Solvent- and Catalyst-free Synthesis, Hybridization and Characterization of Biobased Nonisocyanate Polyurethane (NIPU)
Abstract
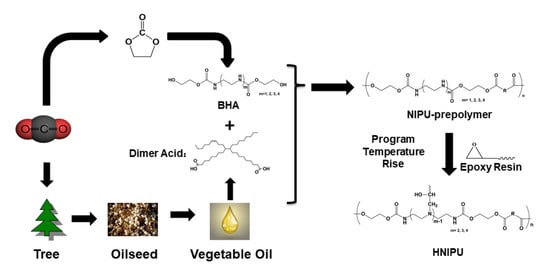
1. Introduction
2. Materials and Methods
2.1. Materials
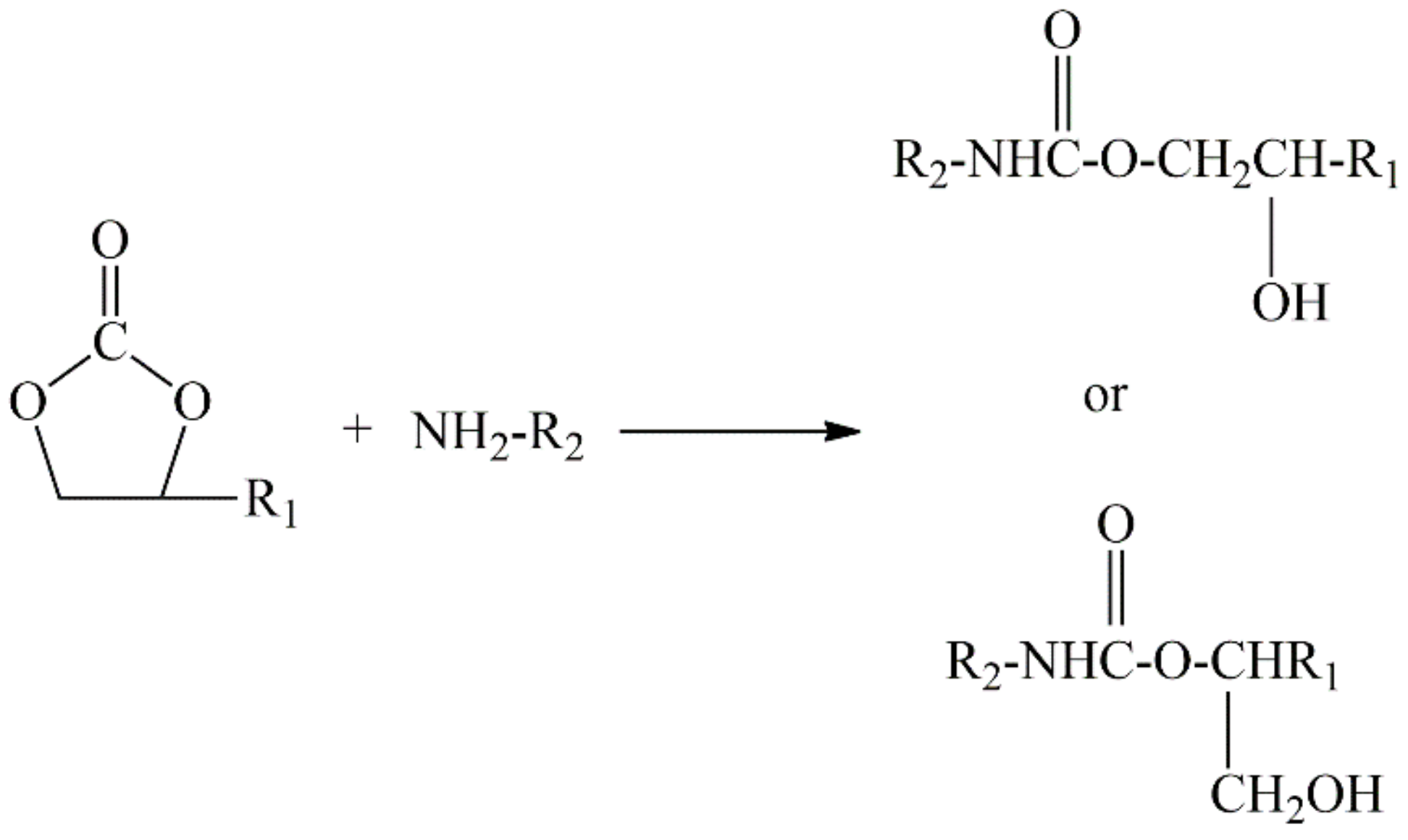
2.2. Synthesis of BHAs
2.3. Synthesis of NIPU Prepolymers
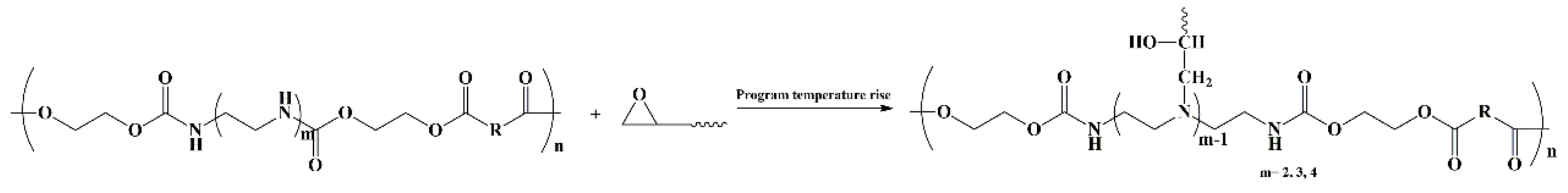
2.4. Synthesis of HNIPUs
2.5. Characterization
3. Results and Discussion
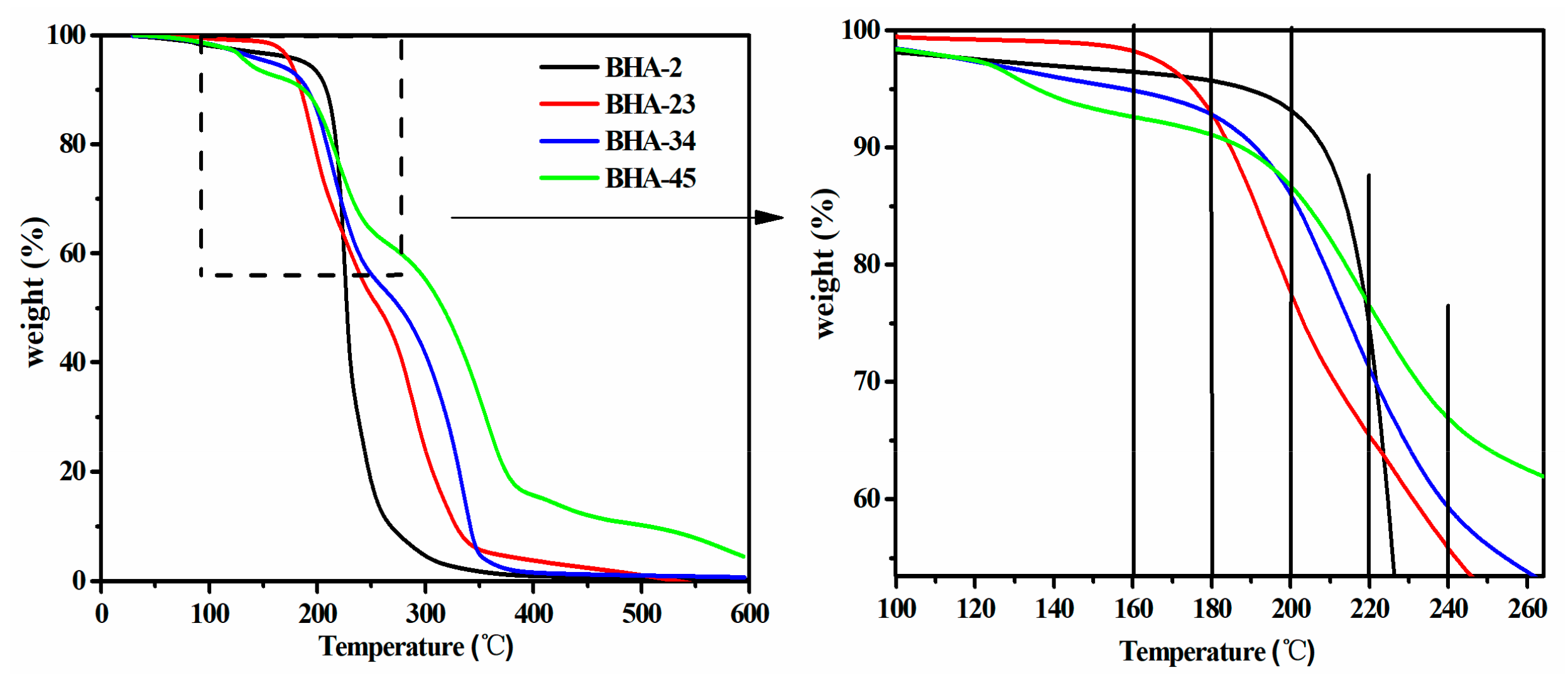
3.1. Synthesis of BHAs
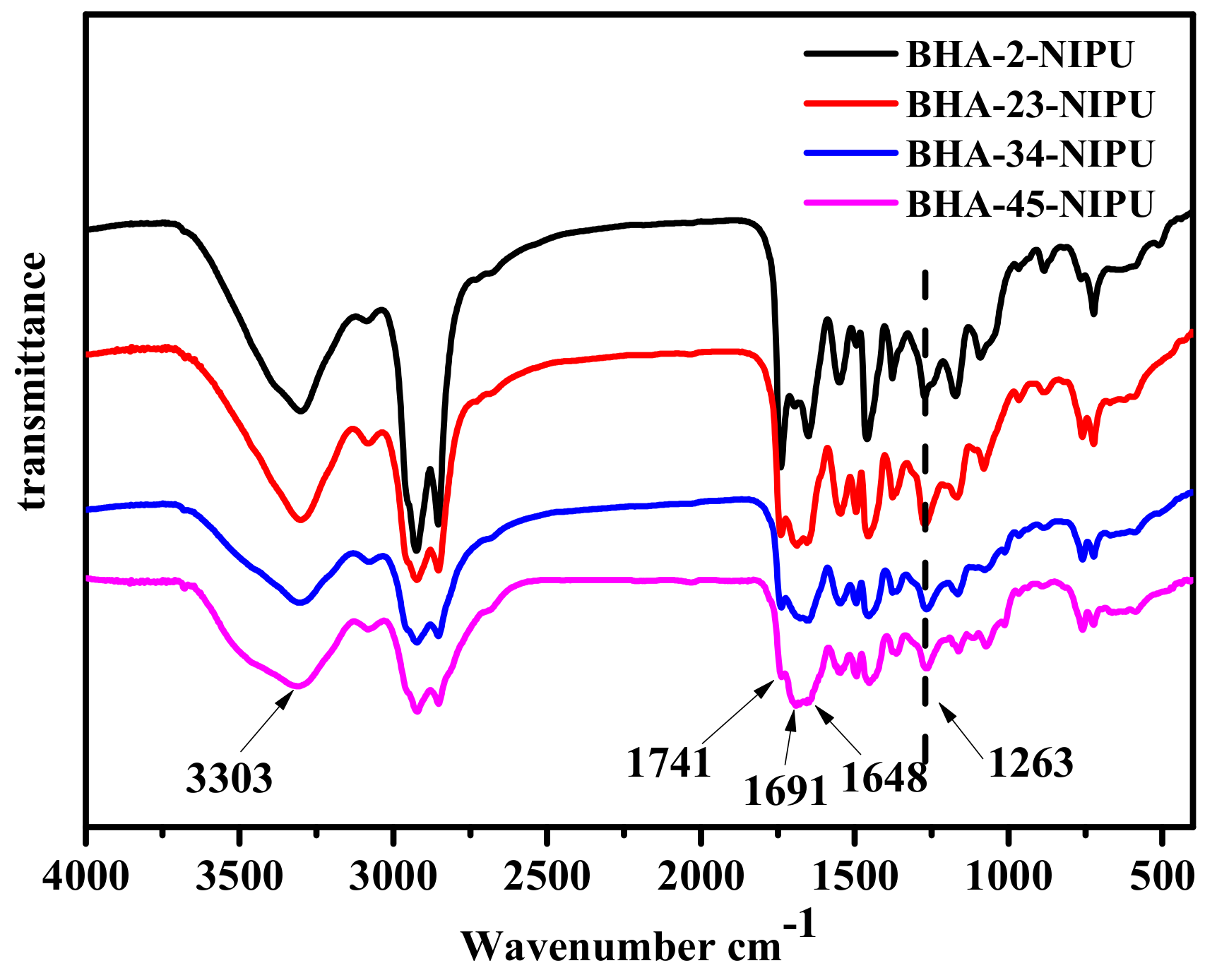
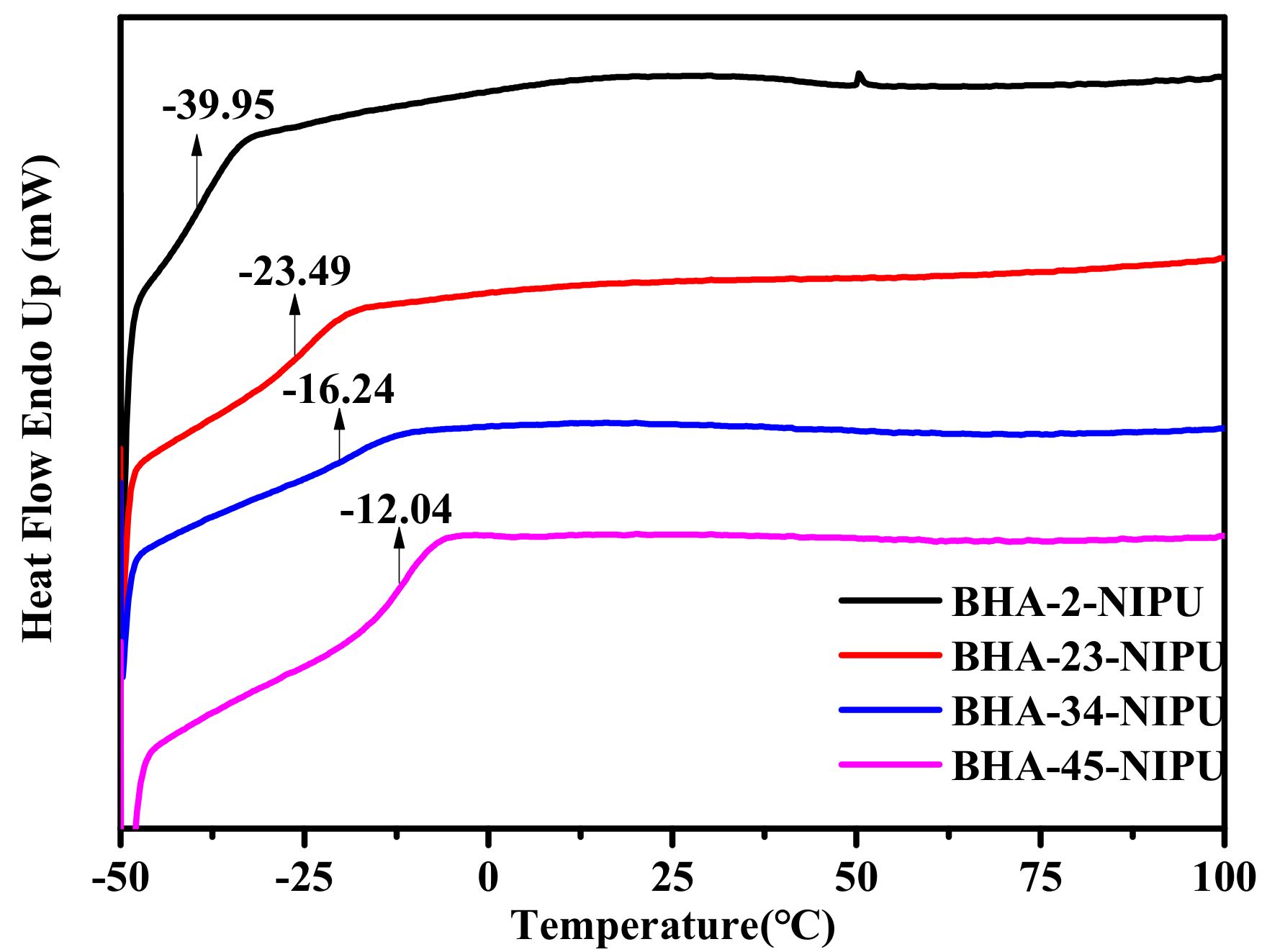
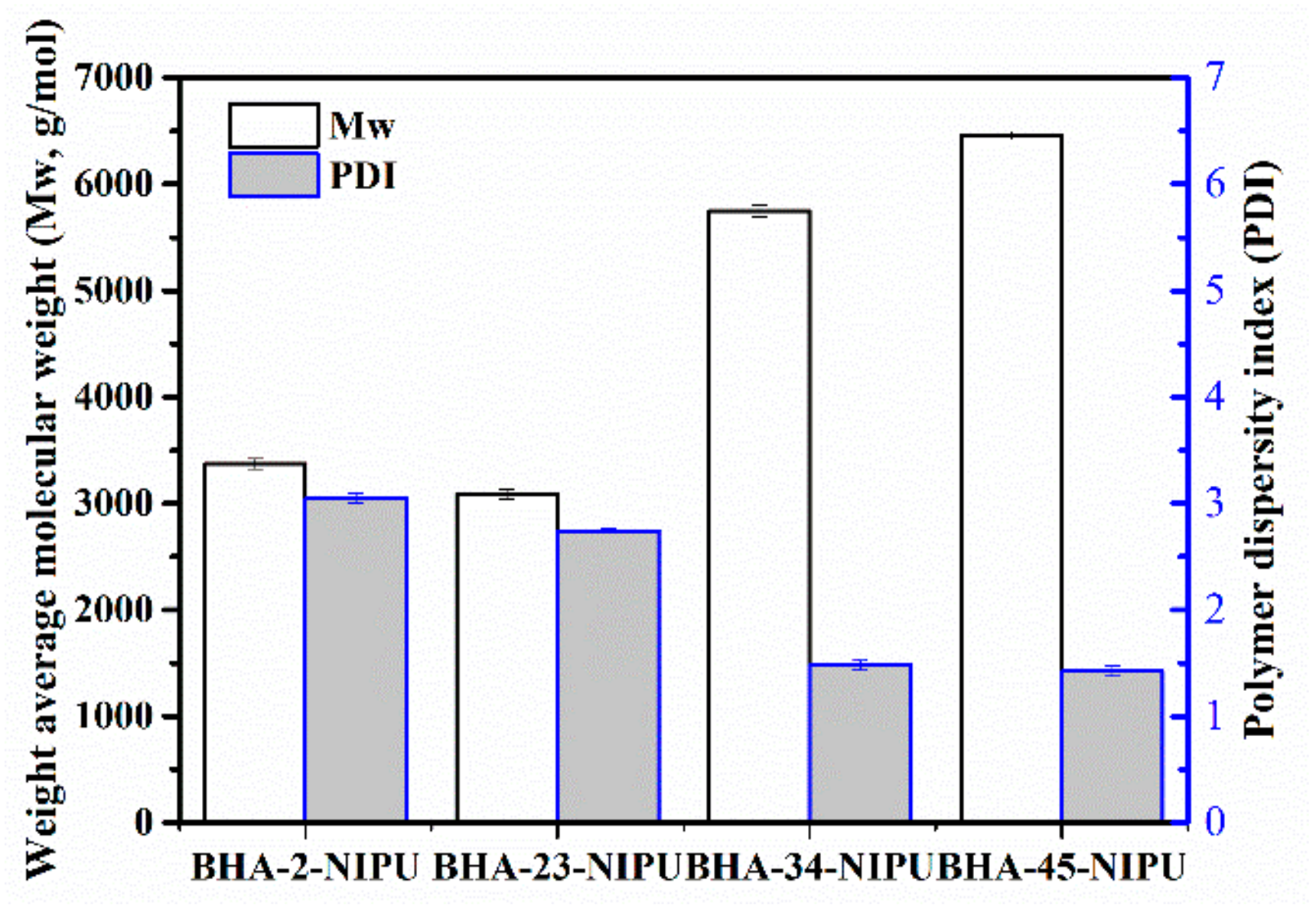
3.2. Synthesis of NIPU-prepolymers
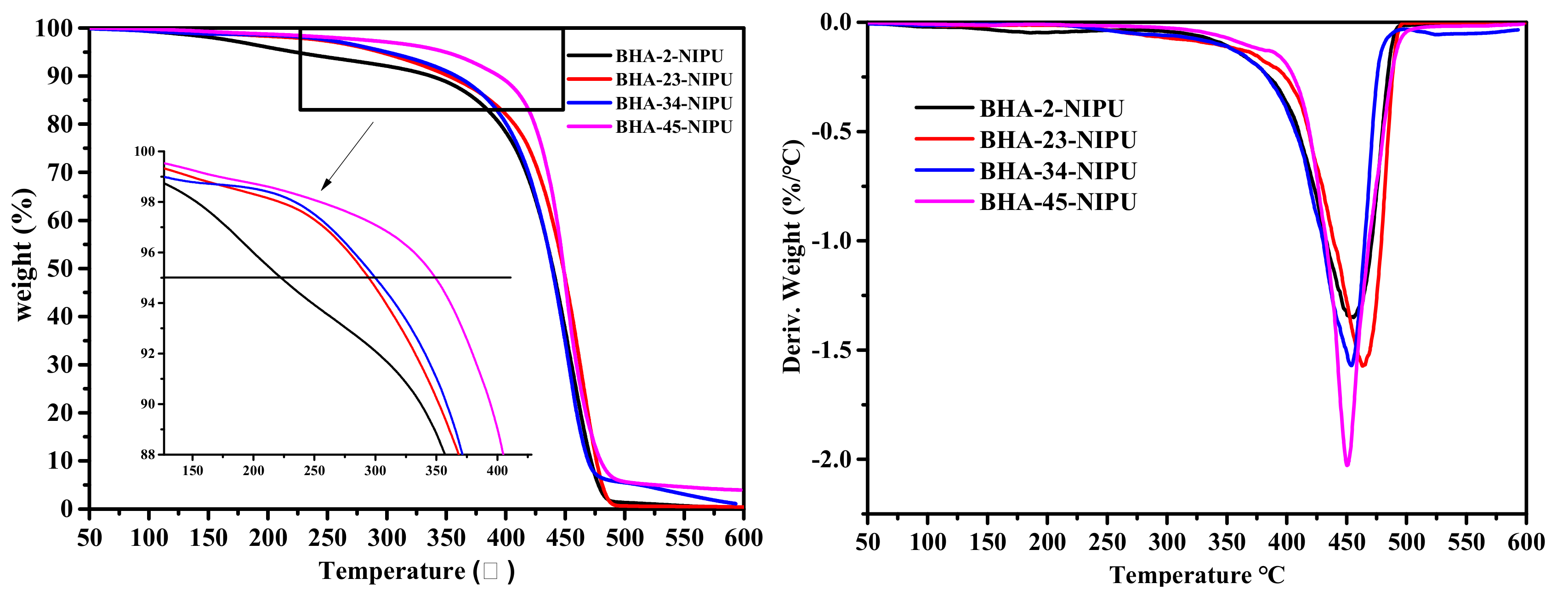
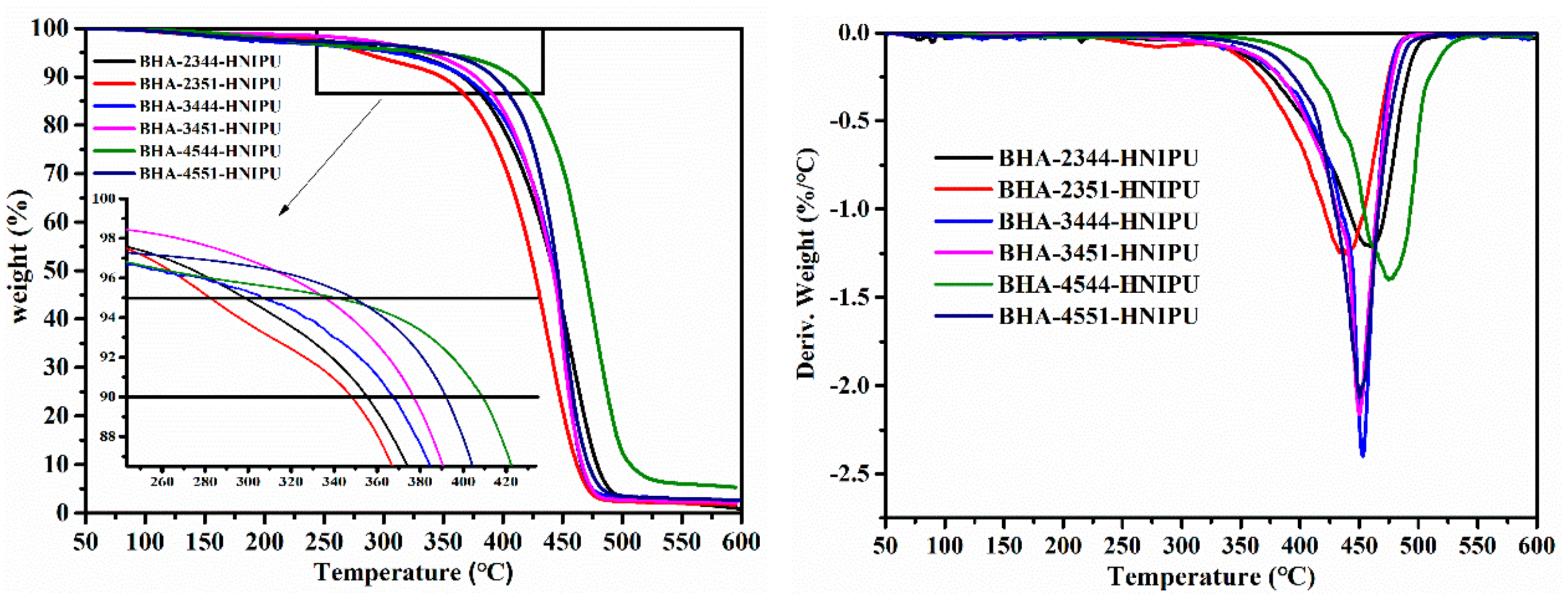
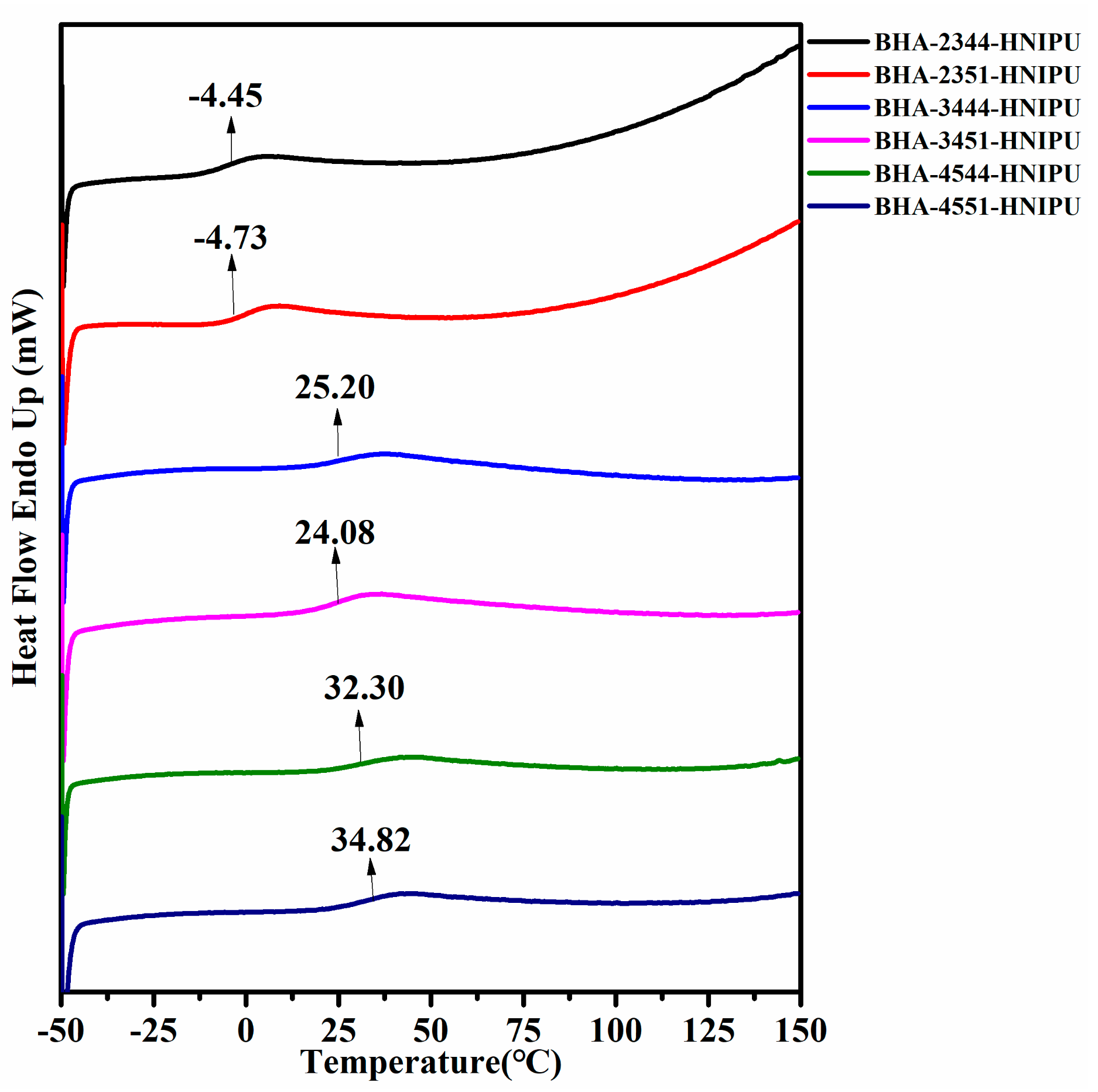
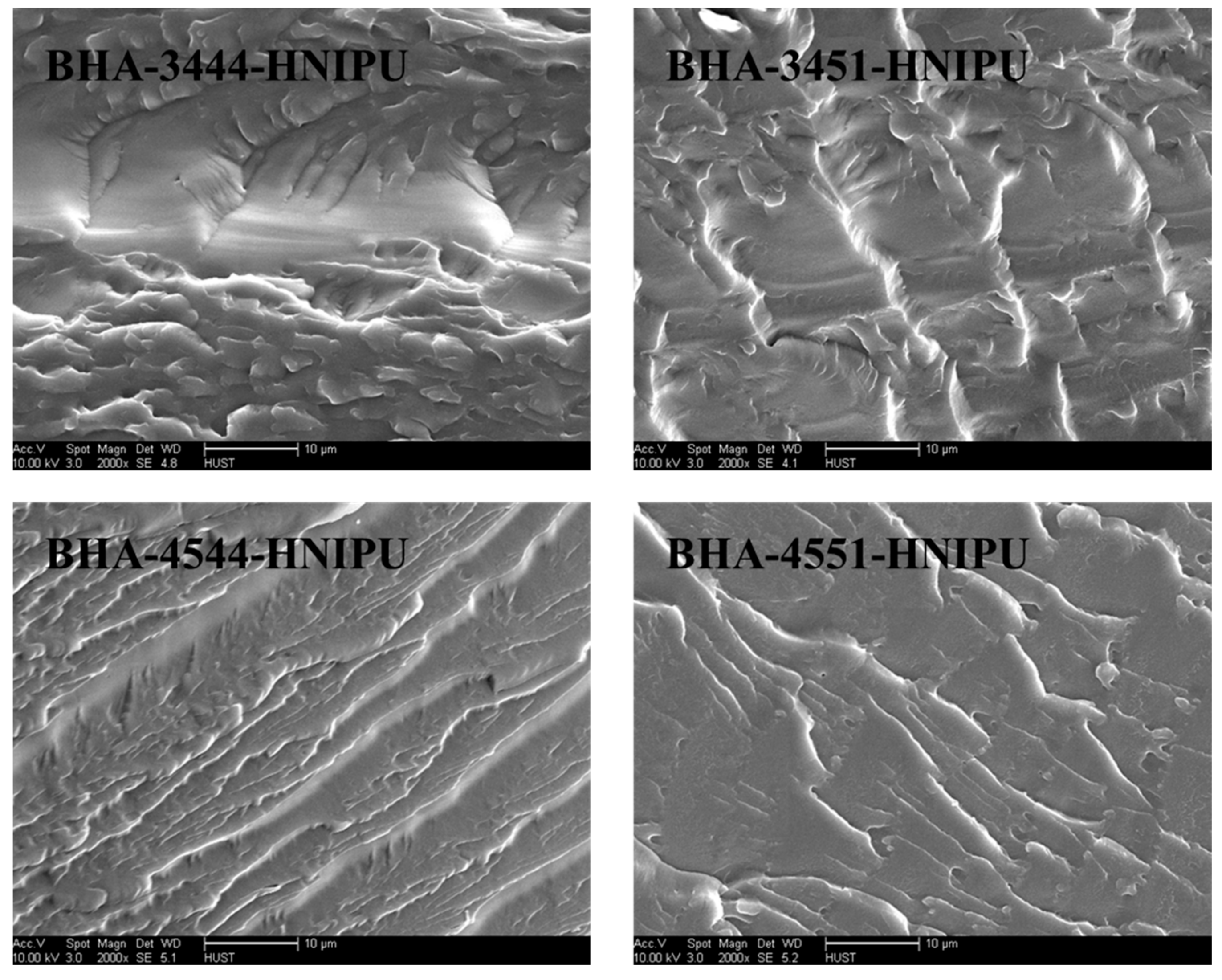
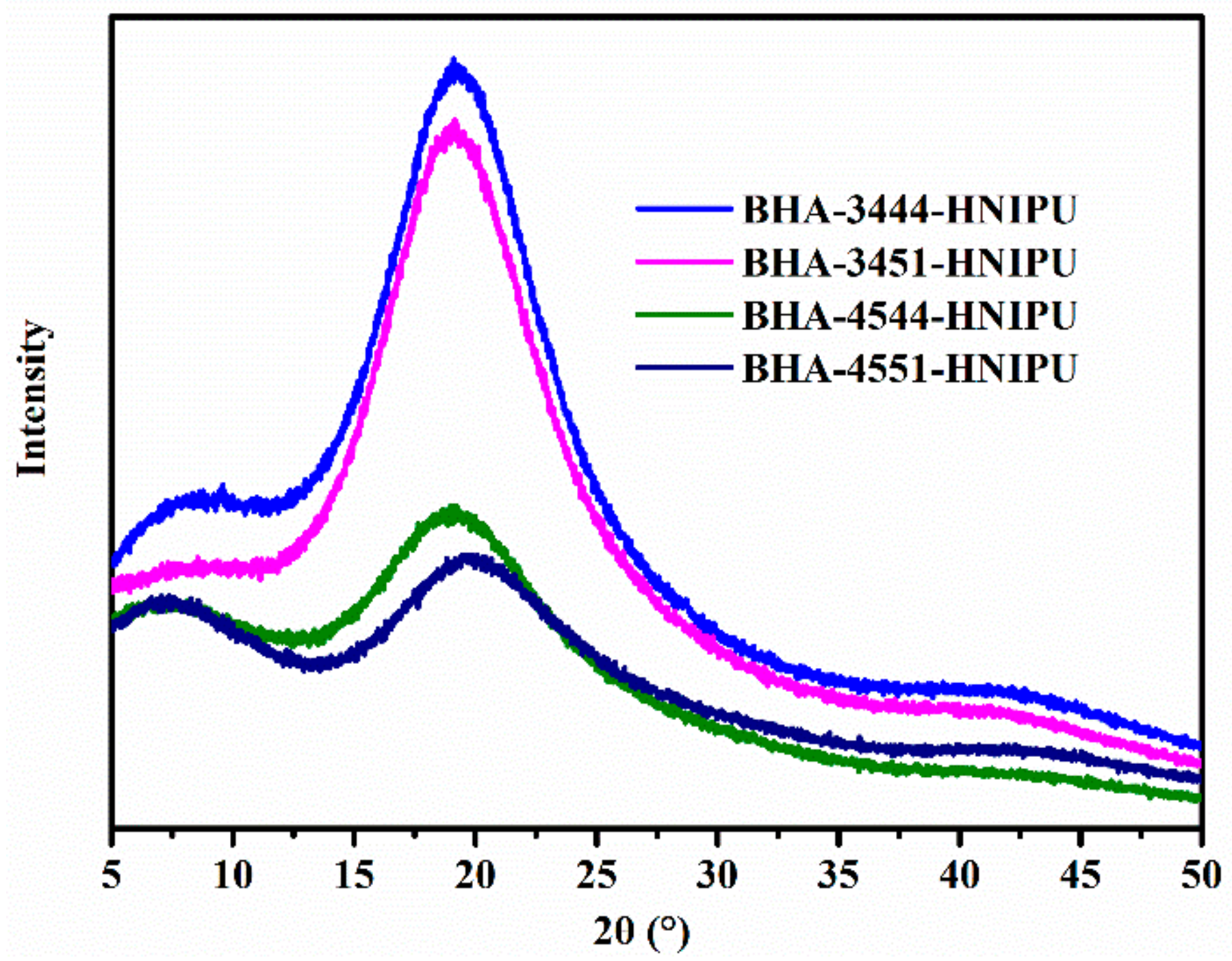
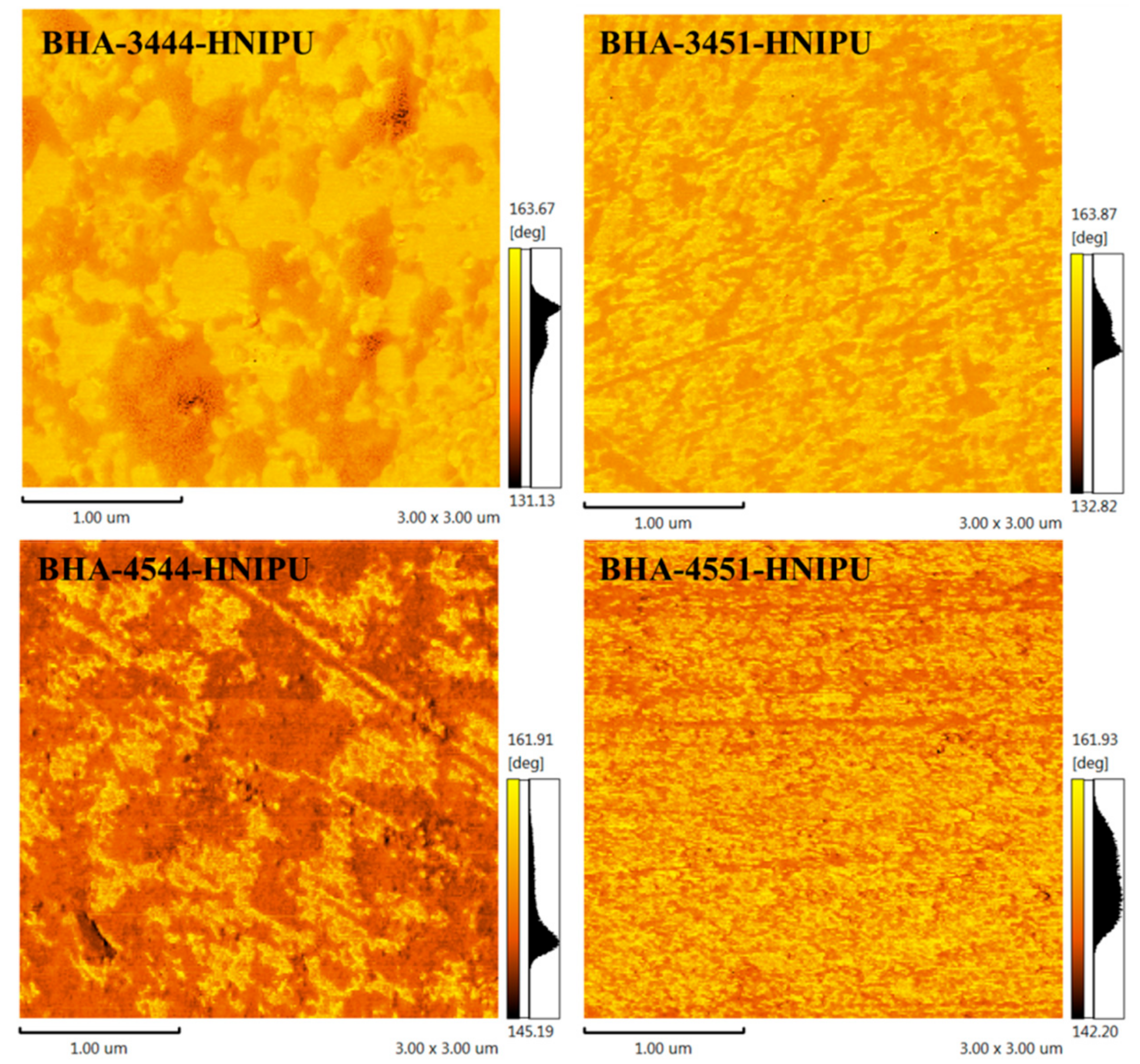
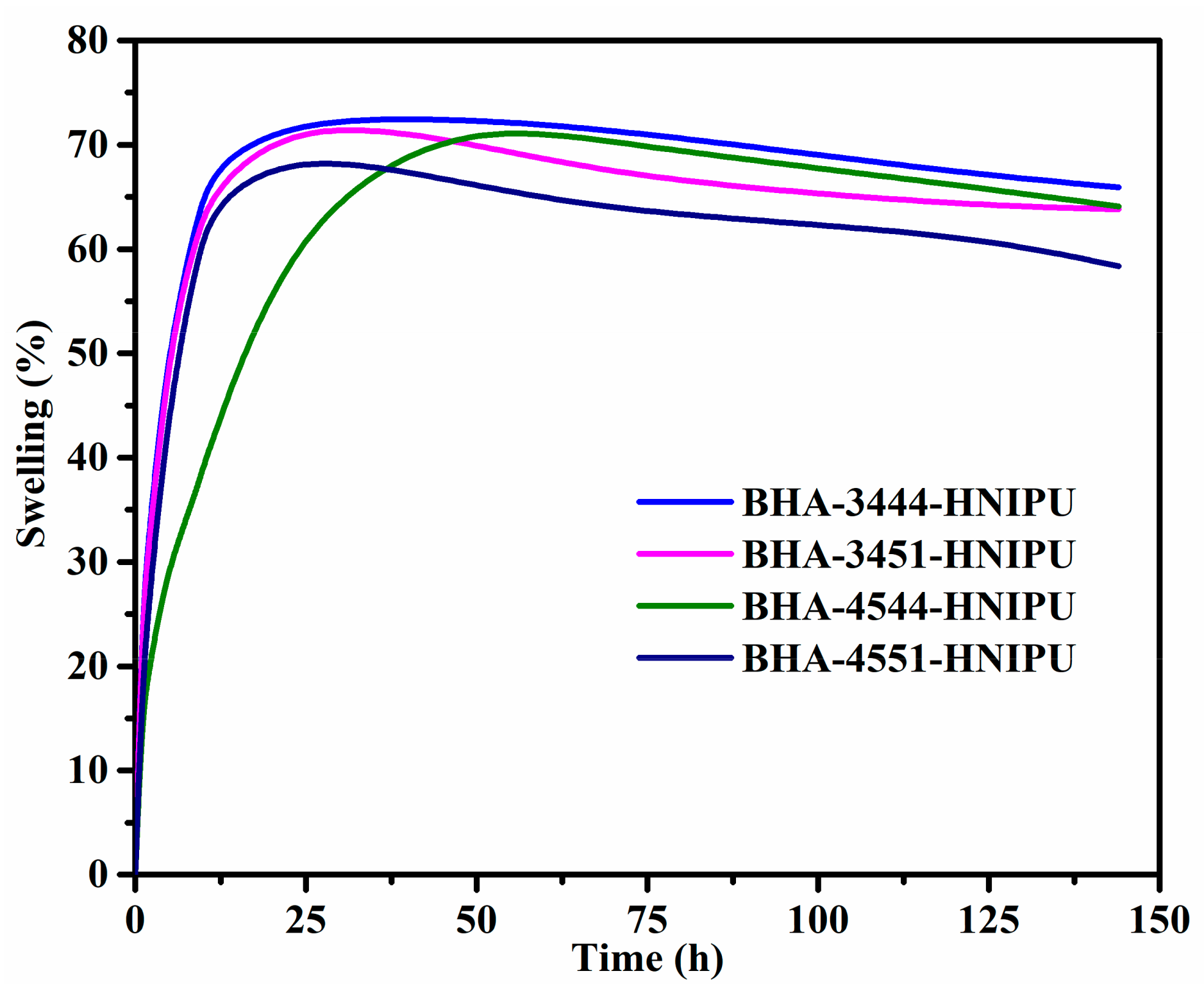
3.3. Synthesis of HNIPUs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, Z.; Cai, W.; Chen, R.; Qu, J. Synthesis and properties of ambient-curable non-isocyanate polyurethanes. Prog. Org. Coat. 2018, 119, 116–122. [Google Scholar] [CrossRef]
- Cornille, A.; Auvergne, R.; Figovsky, O.; Boutevin, B.; Caillol, S. A perspective approach to sustainable routes for non-isocyanate polyurethanes. Eur. Polym. J. 2017, 87, 535–552. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Zhang, L.; Tan, T.; Fong, H. Nonisocyanate Biobased Poly(ester urethanes) with Tunable Properties Synthesized via an Environment-Friendly Route. ACS Sustain. Chem. Eng. 2016, 4, 2762–2770. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Zhao, J.; Zhang, Z.; Zhang, J.; Yang, W. Synthesis and characterization of crystallizable aliphatic thermoplastic poly(ester urethane) elastomers through a non-isocyanate route. Chin. J. Polym. Sci. 2016, 34, 1220–1233. [Google Scholar] [CrossRef]
- Jing, Q.; Liu, Q.; Li, L.; Dong, Z.; Silberschmidt, V.V. Effect of graphene-oxide enhancement on large-deflection bending performance of thermoplastic polyurethane elastomer. Compos. Part B Eng. 2016, 89, 1–8. [Google Scholar] [CrossRef]
- Mazari, F.; Chotebor, M.; Naeem, J.; Mazari, A.; Havelka, A. Effect of Perforated Polyurethane Foam on Moisture Permeability for Car Seat Comfort. Fibres Text. East. Eur. 2016, 24, 165–169. [Google Scholar] [CrossRef]
- Kathalewar, M.S.; Joshi, P.B.; Sabnis, A.S.; Malshe, V.C. Non-isocyanate polyurethanes: From chemistry to applications. RSC Adv. 2013, 3, 4110–4129. [Google Scholar] [CrossRef]
- Hamaviriyapornwattana, N.; Sombatsompop, N.; Markpin, T.; Kositchaiyong, A.; Wimolmala, E. Solar reflectance, surface adhesion, and thermal conductivity of wood/natural rubber composite sheet with Tio2/polyurethane topcoat for roofing applications. J. Vinyl Addit. Technol. 2012, 18, 184–191. [Google Scholar] [CrossRef]
- Guan, J.; Song, Y.; Lin, Y.; Yin, X.; Zuo, M.; Zhao, Y.; Tao, X.; Zheng, Q. Progress in Study of Non-Isocyanate Polyurethane. Ind. Eng. Chem. Res. 2011, 50, 6517–6527. [Google Scholar] [CrossRef]
- Ubaghs, L.; Fricke, N.; Keul, H.; Hocker, H. Polyurethanes with pendant hydroxyl groups: Synthesis and characterization. Macromol. Rapid Comm. 2004, 25, 517–521. [Google Scholar] [CrossRef]
- Nohra, B.; Candy, L.; Blanco, J.; Guerin, C.; Raoul, Y.; Mouloungui, Z. From Petrochemical Polyurethanes to Biobased Polyhydroxyurethanes. Macromolecules 2013, 46, 3771–3792. [Google Scholar] [CrossRef]
- Karol, M.H.; Kramarik, J.A. Phenyl isocyanate is a potent chemical sensitizer. Toxicol. Lett. 1996, 89, 139–146. [Google Scholar] [CrossRef]
- Wunschik, D.S.; Ingenbosch, K.N.; Zähres, M.; Horst, J.; Mayer, C.; Jäger, M.; Strehmel, V.; Dornbusch, M.; Hoffmann-Jacobsen, K. Biocatalytic and solvent-free synthesis of a bio-based biscyclocarbonate. Green Chem. 2018, 20, 4738–4745. [Google Scholar] [CrossRef]
- Zhang, K.; Nelson, A.M.; Talley, S.J.; Chen, M.; Margaretta, E.; Hudson, A.G.; Moore, R.B.; Long, T.E. Non-isocyanate poly(amide-hydroxyurethane)s from sustainable resources. Green Chem. 2016, 18, 4667–4681. [Google Scholar] [CrossRef]
- Furtwengler, P.; Avérous, L. Renewable polyols for advanced polyurethane foams from diverse biomass resources. Polym. Chem.-UK. 2018, 9, 4258–4287. [Google Scholar] [CrossRef]
- Noreen, A.; Zia, K.M.; Zuber, M.; Tabasum, S.; Zahoor, A.F. Bio-based polyurethane: An efficient and environment friendly coating systems: A review. Prog. Org. Coat. 2016, 91, 25–32. [Google Scholar] [CrossRef]
- Kong, X.; Liu, G.; Qi, H.; Curtis, J.M. Preparation and characterization of high-solid polyurethane coating systems based on vegetable oil derived polyols. Prog. Org. Coat. 2013, 76, 1151–1160. [Google Scholar] [CrossRef]
- Wu, G.; He, X.; Yan, Y. Lipase-catalyzed modification of natural Sapium sebiferum oil-based polyol for synthesis of polyurethane with improved properties. RSC Adv. 2017, 7, 1504–1512. [Google Scholar] [CrossRef]
- Campanella, A.; Bonnaillie, L.M.; Wool, R.P. Polyurethane foams from soyoil-based polyols. J. Appl. Polym. Sci. 2009, 112, 2567–2578. [Google Scholar] [CrossRef]
- Kirpluks, M.; Kalnbunde, D.; Benes, H.; Cabulis, U. Natural oil based highly functional polyols as feedstock for rigid polyurethane foam thermal insulation. Ind. Crop. Prod. 2018, 122, 627–636. [Google Scholar] [CrossRef]
- Vanags, E.; Kirpluks, M.; Cabulis, U.; Walterova, Z. Highly functional polyol synthesis from epoxidized tall oil fatty acids. J. Renew. Mater. 2018, 6, 764–771. [Google Scholar] [CrossRef]
- Chaudhari, A.; Kulkarni, R.; Mahulikar, P.; Sohn, D.; Gite, V. Development of PU coatings from neem oil based alkyds prepared by the monoglyceride route. J. Am. Oil Chem. Soc. 2015, 92, 733–741. [Google Scholar] [CrossRef]
- Rajput, S.D.; Mahulikar, P.P.; Gite, V.V. Biobased dimer fatty acid containing two pack polyurethane for wood finished coatings. Prog. Org. Coat. 2014, 77, 38–46. [Google Scholar] [CrossRef]
- Li, Y.; Noordover, B.A.J.; van Benthem, R.A.T.M.; Koning, C.E. Chain extension of dimer fatty acid- and sugar-based polyurethanes in aqueous dispersions. Eur. Polym. J. 2014, 52, 12–22. [Google Scholar] [CrossRef]
- Yao, X.; Wu, G.; Xu, L.; Zhang, H.; Yan, Y. Enzyme-catalyzed preparation of dimeric acid polyester polyol from biodiesel and its further use in the synthesis of polyurethane. RSC Adv. 2014, 4, 31062–31070. [Google Scholar] [CrossRef]
- Yu, A.Z.; Setien, R.A.; Sahouani, J.M.; Docken, J.; Webster, D.C. Catalyzed non-isocyanate polyurethane (NIPU) coatings from bio-based poly(cyclic carbonates). J. Coat. Technol. Res. 2019, 16, 41–57. [Google Scholar] [CrossRef]
- Blattmann, H.; Lauth, M.; Mülhaupt, R. Flexible and bio-based nonisocyanate polyurethane (nipu) foams. Macromol. Mater. Eng. 2016, 301, 944–952. [Google Scholar] [CrossRef]
- Haniffa, M.A.C.M.; Ching, Y.C.; Chuah, C.H.; Kuan, Y.C.; Liu, D.; Liou, N. Synthesis, characterization and the solvent effects on interfacial phenomena of jatropha curcas oil based non-isocyanate polyurethane. Polymers-Basel. 2017, 9, 162. [Google Scholar] [CrossRef]
- Bähr, M.; Bitto, A.; Mülhaupt, R. Cyclic limonene dicarbonate as a new monomer for non-isocyanate oligo- and polyurethanes (NIPU) based upon terpenes. Green Chem. 2012, 14, 1447–1454. [Google Scholar] [CrossRef]
- Fache, M.; Darroman, E.; Besse, V.; Auvergne, R.; Caillol, S.; Boutevin, B. Vanillin, a promising biobased building-block for monomer synthesis. Green Chem. 2014, 16, 1987–1998. [Google Scholar] [CrossRef]
- Bhr, M.; Mlhaupt, R. Linseed and soybean oil-based polyurethanes prepared via the non-isocyanate route and catalytic carbon dioxide conversion. Green Chem. 2012, 14, 483–489. [Google Scholar] [CrossRef]
- Rokicki, G.; Piotrowska, A. A new route to polyurethanes from ethylene carbonate, diamines and diols. Polymer 2002, 43, 2927–2935. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Poussard, L.; Mariage, J.; Grignard, B.; Detrembleur, C.; Jérôme, C.; Calberg, C.; Heinrichs, B.; De Winter, J.; Gerbaux, P.; Raquez, J.M.; et al. Non-isocyanate polyurethanes from carbonated soybean oil using monomeric or oligomeric diamines to achieve thermosets or thermoplastics. Macromolecules 2016, 49, 2162–2171. [Google Scholar] [CrossRef]
- Carré, C.; Bonnet, L.; Avérous, L. Solvent- and catalyst-free synthesis of fully biobased nonisocyanate polyurethanes with different macromolecular architectures. RSC Adv. 2015, 5, 100390–100400. [Google Scholar] [CrossRef]
- North, M.; Pasquale, R.; Young, C. Synthesis of cyclic carbonates from epoxides and CO2. Green Chem. 2010, 12, 1514–1539. [Google Scholar] [CrossRef]
- Sakakura, T.; Kohno, K. The synthesis of organic carbonates from carbon dioxide. Chem. Commun. 2009, 1312–1330. [Google Scholar] [CrossRef]
- Xia, H.; Song, M. Preparation and characterization of polyurethane—carbon nanotube composites. Soft Matter. 2005, 1, 386–394. [Google Scholar] [CrossRef]
- Beniah, G.; Uno, B.E.; Lan, T.; Jeon, J.; Heath, W.H.; Scheidt, K.A.; Torkelson, J.M. Tuning nanophase separation behavior in segmented polyhydroxyurethane via judicious choice of soft segment. Polymer 2017, 110, 218–227. [Google Scholar] [CrossRef]
- Asemani, H.; Zareanshahraki, F.; Mannari, V. Design of hybrid nonisocyanate polyurethane coatings for advanced ambient temperature curing applications. J. Appl. Polym. Sci. 2019, 136, 47266. [Google Scholar] [CrossRef]
- Mó, O.; Yáñez, M.; Eckert-Maksić, M.; Maksić, Z.B.; Alkorta, I.; Elguero, J. Periodic Trends in Bond Dissociation Energies. A Theoretical Study. J. Phys. Chem. A. 2005, 109, 4359–4365. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Li, X.; Jiang, S.; Liang, C.; Wang, J.; Kang, M.; Li, Q.; Zhao, Y. Promising approaches to improve the performances of hybrid non-isocyanate polyurethane. Polym. Int. 2018, 68, 651–660. [Google Scholar] [CrossRef]
- Jin, K.; Leitsch, E.K.; Chen, X.; Heath, W.H.; Torkelson, J.M. Segmented Thermoplastic Polymers Synthesized by Thiol-Ene Click Chemistry: Examples of Thiol–Norbornene and Thiol—Maleimide Click Reactions. Macromolecules 2018, 51, 3620–3631. [Google Scholar] [CrossRef]
- Aoyagi, N.; Furusho, Y.; Endo, T. Convenient synthesis of cyclic carbonates from CO2 and epoxides by simple secondary and primary ammonium iodides as metal-free catalysts under mild conditions and its application to synthesis of polymer bearing cyclic carbonate moiety. J. Polym. Sci. Pol. Chem. 2013, 51, 1230. [Google Scholar] [CrossRef]
- Cornille, A.; Michaud, G.; Simon, F.; Fouquay, S.; Auvergne, R.; Boutevin, B.; Caillol, S. Promising mechanical and adhesive properties of isocyanate-free poly(hydroxyurethane). Eur. Polym. J. 2016, 84, 404–420. [Google Scholar] [CrossRef]
- Karami, Z.; Zohuriaan-Mehr, M.J.; Rostami, A. Bio-based thermo-healable non-isocyanate polyurethane DA network in comparison with its epoxy counterpart. J. CO2 Utilization 2017, 18, 294–302. [Google Scholar] [CrossRef]
- Lee, J.H.; Ju, Y.M.; Kim, D.M. Platelet adhesion onto segmented polyurethane film surfaces modfied by addition and crosslinking of PEO-containing block copolymers. Biomaterials 2000, 21, 683–691. [Google Scholar] [CrossRef]
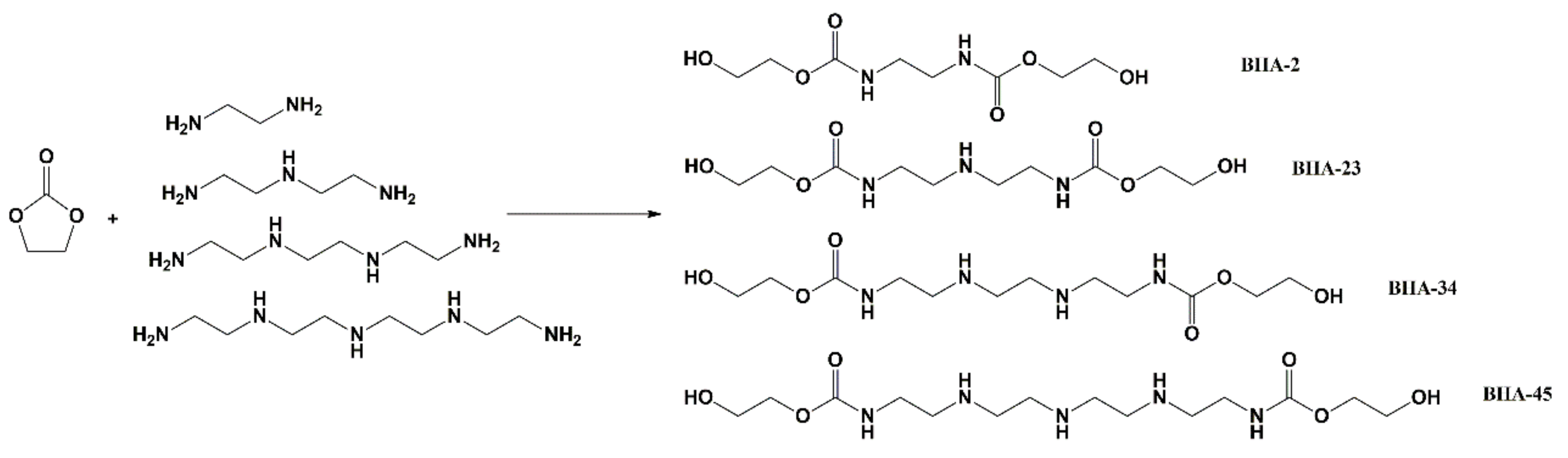

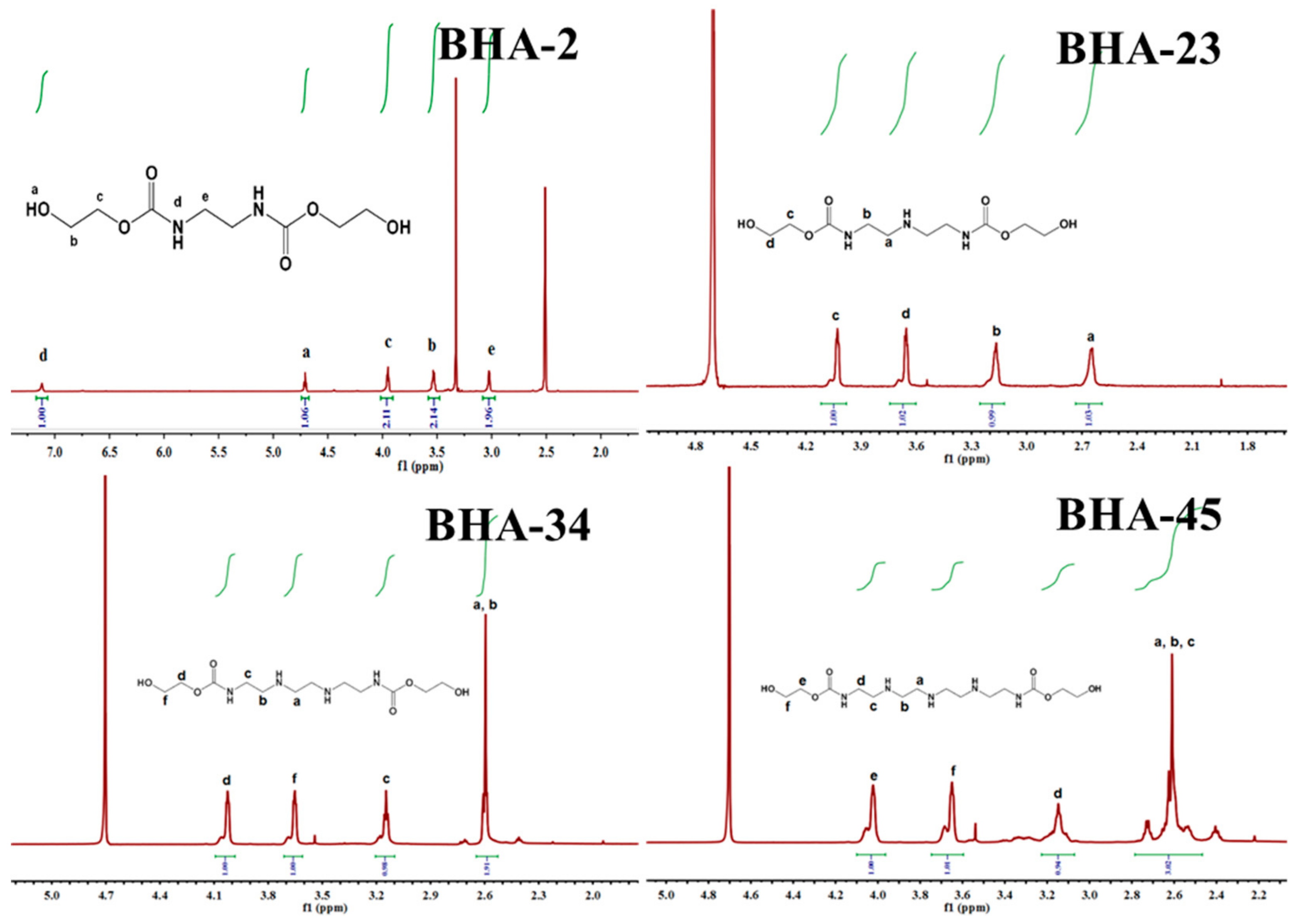



| BHA Type | Amine Type | Mole Ratio (Amine:EC) | Mole Ratio | EC Mass | Amine Mass | BHA Mass | Yield |
|---|---|---|---|---|---|---|---|
| BHA-2 | EDA | 1:2 | 1:1 | 100.0 g | 34.1 g | 127.4 g | 95% |
| BHA-23 | DETA | 1:2 | 1:1 | 100.0 g | 58.0 g | 148.5 g | 94% |
| BHA-34 | TETA | 1:2 | 1:1 | 100.0 g | 82.2 g | 174.9 g | 96% |
| BHA-45 | TEPA | 1:2 | 1:1 | 100.0 g | 118.2 g | 207.3 g | 95% |
| NIPU Type | BHA Type | Mole Ratio (BHA:DA) | BHA Mass | DA Mass | NIPU Mass | Yield |
|---|---|---|---|---|---|---|
| NIPU-2 | BHA-2 | 1:1.05 | 10.0 g | 38.9 g | 43.3 g | 89% |
| NIPU-23 | BHA-23 | 1:1.05 | 10.0 g | 28.0 g | 32.9 g | 87% |
| NIPU-34 | BHA-34 | 1:1.05 | 10.0 g | 17.9 g | 25.0 g | 90% |
| NIPU-45 | BHA-45 | 1:1.05 | 10.0 g | 15.7 g | 21.8 g | 85% |
| HNIPU Type | NIPU Type | EP Type | Mass Ratio (NIPU:EP) | NIPU Mass | EP Mass | HNIPU Mass | Yield |
|---|---|---|---|---|---|---|---|
| HNIPU-2344 | NIPU-23 | E44 | 1:0.7 | 2.0 g | 1.4 g | 3.4 g | 100% |
| HNIPU-2351 | NIPU-23 | E51 | 1:0.7 | 2.0 g | 1.4 g | 3.4 g | 100% |
| HNIPU-3444 | NIPU-34 | E44 | 1:0.7 | 2.0 g | 1.4 g | 3.4 g | 100% |
| HNIPU-3451 | NIPU-34 | E51 | 1:0.7 | 2.0 g | 1.4 g | 3.4 g | 100% |
| HNIPU-4544 | NIPU-45 | E44 | 1:0.7 | 2.0 g | 1.4 g | 3.4 g | 100% |
| HNIPU-4551 | NIPU-45 | E51 | 1:0.7 | 2.0 g | 1.4 g | 3.4 g | 100% |
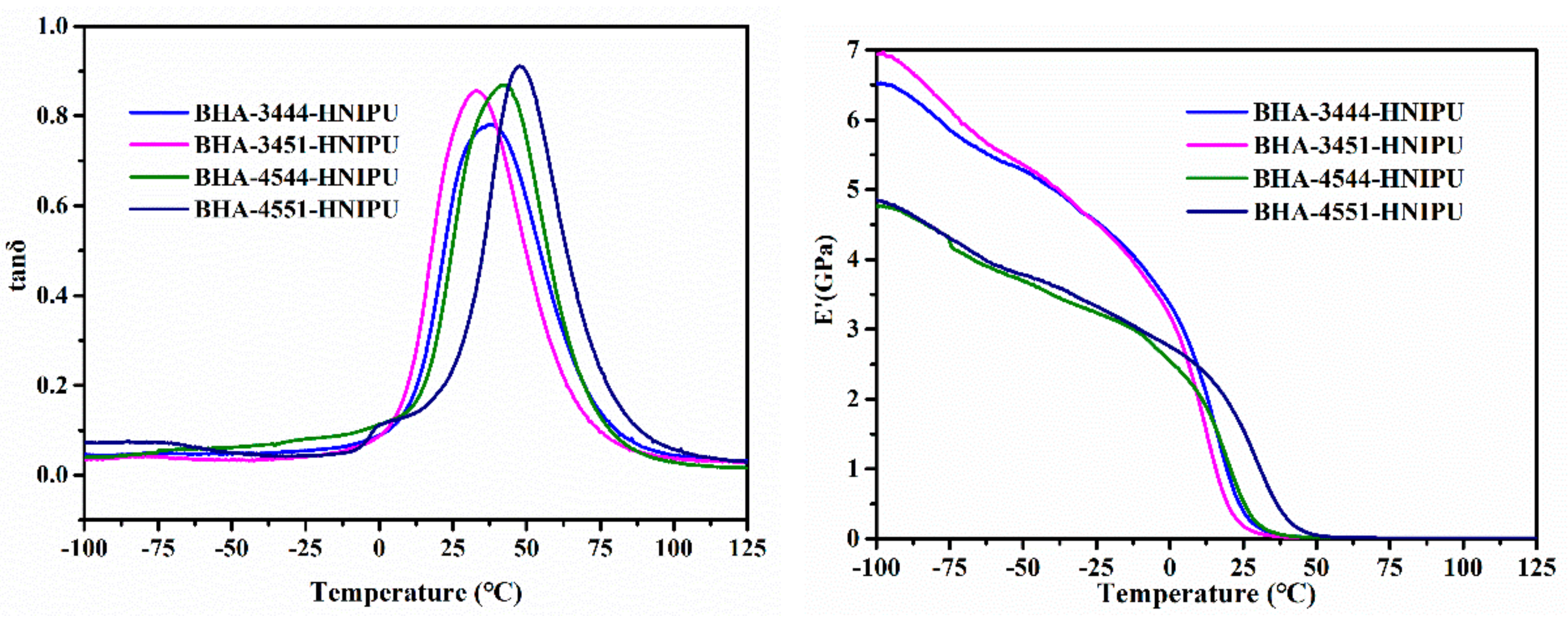
| Sample | Tα (°C) | Tα + 50 (°C) | E’ at Tα+50 (MPa) | νe (mol/m3) |
|---|---|---|---|---|
| BHA-3444-HNIPU | 38.04 | 88.04 | 7.95 | 881.30 |
| BHA-3451-HNIPU | 32.87 | 82.87 | 7.29 | 821.74 |
| BHA-4544-HNIPU | 42.32 | 92.32 | 6.35 | 696.34 |
| BHA-4551-HNIPU | 47.50 | 97.50 | 8.56 | 925.33 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Xu, X.; Wan, Q.; Bo, G.; Yan, Y. Solvent- and Catalyst-free Synthesis, Hybridization and Characterization of Biobased Nonisocyanate Polyurethane (NIPU). Polymers 2019, 11, 1026. https://doi.org/10.3390/polym11061026
He X, Xu X, Wan Q, Bo G, Yan Y. Solvent- and Catalyst-free Synthesis, Hybridization and Characterization of Biobased Nonisocyanate Polyurethane (NIPU). Polymers. 2019; 11(6):1026. https://doi.org/10.3390/polym11061026
Chicago/Turabian StyleHe, Xin, Xiaoling Xu, Qian Wan, Guangxu Bo, and Yunjun Yan. 2019. "Solvent- and Catalyst-free Synthesis, Hybridization and Characterization of Biobased Nonisocyanate Polyurethane (NIPU)" Polymers 11, no. 6: 1026. https://doi.org/10.3390/polym11061026
APA StyleHe, X., Xu, X., Wan, Q., Bo, G., & Yan, Y. (2019). Solvent- and Catalyst-free Synthesis, Hybridization and Characterization of Biobased Nonisocyanate Polyurethane (NIPU). Polymers, 11(6), 1026. https://doi.org/10.3390/polym11061026