Fabrication of Polymer Micelles with Zwitterionic Shell and Biodegradable Core for Reductively Responsive Release of Doxorubicin
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Midifaction of PCL-Diol with Cystamine (Cys-PCL-Cys)
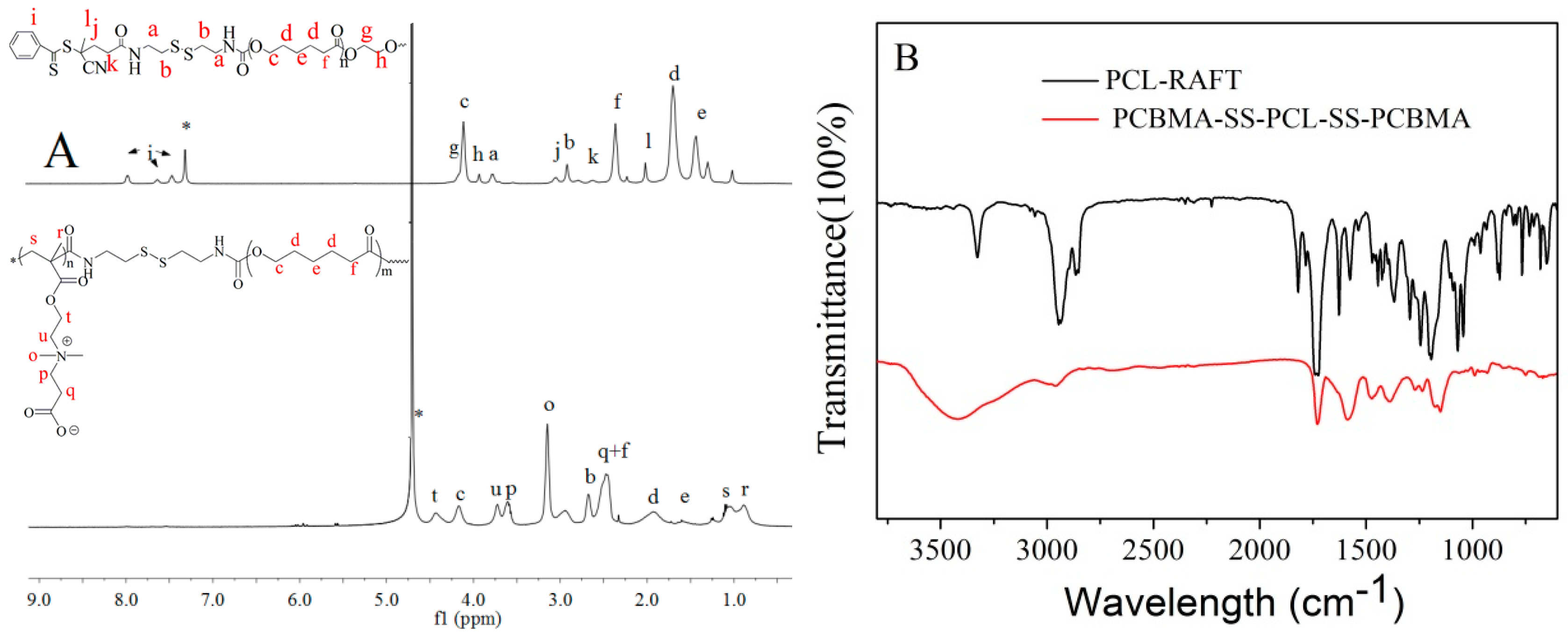
2.3. Synthesis of CPADB-SS-PCL-SS-CPADB Macro-RAFT Agent.
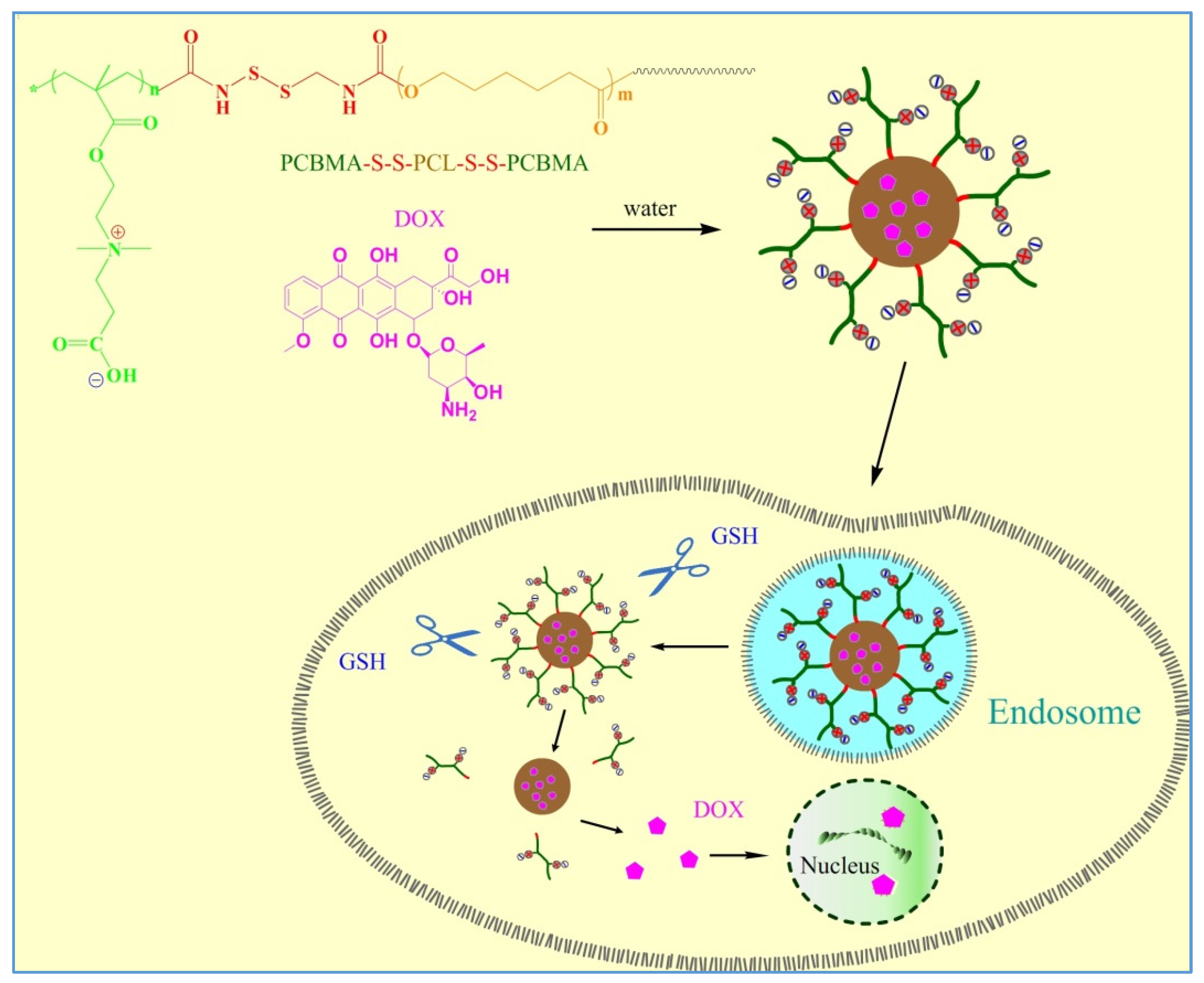
2.4. Synthesis of PCBMA-SS-PCL-SS-PCBMA
2.5. Preparation of PCBMA-SS-PCL-SS-PCBMA Micelles
2.6. DOX Loading and Release
2.7. Cell Uptake Studies
2.8. Cell Viability Assays
3. Results and Discussion
3.1. Polymer Synthesis and Characterization
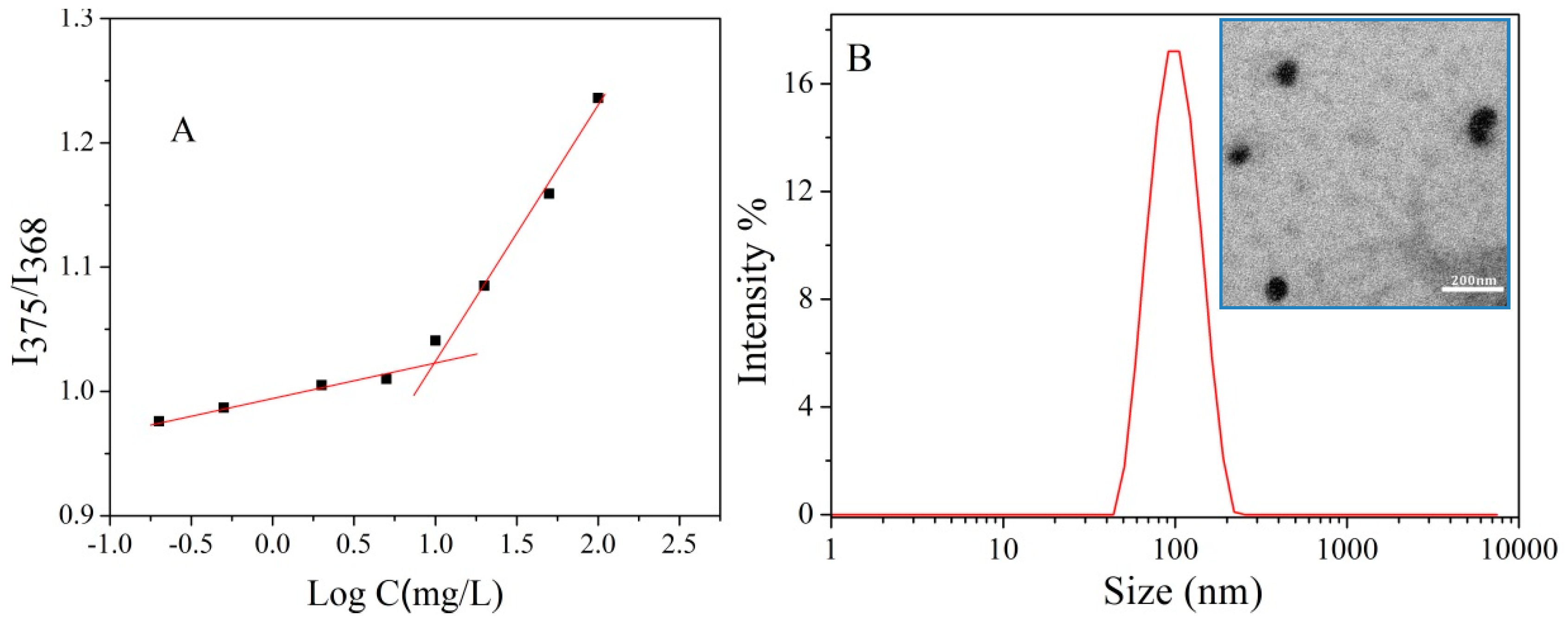
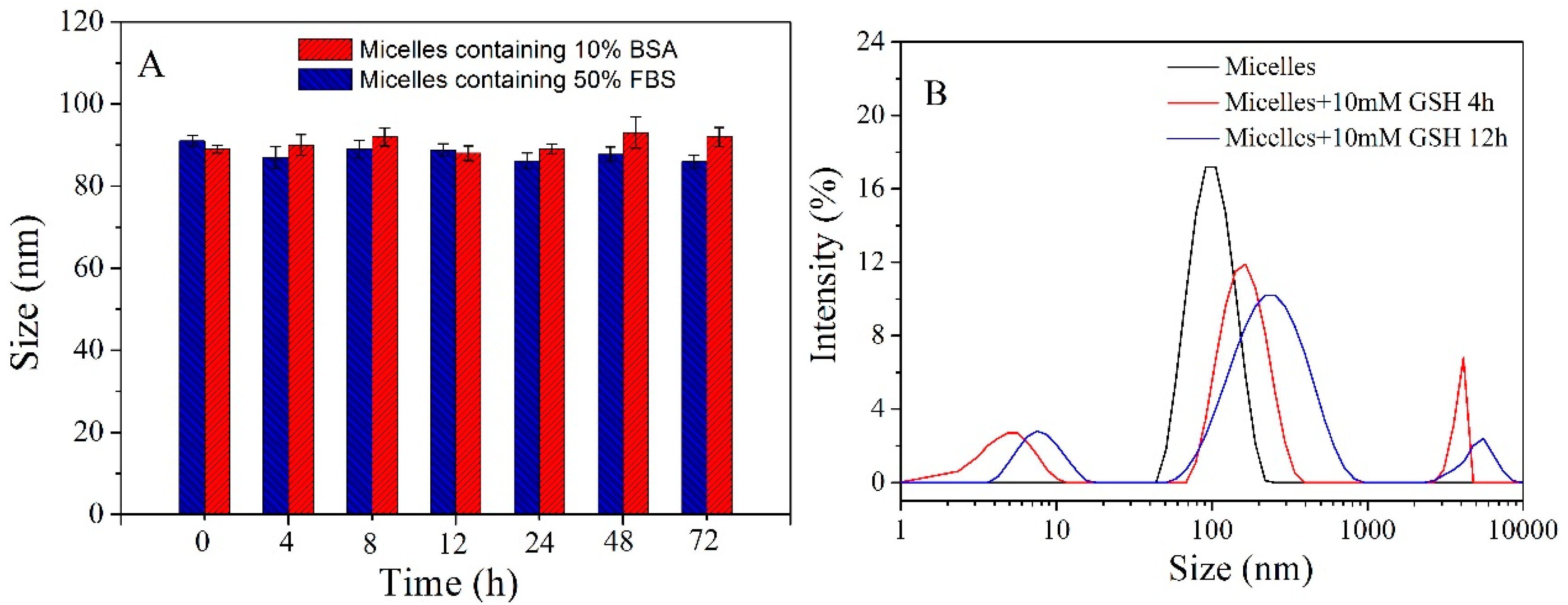
3.2. Characterization of Polymeric Micelles
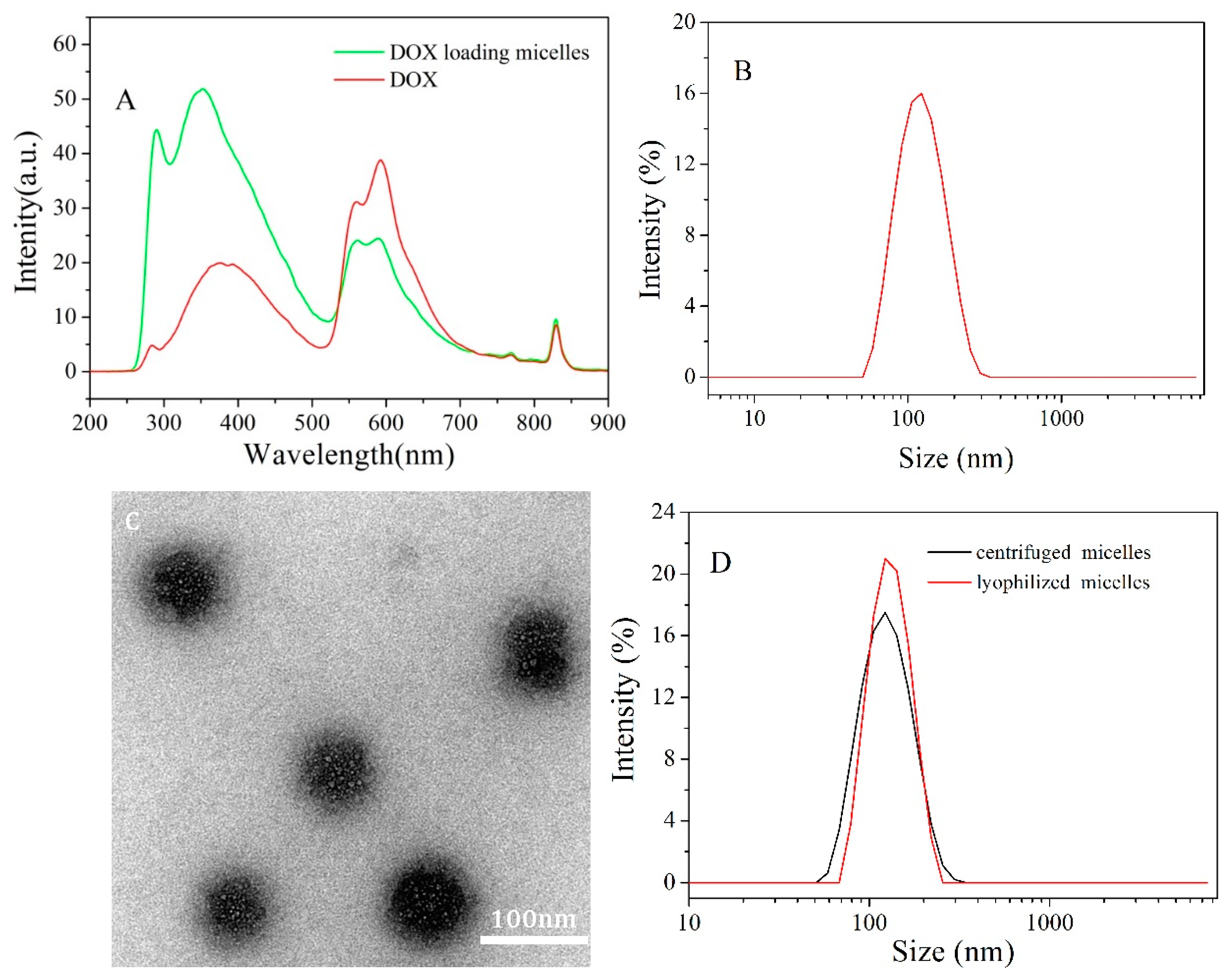
3.3. Characterization of DOX Loaded Micelles
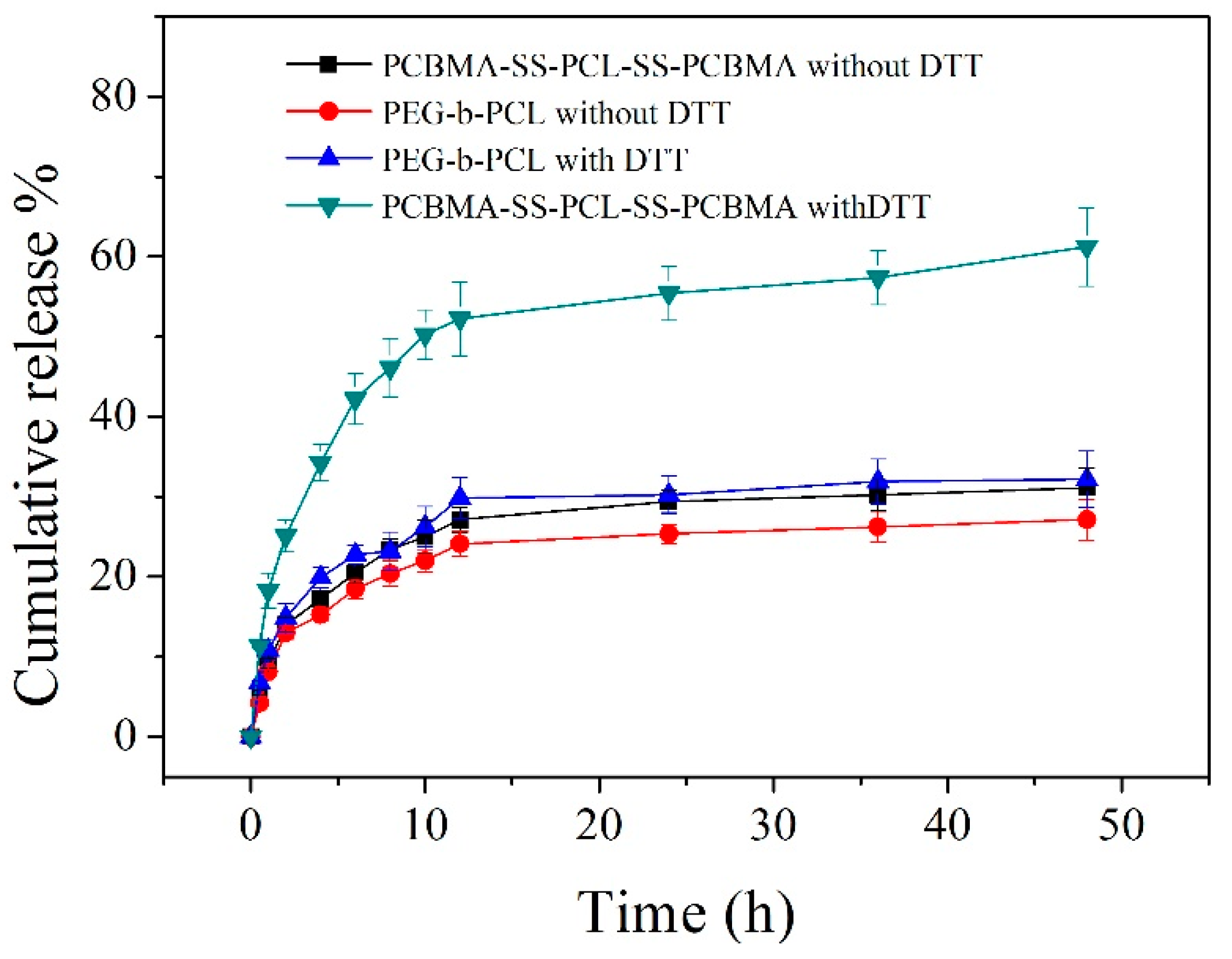
3.4. In-Vitro Drug Release
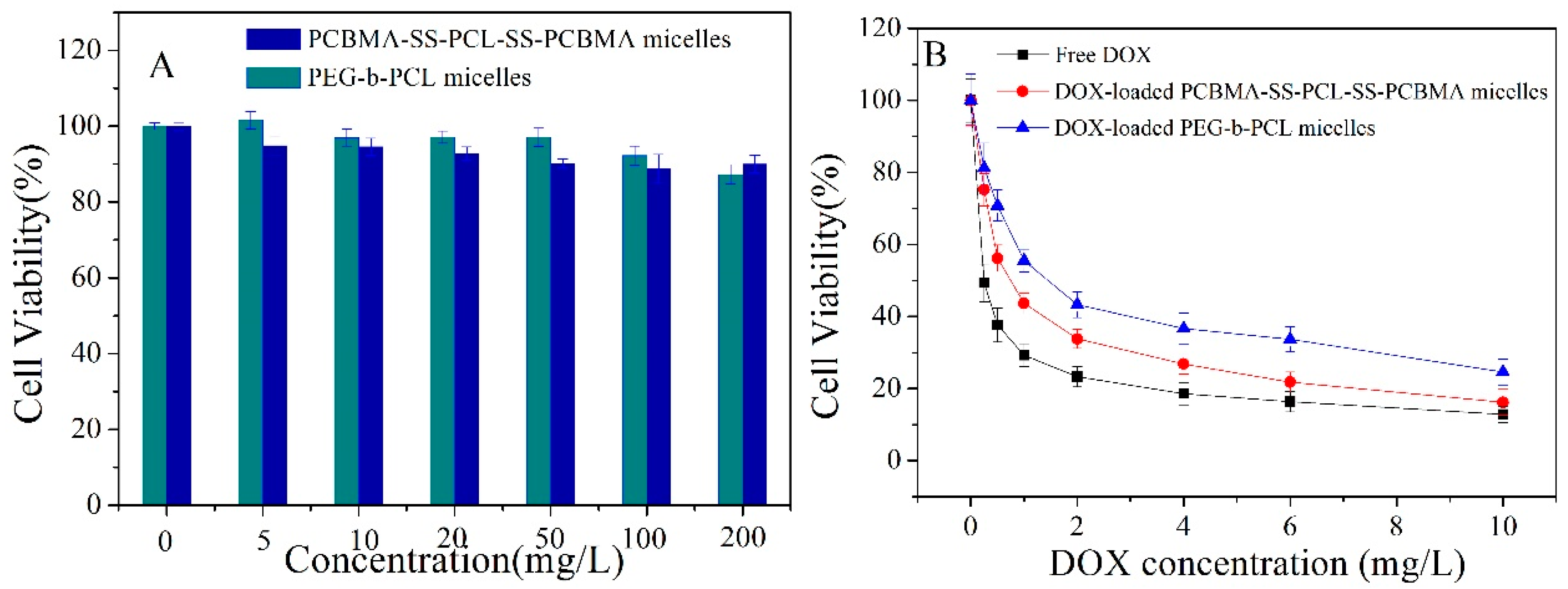
3.5. In Vitro Cytotoxicity and Cell Uptake
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, D.; Poon, C.; Lu, K.; He, C.; Lin, W. Self-assembled nanoscale coordination polymers with trigger release properties for effective anticancer therapy. Nat. Commun. 2014, 5, 4182. [Google Scholar] [CrossRef] [PubMed]
- Poon, C.; He, C.; Liu, D.; Lu, K.; Lin, W. Self-assembled nanoscale coordination polymers carrying oxaliplatin and gemcitabine for synergistic combination therapy of pancreatic cancer. J. Control. Release 2015, 201, 90–99. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Liu, D.; Lin, W. Self-assembled nanoscale coordination polymers carrying siRNAs and cisplatin for effective treatment of resistant ovarian cancer. Biomaterials 2015, 36, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Chunbai, H.; Demin, L.; Wenbin, L. Self-assembled core-shell nanoparticles for combined chemotherapy and photodynamic therapy of resistant head and neck cancers. ACS Nano 2015, 9, 991–1003. [Google Scholar]
- He, C.; Poon, C.; Chan, C.; Yamada, S.D.; Lin, W. Nanoscale Coordination Polymers Codeliver Chemotherapeutics and siRNAs to Eradicate Tumors of Cisplatin-Resistant Ovarian Cancer. J. Am. Chem. Soc. 2016, 138, 6010. [Google Scholar] [CrossRef]
- Li, X.; Yang, Z.; Yang, K.; Zhou, Y.; Chen, X.; Zhang, Y.; Fei, W.; Yan, L.; Ren, L. Self-Assembled Polymeric Micellar Nanoparticles as Nanocarriers for Poorly Soluble Anticancer Drug Ethaselen. Nanoscale Res. Lett. 2009, 4, 1502–1511. [Google Scholar] [CrossRef]
- Xiong, D.; Yao, N.; Gu, H.; Wang, J.; Zhang, L. Stimuli-responsive shell cross-linked micelles from amphiphilic four-arm star copolymers as potential nanocarriers for “pH/redox-triggered” anticancer drug release. Polymer 2017, 114, 161–172. [Google Scholar] [CrossRef]
- Cabral, H.; Kataoka, K. Progress of drug-loaded polymeric micelles into clinical studies. J. Control. Release 2014, 190, 465–476. [Google Scholar] [CrossRef]
- Kwon, G.S.; Kataoka, K. Block copolymer micelles as long-circulating drug vehicles. Adv. Drug Deliv. Rev. 2012, 64, 237–245. [Google Scholar] [CrossRef]
- Deng, C.; Jiang, Y.; Cheng, R.; Meng, F.; Zhong, Z. Biodegradable polymeric micelles for targeted and controlled anticancer drug delivery: Promises, progress and prospects. Nano Today 2012, 7, 467–480. [Google Scholar] [CrossRef]
- Otsuka, H. PEGylated Nanoparticles for Biological and Pharmaceutical Applications. Adv. Drug Deliv. Rev. 2003, 55, 403–419. [Google Scholar] [CrossRef]
- Petros, R.A.; Desimone, J.M. Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 2010, 9, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Lin-Lin, B.; Jun-Hua, X.; Bo, C.; Guang-Tao, Y.; Xiaolei, Y.; Zhaobo, H.; Qinqin, H.; Andrew, L.; Shi-Shang, G. Red Blood Cell Membrane as a Biomimetic Nanocoating for Prolonged Circulation Time and Reduced Accelerated Blood Clearance. Small 2016, 11, 6225–6236. [Google Scholar]
- Ma, J.; Kang, K.; Yi, Q.; Zhang, Z.; Gu, Z. Multiple pH responsive zwitterionic micelles for stealth delivery of anticancer drugs. RSC Adv. 2016, 6, 64778–64790. [Google Scholar] [CrossRef]
- Ou, H.; Cheng, T.; Zhang, Y.; Liu, J.; Ding, Y.; Zhen, J.; Shen, W.; Xu, Y.; Yang, W.; Niu, P. Surface-adaptive zwitterionic nanoparticles for prolonged blood circulation time and enhanced cellular uptake in tumor cells. Acta Biomater. 2018, 65, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chang, M.Y.Z.; Cheng, W.I.; Wang, Q.; Commisso, A.; Capeling, M.; Yun, W.; Chong, C. Biodegradable zwitterionic sulfobetaine polymer and its conjugate with paclitaxel for sustained drug delivery. Acta Biomater. 2017, 64, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Ma, G.; Wu, J.; Chen, S. Different in vitro and in vivo behaviors between Poly(carboxybetaine methacrylate) and poly(sulfobetaine methacrylate). Colloids Surf. B Biointerfaces 2016, 146, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Ma, Y.; Sun, C.; Lu, Z.; Yao, Z.; Wang, J.X.; Li, D.; Yuan, Y.; Yang, X. ROS-Sensitive Polymeric Nanocarriers with Red Light-Activated Size Shrinkage for Remotely Controlled Drug Release. Chem. Mater. 2017, 30, 517–525. [Google Scholar] [CrossRef]
- Adams, M.L.; Lavasanifar, A.; Kwon, G.S. Amphiphilic block copolymers for drug delivery. J. Pharm. Sci. 2010, 92, 1343–1355. [Google Scholar] [CrossRef]
- Zhiqiang, C.; Qiuming, Y.; Hong, X.; Gang, C.; Shaoyi, J. Nanoparticles for drug delivery prepared from amphiphilic PLGA zwitterionic block copolymers with sharp contrast in polarity between two blocks. Angew. Chem. 2010, 122, 3859–3864. [Google Scholar]
- Zhu, X.; Fryd, M.; Wayland, B.B. Kinetic-mechanistic studies of lipase-polymer micelle binding and catalytic degradation: Enzyme interfacial activation. Polym. Degrad. Stab. 2013, 98, 1173–1181. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, C.; Wang, F.; Ying, A.; Xu, C.; Liu, S. Fabrication of biofunctional complex micelles with tunable structure for application in controlled drug release. Colloid Polym. Sci. 2014, 292, 1675–1683. [Google Scholar] [CrossRef]
- Staff, R.H.; Gallei, M.; Landfester, K.; Crespy, D. Hydrophobic Nanocontainers for Stimulus-Selective Release in Aqueous Environments. Macromolecules 2014, 47, 4876–4883. [Google Scholar] [CrossRef]
- Lallana, E.; Tirelli, N. Oxidation-Responsive Polymers: Which Groups to Use, How to Make Them, What to Expect from Them (Biomedical Applications). Macromol. Chem. Phys. 2013, 214, 143–158. [Google Scholar] [CrossRef]
- Li-Ping, L.; Yi, Z.; Nicole, V.; Markus, G.; Ashokanand, V.; Michael, R.; Katharina, L.; Daniel, C. Redox responsive release of hydrophobic self-healing agents from polyaniline capsules. J. Am. Chem. Soc. 2013, 135, 14198–14205. [Google Scholar]
- Scheid, D.; Von, D.L.M.; Gallei, M. Synthesis of Breathing Metallopolymer Hollow Spheres for Redox-Controlled Release. Macromol. Rapid Commun. 2016, 37, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Staff, R.H.; Gallei, M.; Mazurowski, M.; Rehahn, M.; Berger, R.; Landfester, K.; Crespy, D. Patchy nanocapsules of poly(vinylferrocene)-based block copolymers for redox-responsive release. ACS Nano 2012, 6, 9042. [Google Scholar] [CrossRef]
- Marzbali, M.Y.; Khosroushahi, A.Y. Polymeric micelles as mighty nanocarriers for cancer gene therapy: A review. Cancer Chemother. Pharmacol. 2017, 79, 637–649. [Google Scholar] [CrossRef]
- Ju, Y.C.; Thapa, R.K.; Yong, C.S.; Kim, J.O. Nanoparticle-based combination drug delivery systems for synergistic cancer treatment. J. Pharm. Investig. 2016, 46, 1–15. [Google Scholar]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- Gulfam, M.; Sahle, F.F.; Lowe, T.L. Design strategies for chemical-stimuli-responsive programmable nanotherapeutics. Drug Discov. Today 2019, 24, 129–147. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.W.; Du, Y.Z.; Liu, N.; Liu, X.; Meng, T.T.; Cheng, B.L.; He, J.B.; You, J.; Yuan, H.; Hu, F.Q. Selective redox-responsive drug release in tumor cells mediated by chitosan based glycolipid-like nanocarrier. J. Control. Release 2015, 206, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Huanli, S.; Bingnan, G.; Xiaoqing, L.; Ru, C.; Fenghua, M.; Haiyan, L.; Zhiyuan, Z. Shell-sheddable micelles based on dextran-SS-poly(epsilon-caprolactone) diblock copolymer for efficient intracellular release of doxorubicin. Biomacromolecules 2010, 11, 848–854. [Google Scholar]
- Davoodi, P.; Srinivasan, M.P.; Wang, C.-H. Synthesis of intracellular reduction-sensitive amphiphilic polyethyleneimine and poly(ε-caprolactone) graft copolymer for on-demand release of doxorubicin and p53 plasmid DNA. Acta Biomater. 2016, 39, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Shun-Li, W.; Chi-Hsiang, C.; Shan-Yang, L. PH-dependent coordination of metal-lisinopril complex investigated by attenuated total reflection/Fourier transform infrared spectroscopy. Chem. Pharm. Bull. 2002, 50, 78. [Google Scholar]
- Neagu, V.; Vasiliu, S.; Racovita, S. Adsorption studies of some inorganic and organic salts on new zwitterionic ion exchangers with carboxybetaine moieties. Chem. Eng. J. 2010, 162, 965–973. [Google Scholar] [CrossRef]
- Torchilin, V.P. Structure and design of polymeric surfactant-based drug delivery systems. J. Control. Release 2001, 73, 137–172. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.; Li, Y.K.; Wang, C.Q. Reduction and pH dual-responsive nanoparticles based chitooligosaccharide-based graft copolymer for doxorubicin delivery. Colloids Surf. A Physicochem. Eng. Asp. 2016, 497, 8–15. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Huang, Y.; Gao, F.; Fang, X. In Vivo Biodistribution and Anti-Tumor Efficacy Evaluation of Doxorubicin and Paclitaxel-Loaded Pluronic Micelles Decorated with c(RGDyK) Peptide. PLoS ONE 2016, 11, e0149952. [Google Scholar] [CrossRef]
- Cheng, J.; Teply, B.A.; Sherifi, I.; Sung, J.; Luther, G.; Gu, F.X.; Levy-Nissenbaum, E.; Radovic-Moreno, A.F.; Langer, R.; Farokhzad, O.C. Formulation of functionalized PLGA–PEG nanoparticles for in vivo targeted drug delivery. Biomaterials 2007, 28, 869–876. [Google Scholar] [CrossRef]
- Simona, M.; Julien, N.; Patrick, C. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar]
- Tewey, K.M.; Rowe, T.C.; Yang, L.; Halligan, B.D.; Liu, L.F. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science 1984, 226, 466–468. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Duan, J.; Wang, Y.; Zhang, Y.; Guo, Y.; Guo, H.; Kang, X. Nanopore Single-Molecule Analysis of DNA–Doxorubicin Interactions. Anal. Chem. 2015, 87, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Xianzheng, Z. Design and development of polymeric micelles with cleavable links for intracellular drug delivery. Prog. Polym. Sci. 2013, 38, 503–535. [Google Scholar]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Li, J.; Zhou, B.; Niu, C.; Wang, W.; Wu, W.; Liang, J. Fabrication of Polymer Micelles with Zwitterionic Shell and Biodegradable Core for Reductively Responsive Release of Doxorubicin. Polymers 2019, 11, 1019. https://doi.org/10.3390/polym11061019
Jiang J, Li J, Zhou B, Niu C, Wang W, Wu W, Liang J. Fabrication of Polymer Micelles with Zwitterionic Shell and Biodegradable Core for Reductively Responsive Release of Doxorubicin. Polymers. 2019; 11(6):1019. https://doi.org/10.3390/polym11061019
Chicago/Turabian StyleJiang, Junting, Junbo Li, Biyu Zhou, Chaohuang Niu, Wendi Wang, Wenlan Wu, and Ju Liang. 2019. "Fabrication of Polymer Micelles with Zwitterionic Shell and Biodegradable Core for Reductively Responsive Release of Doxorubicin" Polymers 11, no. 6: 1019. https://doi.org/10.3390/polym11061019
APA StyleJiang, J., Li, J., Zhou, B., Niu, C., Wang, W., Wu, W., & Liang, J. (2019). Fabrication of Polymer Micelles with Zwitterionic Shell and Biodegradable Core for Reductively Responsive Release of Doxorubicin. Polymers, 11(6), 1019. https://doi.org/10.3390/polym11061019




