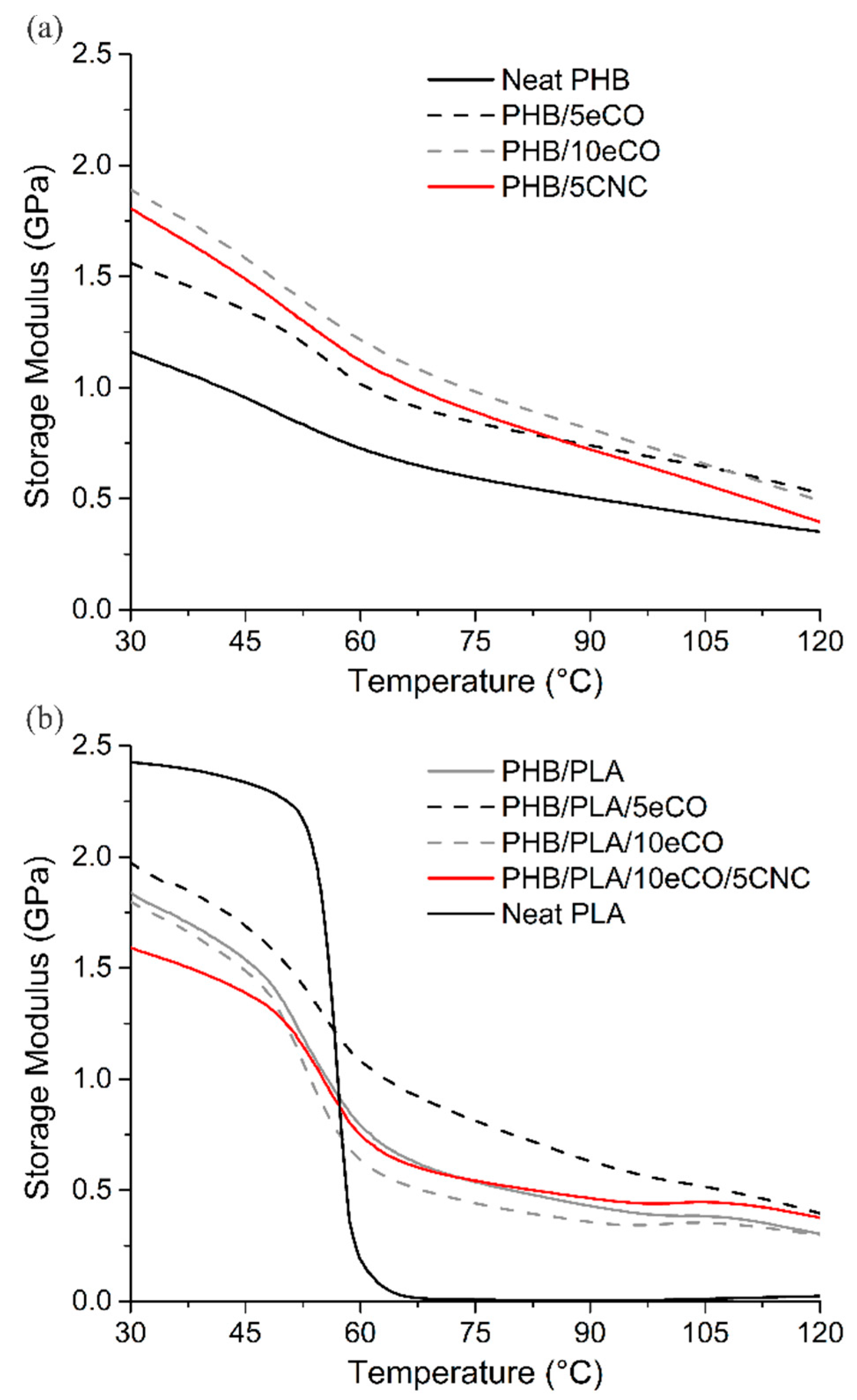
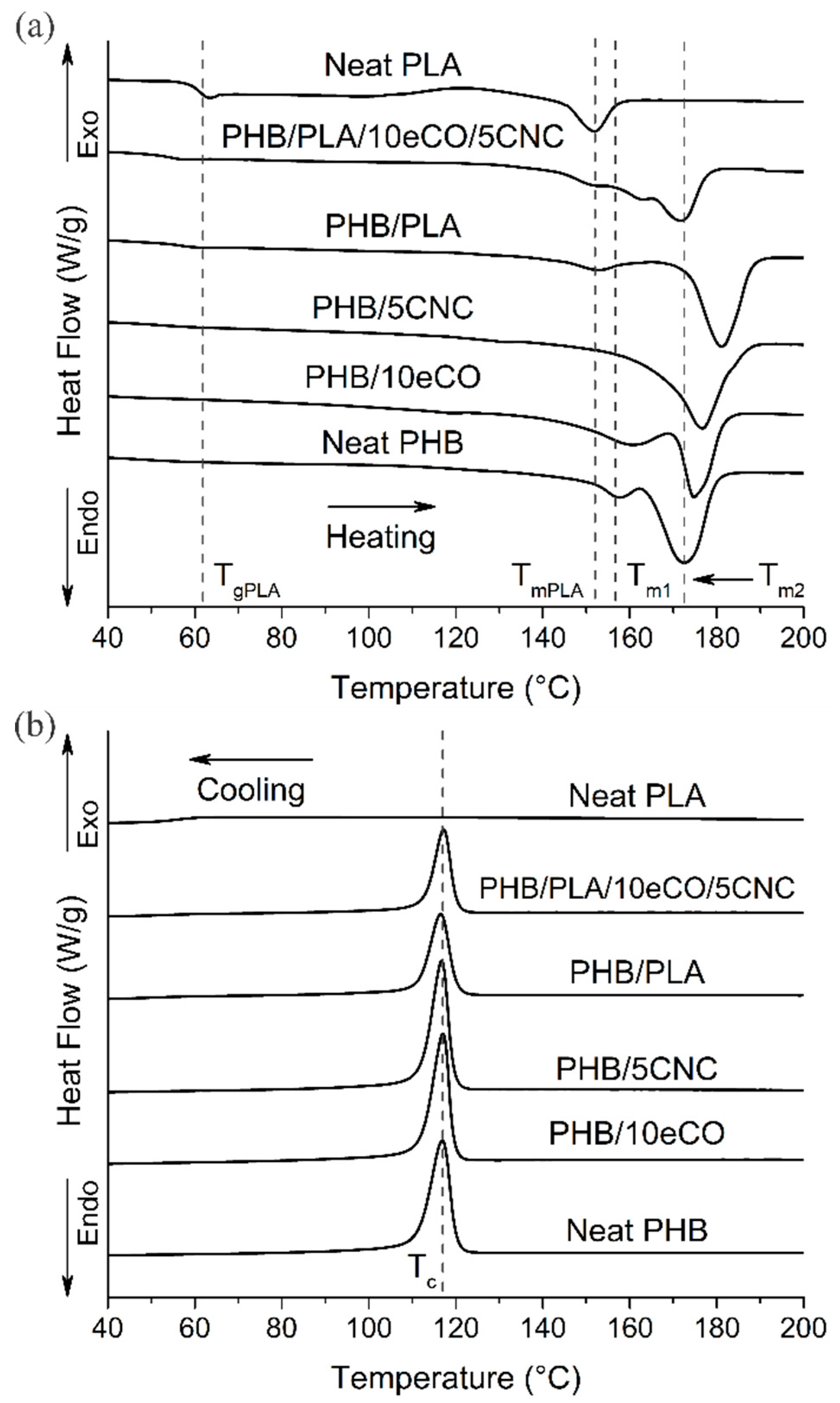
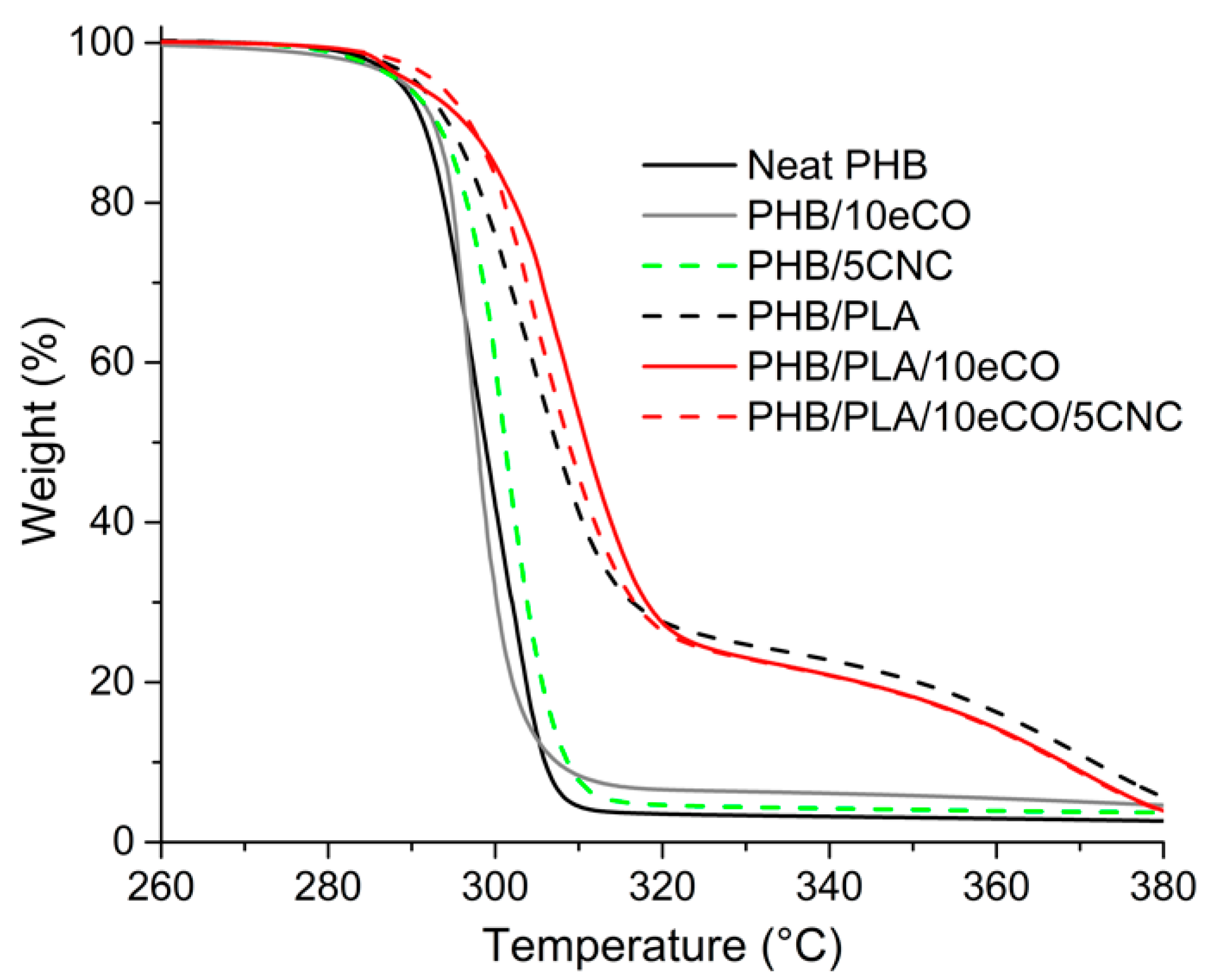
3.3. Crystallinity and Thermal Stability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ŝprajcar, M.; Horvat, P.; Kržan, A. Biopolymers and Bioplastics—Plastics Aligned with Nature, Plastice Project; National Institute of Chemistry: Ljubljana, Slovenia, 2012; pp. 1–32. [Google Scholar]
- Karlsson, S.; Albertsson, A.C. Biodegradable polymers and environmental interaction. Polym. Eng. Sci. 1998, 38, 1251–1253. [Google Scholar] [CrossRef]
- Martínez-Tobón, D.I.; Gul, M.; Elias, A.L.; Sauvageau, D. Polyhydroxybutyrate (PHB) biodegradation using bacterial strains with demonstrated and predicted PHB depolymerase activity. Appl. Microbiol. Biotechnol. 2018, 102, 8049–8067. [Google Scholar] [CrossRef]
- Mottin, A.C.; Ayres, E.; Oréfice, R.L.; Câmara, J.J.D. What Changes in Poly(3-Hydroxybutyrate) (PHB) When Processed as Electrospun Nanofibers or Thermo-Compression Molded Film? Mater. Res. 2016, 19, 57–66. [Google Scholar] [CrossRef]
- Anbukarasu, P.; Sauvageau, D.; Elias, A. Tuning the properties of polyhydroxybutyrate films using acetic acid via solvent casting. Sci. Rep. 2016, 5, 1–14. [Google Scholar] [CrossRef]
- Niaounakis, M. Packaging. In Biopolymers: Applications and Trends, 1st ed.; Elsevier: Rijswijk, The Netherlands, 2015; pp. 139–183. [Google Scholar]
- Bucci, D.Z.; Tavares, L.B.B.; Sell, I. PHB packaging for the storage of food products. Polym. Test. 2005, 24, 564–571. [Google Scholar] [CrossRef]
- Gredes, T.; Gedrange, T.; Hinüber, C.; Gelinsky, M.; Kunert-Keil, C. Histological and molecular-biological analyses of poly(3-hydroxybutyrate) (PHB) patches for enhancement of bone regeneration. Ann. Anat.—Anat. Anzeiger. 2015, 199, 36–42. [Google Scholar] [CrossRef]
- Zhang, M.; Thomas, N.L. Blending polylactic acid with polyhydroxybutyrate: The effect on thermal, mechanical, and biodegradation properties. Adv. Polym. Technol. 2011, 30, 67–79. [Google Scholar] [CrossRef]
- Bhardwaj, U.; Dhar, P.; Kumar, A.; Katiyar, V. Polyhydroxyalkanoates (PHA)-Cellulose Based Nanobiocomposites for Food Packaging Applications. In Food Additives and Packaging, 1st ed.; American Chemical Society: Washington, DC, USA, 2014; pp. 275–314. [Google Scholar]
- Chieng, B.W.; Ibrahim, N.A.; Then, Y.Y.; Loo, Y.Y. Epoxidized Vegetable Oils Plasticized Poly(lactic acid) Biocomposites: Mechanical, Thermal and Morphology Properties. Molecules 2014, 19, 16024–16038. [Google Scholar] [CrossRef]
- Seoane, I.T.; Manfredi, L.B.; Cyras, V.P. Effect of two different plasticizers on the properties of poly(3-hydroxybutyrate) binary and ternary blends. J. Appl. Polym. Sci. 2017, 135, 1–12. [Google Scholar] [CrossRef]
- Özgür Seydibeyoǧlu, M.; Misra, M.; Mohanty, A. Synergistic improvements in the impact strength and % elongation of polyhydroxybutyrate-co-valerate copolymers with functionalized soybean oils and poss. Int. J. Plast. Technol. 2010, 14, 1–16. [Google Scholar] [CrossRef]
- Mathieu-Denoncourt, J.; Wallace, S.J.; de Solla, S.R.; Langlois, V.S. Plasticizer endocrine disruption: Highlighting developmental and reproductive effects in mammals and non-mammalian aquatic species. Gen. Comp. Endocrinol. 2015, 219, 74–88. [Google Scholar] [CrossRef]
- Uren-Webster, T.M.; Lewis, C.; Filby, A.L.; Paull, G.C.; Santos, E.M. Mechanisms of toxicity of di(2-ethylhexyl) phthalate on the reproductive health of male zebrafish. Aquat. Toxicol. 2010, 99, 360–369. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Ferri, J.M.; Montanes, N.; Lopez-Martinez, J.; Balart, R. Plasticization effects of epoxidized vegetable oils on mechanical properties of poly(3-hydroxybutyrate). Polym. Int. 2016, 65, 1157–1164. [Google Scholar] [CrossRef]
- Mungroo, R.; Pradhan, N.C.; Goud, V.V.; Dalai, A.K. Epoxidation of Canola Oil with Hydrogen Peroxide Catalyzed by Acidic Ion Exchange Resin. J. Am. Oil Chem. Soc. 2008, 85, 887–896. [Google Scholar] [CrossRef]
- Ren, J. Biodegradable Poly(Lactic Acid): Synthesis, Modification, Processing and Applications, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 38–141. [Google Scholar]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Rayón, E.; López, J.; Kenny, J.M. Multifunctional PLA–PHB/cellulose nanocrystal films: Processing, structural and thermal properties. Carbohydr. Polym. 2014, 107, 16–24. [Google Scholar] [CrossRef]
- Södergård, A.; Stolt, M. Industrial Production of High Molecular Weight Poly(Lactic Acid). In Poly(Lactic Acid), 3rd ed.; Auras, R., Lim, L., Selke, S., Tsuji, H., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 27–41. [Google Scholar]
- Ma, P.; Hristova-Bogaerds, D.G.; Goossens, J.G.P.; Spoelstra, A.B.; Zhang, Y.; Lemstra, P.J. Toughening of poly(lactic acid) by ethylene-co-vinyl acetate copolymer with different vinyl acetate contents. Eur. Polym. J. 2012, 48, 146–154. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Rayón, E.; López, J.; Kenny, J.M. PLA-PHB/cellulose based films: Mechanical, barrier and disintegration properties. Polym. Degrad. Stab. 2014, 107, 139–149. [Google Scholar] [CrossRef]
- Li, L.; Huang, W.; Wang, B.; Wei, W.; Gu, Q.; Chen, P. Properties and structure of polylactide/poly (3-hydroxybutyrate-co-3-hydroxyvalerate) (PLA/PHBV) blend fibers. Polymer 2015, 68, 183–194. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Y.; Wang, J.; Xu, Y. Effect of PBAT on Property of PLA/PHB Film Used for Fruits and Vegetables. MATEC Web Conf. 2017, 88, 1–6. [Google Scholar] [CrossRef]
- Gunaratne, L.M.W.K.; Shanks, R.A. Miscibility, melting, and crystallization behavior of poly(hydroxybutyrate) and poly(D,L-lactic acid) blends. Polym. Eng. Sci. 2008, 48, 1683–1692. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, H.; Zhang, C.; Jiang, Q.; Zhan, Y.; Chen, P.; Wang, D. Transesterification induced mechanical properties enhancement of PLLA/PHBV bio-alloy. Polymer 2016, 83, 230–238. [Google Scholar] [CrossRef]
- Ardal, D.; Yilgör, E.; Yilgör, I. Catalyst effect on the transesterification reactions between polycarbonate and polycaprolactone-B-polydimethylsiloxane triblock copolymers. Polym. Bull. 1999, 43, 207–214. [Google Scholar] [CrossRef]
- Leão, R.M.; Miléo, P.C.; Maia, J.M.L.L.; Luz, S.M. Environmental and technical feasibility of cellulose nanocrystal manufacturing from sugarcane bagasse. Carbohydr. Polym. 2017, 175, 518–529. [Google Scholar] [CrossRef]
- Dhar, P.; Bhardwaj, U.; Kumar, A.; Katiyar, V. Investigations on rheological and mechanical behavior of poly(3-Hydroxybutyrate)/cellulose nanocrystal based nanobiocomposites. Polym. Compos. 2017, 38, 1–10. [Google Scholar] [CrossRef]
- Rodriguez, J.F.; Thomas, J.P.; Renaud, J.E. Characterization of the mesostructure of fused-deposition acrylonitrile-butadienestyrene materials. Rapid Prototyp. J. 2000, 6, 175–185. [Google Scholar] [CrossRef]
- International Organization for Standards. ISO 527-1: Plastics—Determination of Tensile Properties, Part 1: General Principles; International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- International Organization for Standards. ISO 527-2: Plastics—Determination of Tensile Properties, Part 2: General Principles; International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- Hurrell, B.L.; Cameron, R.E. A wide-angle X-ray scattering study of the ageing of poly(hydroxybutyrate). J. Mater. Sci. 1998, 33, 1709–1713. [Google Scholar] [CrossRef]
- Maurício Pachekoski, W.; Dalmolin, C.; Augusto Marcondes Agnelli, J. The Influence of the Industrial Processing on the Degradation of Poly(hidroxybutyrate)—PHB. Mater. Res. 2013, 16, 327–332. [Google Scholar] [CrossRef]
- Zhai, W.; Ko, Y.; Zhu, W.; Wong, A.; Park, C.B. A Study of the Crystallization, Melting, and Foaming Behaviors of Polylactic Acid in Compressed CO2. Int. J. Mol. Sci. 2009, 10, 5381–5397. [Google Scholar] [CrossRef]
- Battegazzore, D.; Bocchini, S.; Frache, A. Crystallization kinetics of poly(lactic acid)-talc composites. Express Polym. Lett. 2011, 5, 849–858. [Google Scholar] [CrossRef]
- D’Amico, D.A.; Iglesias Montes, M.L.; Manfredi, L.B.; Cyras, V.P. Fully bio-based and biodegradable polylactic acid/poly(3-hydroxybutirate) blends: Use of a common plasticizer as performance improvement strategy. Polym. Test. 2016, 49, 22–28. [Google Scholar] [CrossRef]
- Gan, L.; Liao, J.; Lin, N.; Hu, C.; Wang, H.; Huang, J. Focus on Gradientwise Control of the Surface Acetylation of Cellulose Nanocrystals to Optimize Mechanical Reinforcement for Hydrophobic Polyester-Based Nanocomposites. ACS Omega 2017, 2, 4725–4736. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Garrido, L.; Alvarado, N.; Romero, J.; Palma, J.; Galotto, M. Improvement of Polylactide Properties through Cellulose Nanocrystals Embedded in Poly(Vinyl Alcohol) Electrospun Nanofibers. Nanomaterials 2017, 7, 1–16. [Google Scholar] [CrossRef]
- Orts, W.J.; Nobes, G.A.; Kawada, J.; Nguyen, S.; Yu, G.; Ravenelle, F. Poly(hydroxyalkanoates): Biorefinery polymers with a whole range of applications. The work of Robert H. Marchessault. Can. J. Chem. 2008, 86, 628–640. [Google Scholar] [CrossRef]
- Hankermeyer, C.R.; Tjeerdema, R.S. Polyhydroxybutyrate: plastic made and degraded by microorganisms. Rev. Environ. Contam. Toxicol. 1999, 159, 1–24. [Google Scholar] [CrossRef]
- Niaounakis, M. Properties. In Biopolymers: Applications and Trends, 1st ed.; Elsevier: Rijswijk, The Netherlands, 2015; pp. 91–138. [Google Scholar]
- Lopera-Valle, A.; McDonald, A. Application of Flame-Sprayed Coatings as Heating Elements for Polymer-Based Composite Structures. J. Therm. Spray Technol. 2015, 24, 1289–1301. [Google Scholar] [CrossRef]
- Mazinani, S.; Ramezani, R.; Darvishmanesh, S.; Molelekwa, G.F.; Di Felice, R.; Van der Bruggen, B. A ground breaking polymer blend for CO2/N2 separation. J. CO2 Util. 2018, 27, 536–546. [Google Scholar] [CrossRef]
- Battegazzore, D.; Frache, A.; Abt, T.; Maspoch, M.L. Epoxy coupling agent for PLA and PHB copolymer-based cotton fabric bio-composites. Compos. Part B Eng. 2018, 148, 188–197. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; Hernández, A.; Rayón, E. Ternary PLA–PHB–Limonene blends intended for biodegradable food packaging applications. Eur. Polym. J. 2014, 50, 255–270. [Google Scholar] [CrossRef]
- Menard, K.P. Dynamic Mechanical Analysis: A Practical Introduction, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; p. 240. [Google Scholar]
- Wittbrodt, B.; Pearce, J.M. The effects of PLA color on material properties of 3-D printed components. Addit. Manuf. 2015, 8, 110–116. [Google Scholar] [CrossRef]
- Zhao, H.; Cui, Z.; Wang, X.; Turng, L.-S.; Peng, X. Processing and characterization of solid and microcellular poly(lactic acid)/polyhydroxybutyrate-valerate (PLA/PHBV) blends and PLA/PHBV/Clay nanocomposites. Compos. Part B Eng. 2013, 51, 79–91. [Google Scholar] [CrossRef]
- Bourbigot, S.; Fontaine, G.; Bellayer, S.; Delobel, R. Processing and nanodispersion: A quantitative approach for polylactide nanocomposite. Polym. Test. 2008, 27, 2–10. [Google Scholar] [CrossRef]
- Mofokeng, J.P.; Luyt, A.S. Dynamic mechanical properties of PLA/PHBV, PLA/PCL, PHBV/PCL blends and their nanocomposites with TiO2 as nanofiller. Thermochim. Acta 2015, 613, 41–53. [Google Scholar] [CrossRef]
- Grassie, N.; Murray, E.J.; Holmes, P.A. The thermal degradation of poly(-(d)-β-hydroxybutyric acid): Part 2—Changes in molecular weight. Polym. Degrad. Stab. 1984, 6, 95–103. [Google Scholar] [CrossRef]
- Mofokeng, J.P.; Luyt, A.S. Morphology and thermal degradation studies of melt-mixed poly(lactic acid) (PLA)/poly(ε-caprolactone) (PCL) biodegradable polymer blend nanocomposites with TiO2 as filler. Polym. Test. 2015, 45, 93–100. [Google Scholar] [CrossRef]
- Hosney, H.; Nadiem, B.; Ashour, I.; Mustafa, I.; El-Shibiny, A. Epoxidized vegetable oil and bio-based materials as PVC plasticizer. J. Appl. Polym. Sci. 2018, 135, 1–12. [Google Scholar] [CrossRef]
| Sample Name | PHB:PLA (wt:wt) | eCO wt % | CNCs wt % |
|---|---|---|---|
| Neat PHB | 1:0 | 0 | 0 |
| PHB/5eCO | 1:0 | 5 | 0 |
| PHB/10eCO | 1:0 | 10 | 0 |
| PHB/5CNC | 1:0 | 0 | 5 |
| PHB/PLA | 3:1 | 0 | 0 |
| PHB/PLA/5eCO | 3:1 | 5 | 0 |
| PHB/PLA/10eCO | 3:1 | 10 | 0 |
| PHB/PLA/10eCO/5CNC | 3:1 | 10 | 5 |
| Neat PLA | 0:1 | 0 | 0 |
| Composition | Tc1 (°C) | Tm11 (°C) | Tm21 (°C) | Xc,1 Day2 (%) | Xc,7 Days2 (%) |
|---|---|---|---|---|---|
| Neat PHB | 117 | 157 | 172 | 20.5 | 28.2 |
| PHB/5eCO | 117 | 158 | 173 | 21.4 | 34.0 |
| PHB/10eCO | 116 | 161 | 175 | 20.8 | 37.1 |
| PHB/5CNC | 117 | - | 176 | 26.4 | 39.6 |
| PHB/PLA | 116 | - | 181 | 22.9 | 23.5 |
| PHB/PLA/5eCO | 116 | 159 | 171 | 19.2 | 41.8 |
| PHB/PLA/10eCO | 115 | 161 | 170 | 20.7 | 42.4 |
| PHB/PLA/5CNC/10eCO | 115 | 161 | 171 | 20.3 | 43.4 |
| Neat PLA3 | - | 151 | - | 1.4 | 1.2 |
| 1: Max standard deviation = 3 °C. 2: Max standard deviation = 2.5%. 3: Crystallinity of neat PLA with [39] | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopera-Valle, A.; Caputo, J.V.; Leão, R.; Sauvageau, D.; Luz, S.M.; Elias, A. Influence of Epoxidized Canola Oil (eCO) and Cellulose Nanocrystals (CNCs) on the Mechanical and Thermal Properties of Polyhydroxybutyrate (PHB)—Poly(lactic acid) (PLA) Blends. Polymers 2019, 11, 933. https://doi.org/10.3390/polym11060933
Lopera-Valle A, Caputo JV, Leão R, Sauvageau D, Luz SM, Elias A. Influence of Epoxidized Canola Oil (eCO) and Cellulose Nanocrystals (CNCs) on the Mechanical and Thermal Properties of Polyhydroxybutyrate (PHB)—Poly(lactic acid) (PLA) Blends. Polymers. 2019; 11(6):933. https://doi.org/10.3390/polym11060933
Chicago/Turabian StyleLopera-Valle, Adrián, Joseph V. Caputo, Rosineide Leão, Dominic Sauvageau, Sandra Maria Luz, and Anastasia Elias. 2019. "Influence of Epoxidized Canola Oil (eCO) and Cellulose Nanocrystals (CNCs) on the Mechanical and Thermal Properties of Polyhydroxybutyrate (PHB)—Poly(lactic acid) (PLA) Blends" Polymers 11, no. 6: 933. https://doi.org/10.3390/polym11060933
APA StyleLopera-Valle, A., Caputo, J. V., Leão, R., Sauvageau, D., Luz, S. M., & Elias, A. (2019). Influence of Epoxidized Canola Oil (eCO) and Cellulose Nanocrystals (CNCs) on the Mechanical and Thermal Properties of Polyhydroxybutyrate (PHB)—Poly(lactic acid) (PLA) Blends. Polymers, 11(6), 933. https://doi.org/10.3390/polym11060933





