Surface Modification by Polyzwitterions of the Sulfabetaine-Type, and Their Resistance to Biofouling
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthetic Methods and Procedures
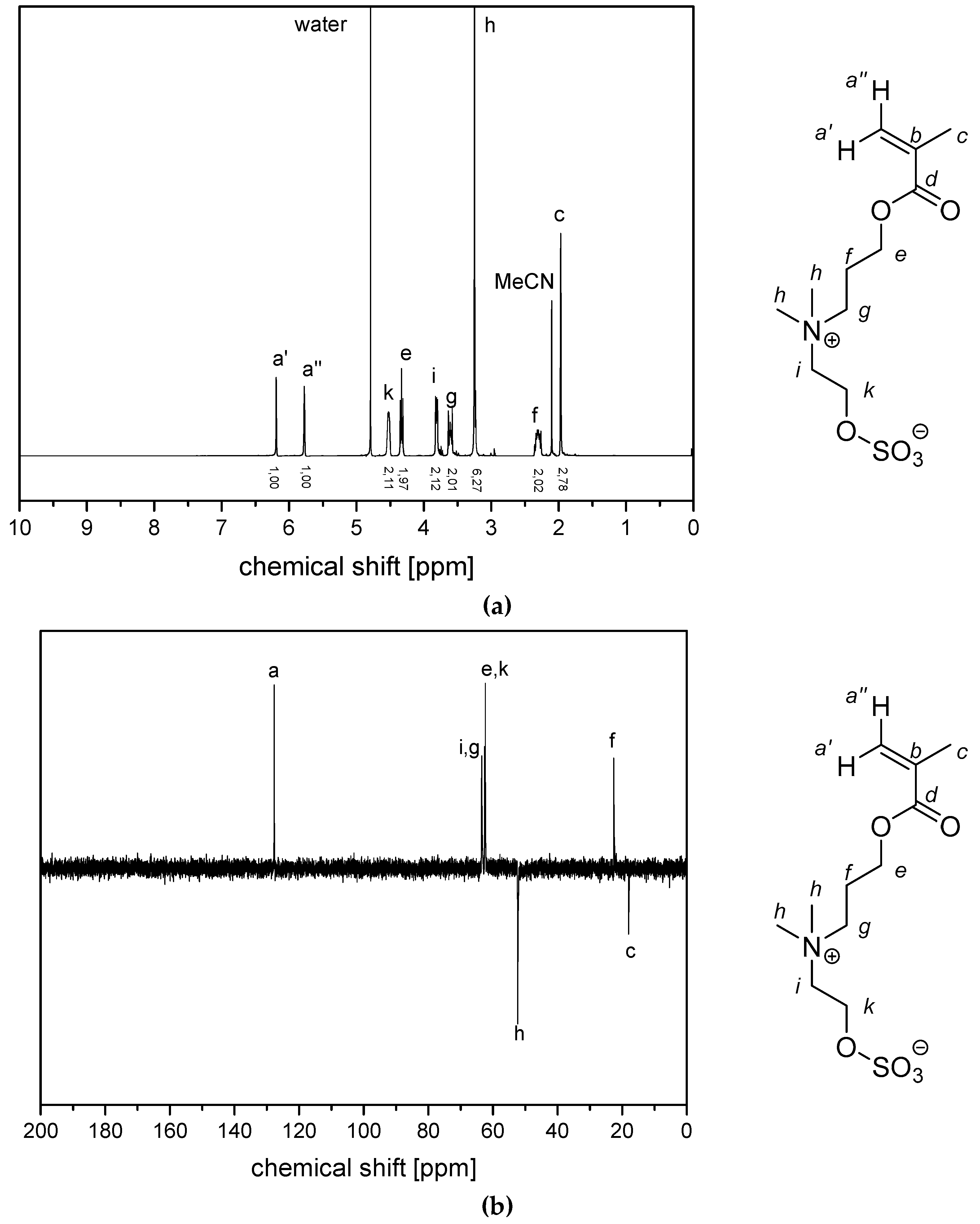
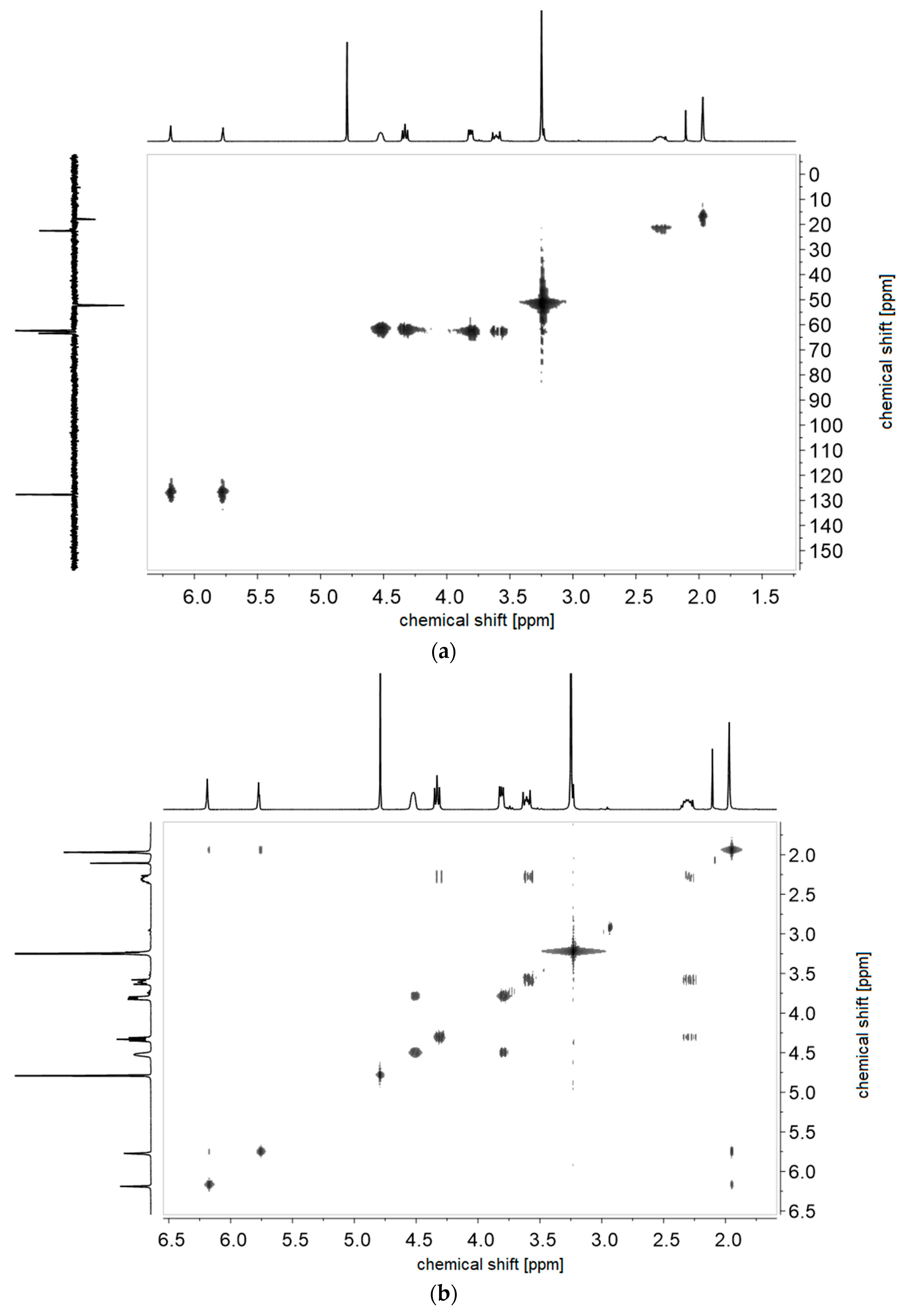
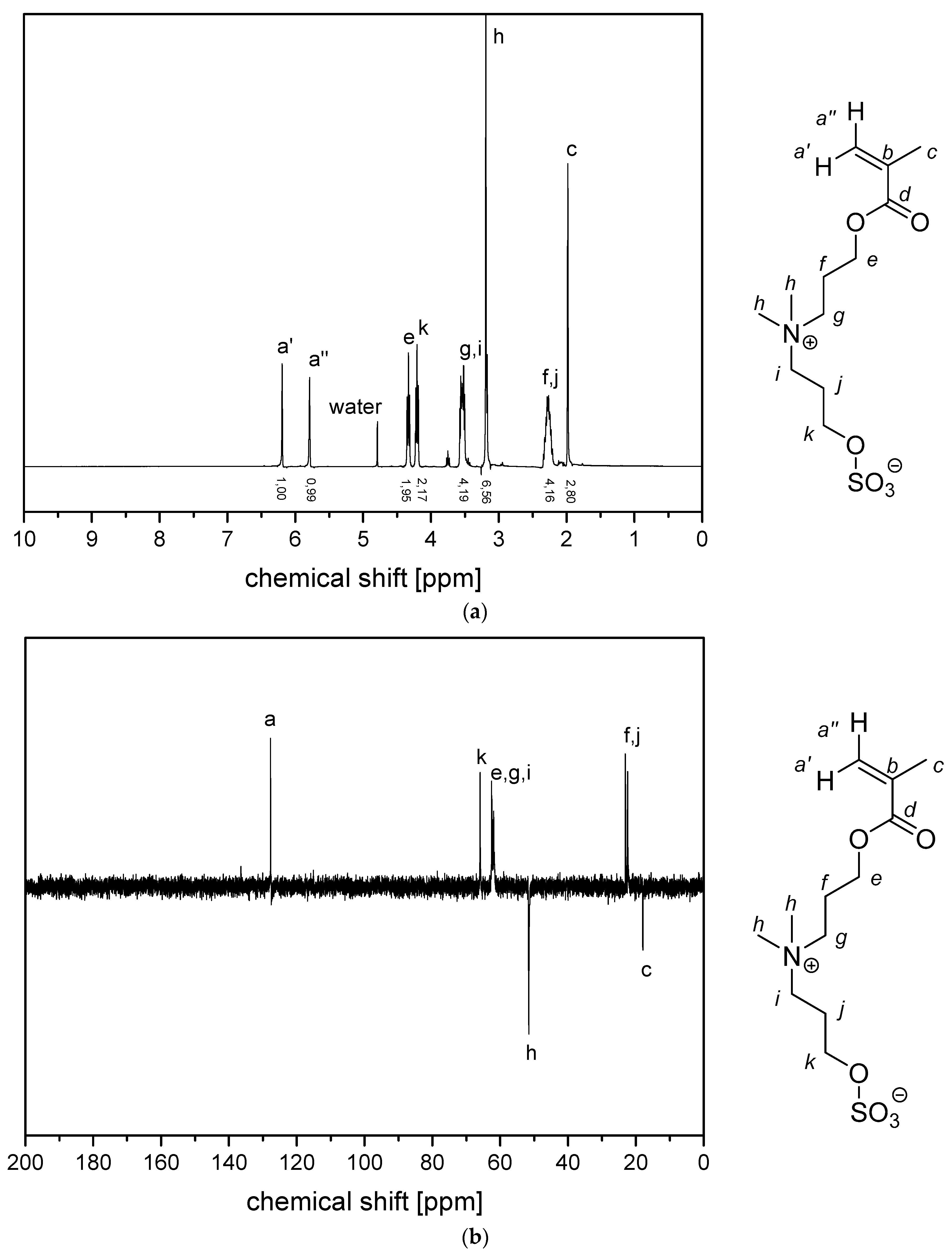
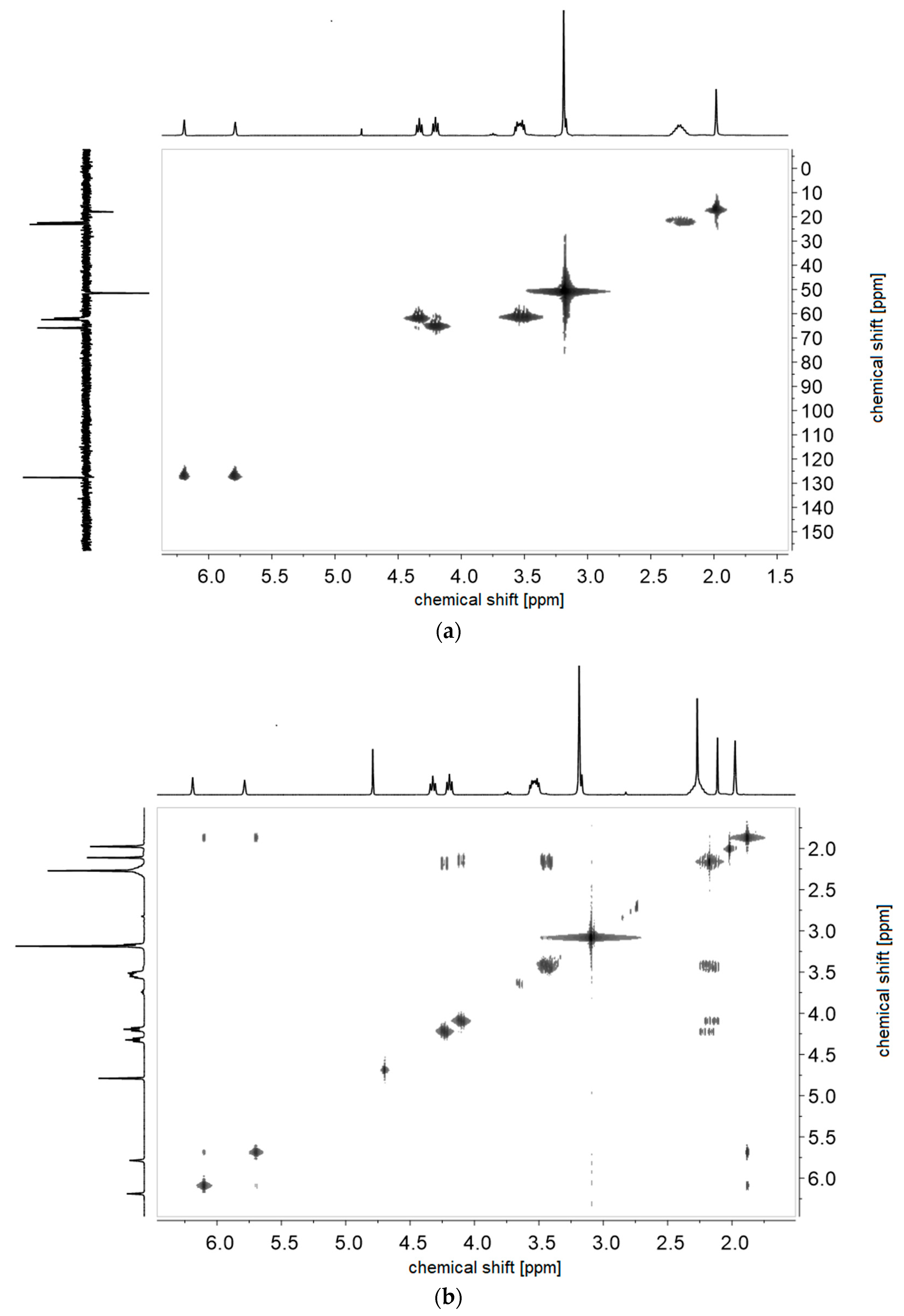
2.2.1. Synthesis of Sulfabetaine Monomers
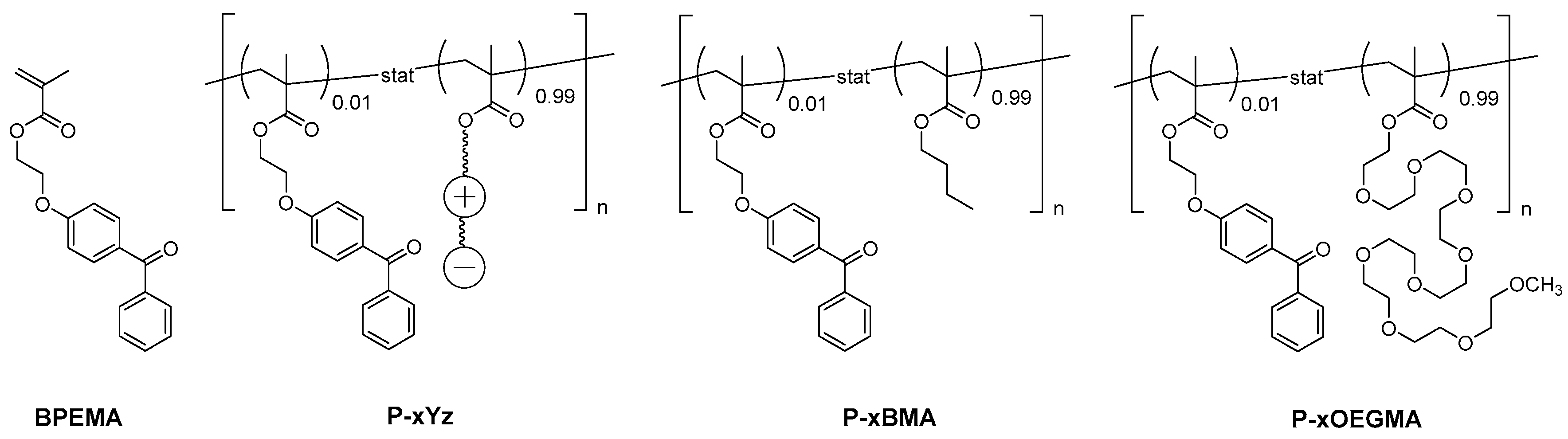
2.2.2. Polymer Synthesis
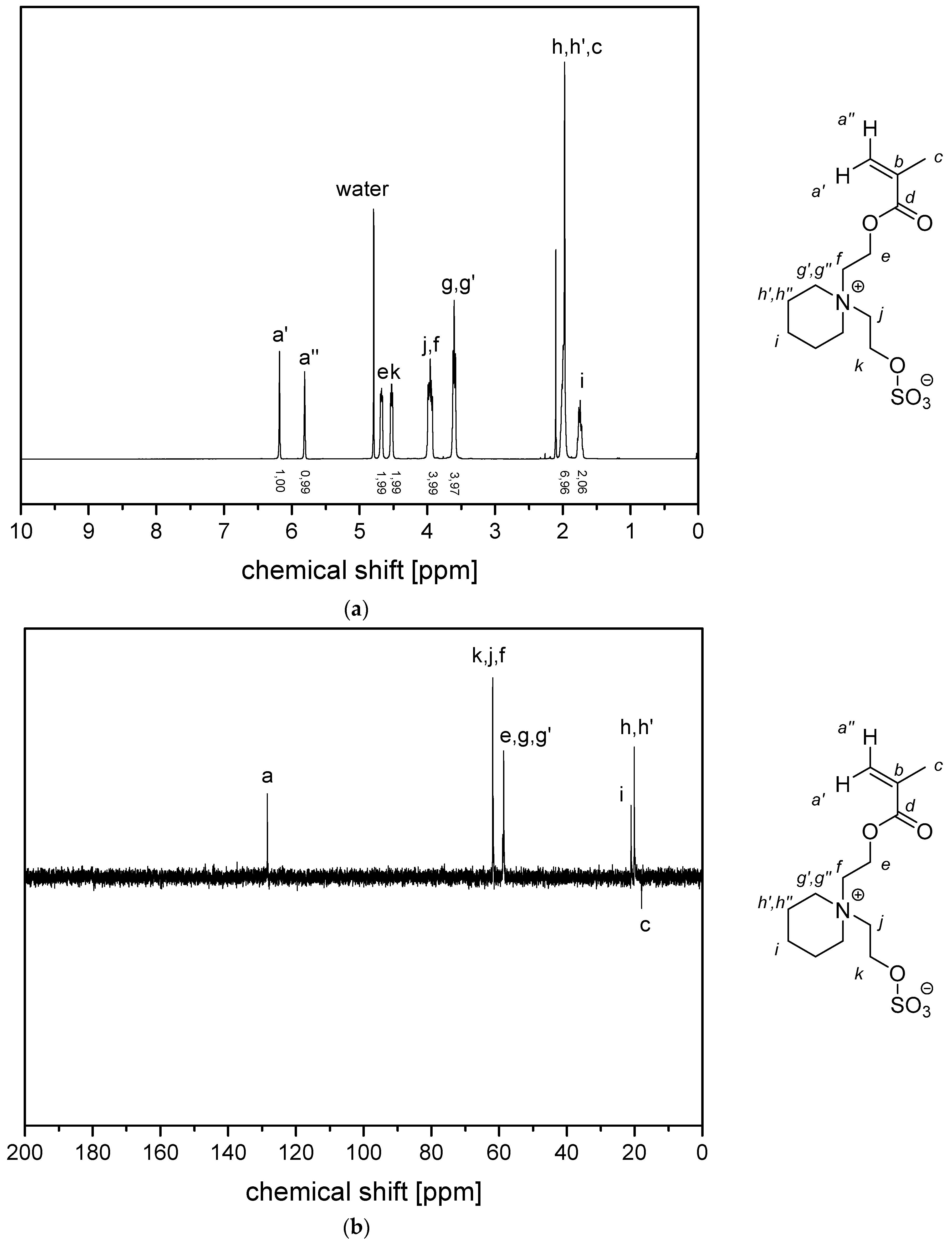
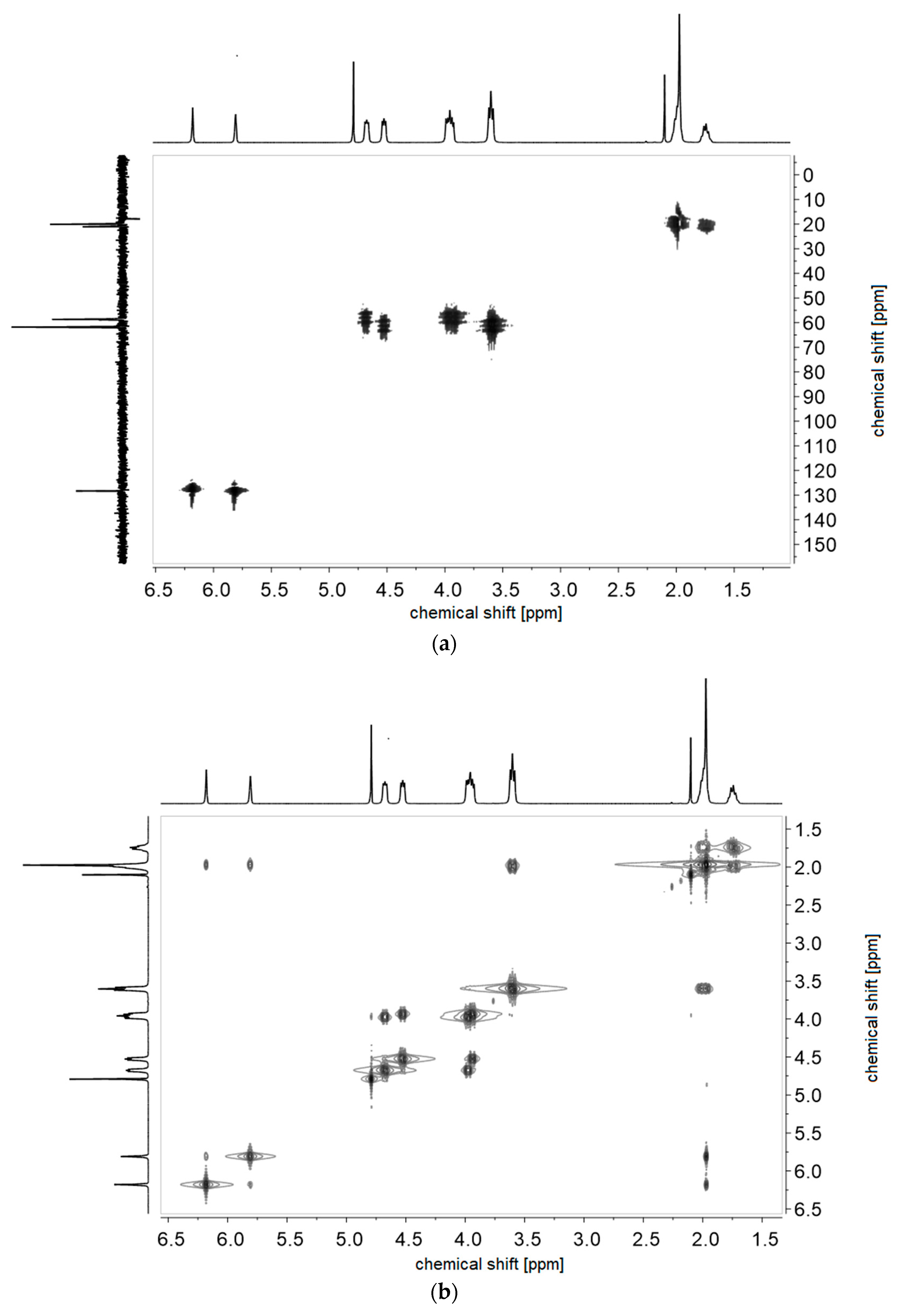
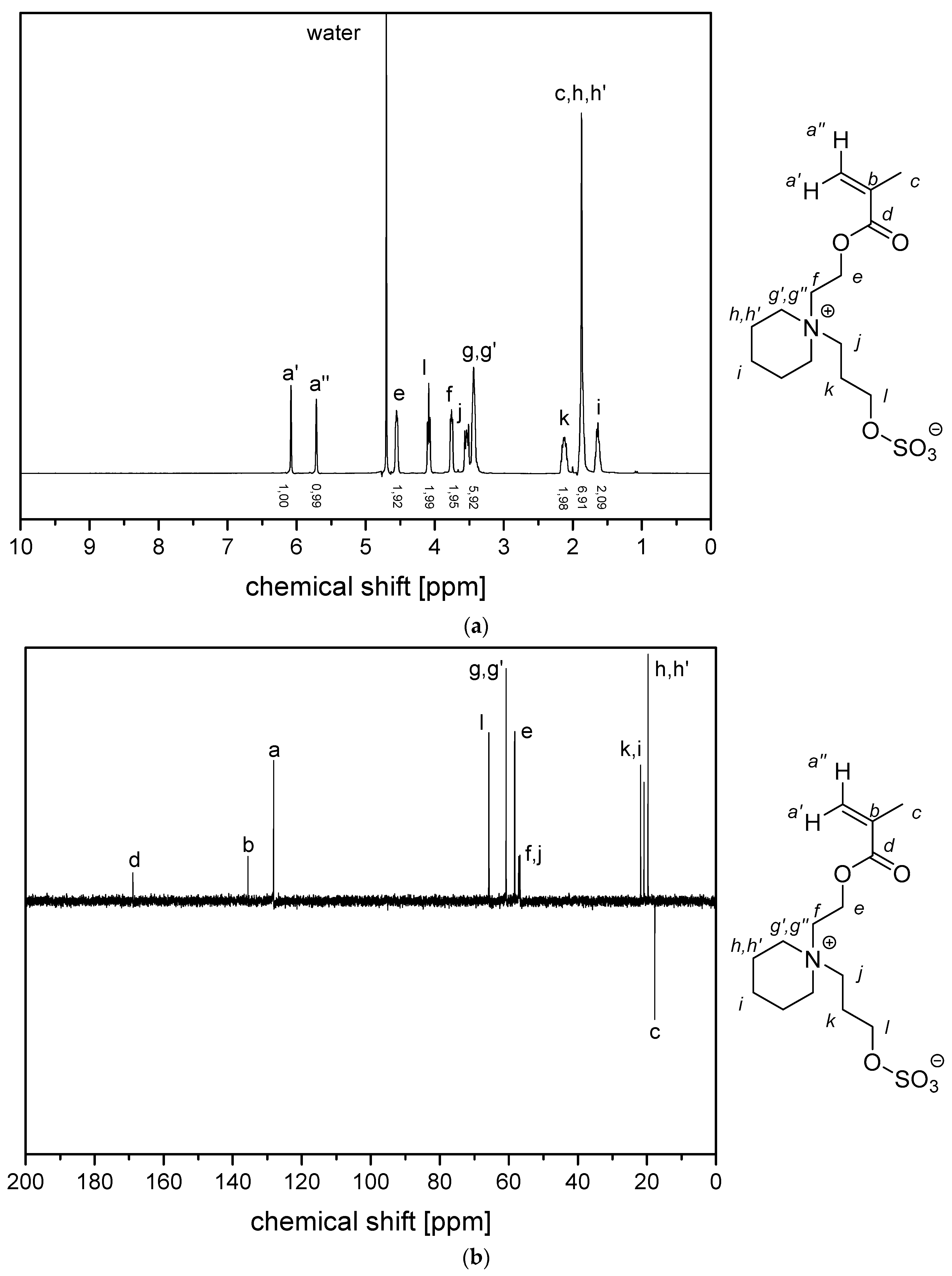
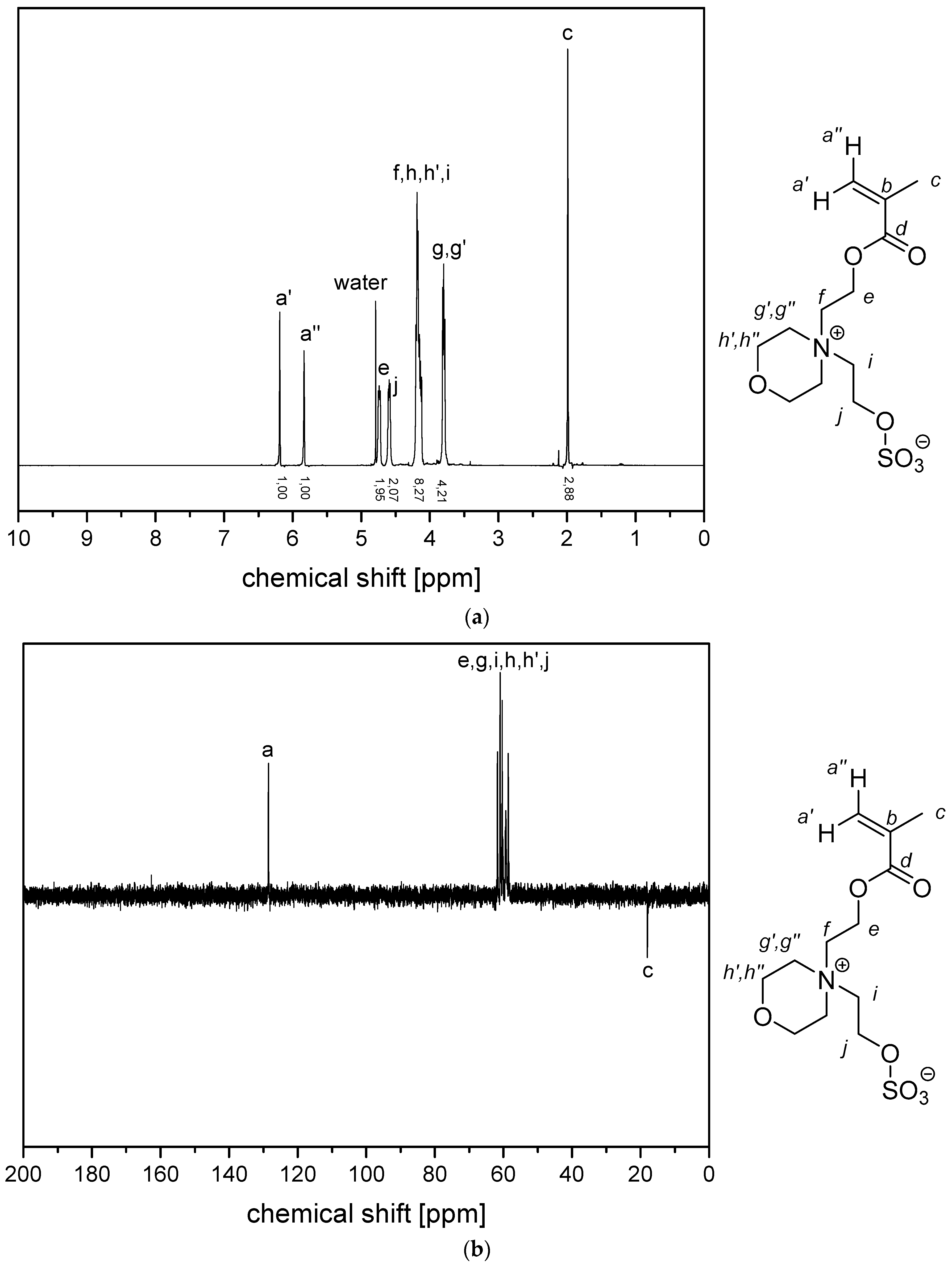
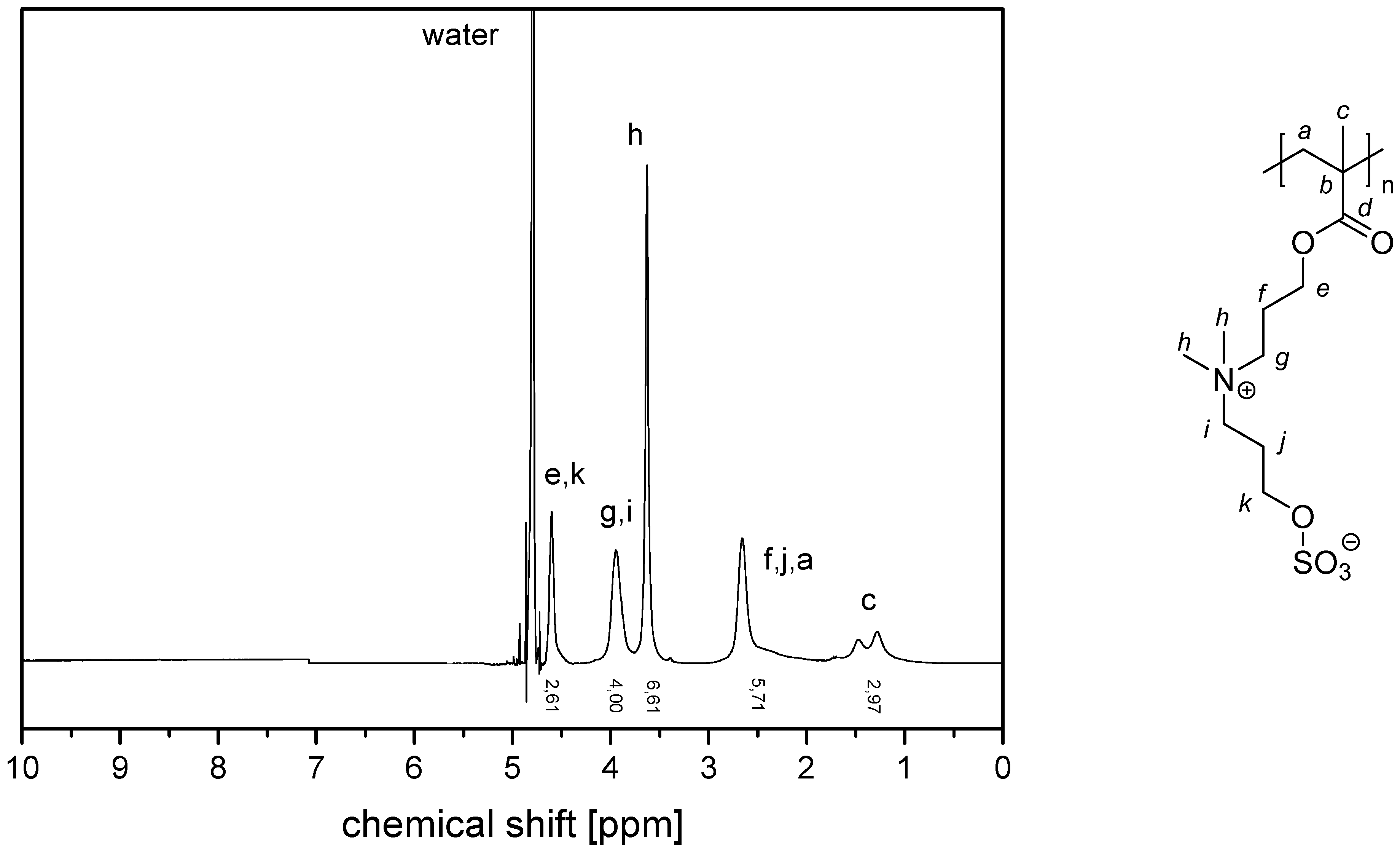
- P−x2c:1H NMR (300 MHz, D2O (saturated with NaCl)) δ = 5.1–4.5 (br, 4H, k + e), 4.4–4.1 (br, 2H, i), 4.1–3.9 (br, 2H, g), 3.9–3.5 (br, 6H, h), 3.1–1.6 (br, 7H, f + a), 1.9–0.6 (br, 3H, c).Elemental analysis (C11H21NO6S, MCRU = 295.11 g∙mol−1): Calculated: C = 44.73%, H = 7.17%, N = 4.74%, S = 10.85%, O = 32.50%; found: C = 42.81%, H = 7.61%, N = 4.49%, S = 10.65%.FT-IR (selected bands, cm−1): 2972, 1727, 1965, 1491, 1263, 1232, 1160, 1068, and 1035.
- P−x2d:1H NMR (300 MHz, D2O (saturated with NaCl)) δ = 4.7–4.4 (br, 4H, e + k), 4.1–3.8 (br, 4H, g + i), 3.7–3.5 (br, 6H, h), 2.9–2.0 (br, 6H, f + j + a), 1.8–1.0 (br, 3H, c).Elemental analysis (C12H23NO6S, MCRU = 309.12 g∙mol−1): Calculated: C = 46.59%, H = 7.49%, N = 4.53%, S = 10.36%, O = 31.03%; found: C = 42.33%, H = 8.06%, N = 4.11%, S = 9.76%.FT-IR (selected bands, cm−1): 2967, 2894, 1721, 1640, 1484, 1220, 1161, 1065, and 1027.
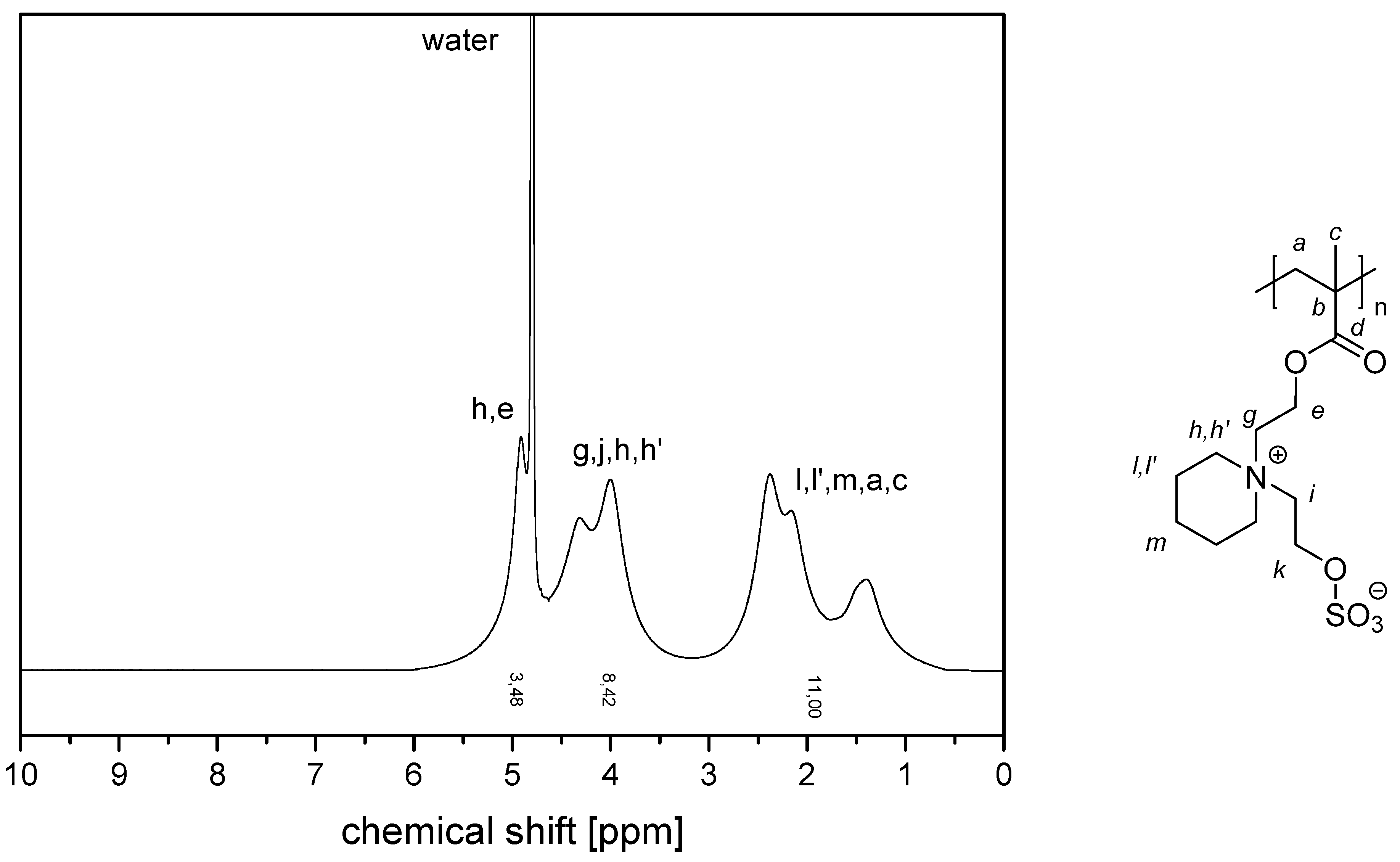
- P−x4c:1H NMR (300 MHz, D2O (saturated with NaCl)) δ = 5.3–4.9 (br, 4H, k + e), 4.6–3.2 (br, 8H, g + i + h‘ + h‘‘), 3.2–0.6 (br, 11H, l‘ + l‘‘ + m + a + c).Elemental analysis (C13H23NO6S, MCRU = 321.12 g∙mol−1): Calculated: C = 48.58%, H = 7.21%, N = 4.36%, S = 9.98%, O = 29.87%; found: C = 45.39%, H = 7.56%, N = 4.09%, S = 9.70%.FT-IR (selected bands, cm−1): 2959, 1729, 1647, 1484, 1263, 1229, 1149, and 1031.
- P−x4d:1H NMR (300 MHz, D2O (saturated with NaCl)) δ = 5.9–3.3 (br, e + g + i + k + h‘ + h‘‘), 3.3–0.2 (br, a + c + l‘ + l‘‘ + m + j).Elemental analysis (C14H25NO6S, MCRU = 335.14 g∙mol−1): Calculated: C = 50.13%, H = 7.51%, N = 4.18%, S = 9.56%, O = 28.62%; found: C = 45.48%, H = 7.68%, N = 3.71%, S = 8.45%.FT-IR (selected bands, cm−1): 2954, 1725, 1643, 1463, 1222, 1176, 1149, 1061, and 1030.
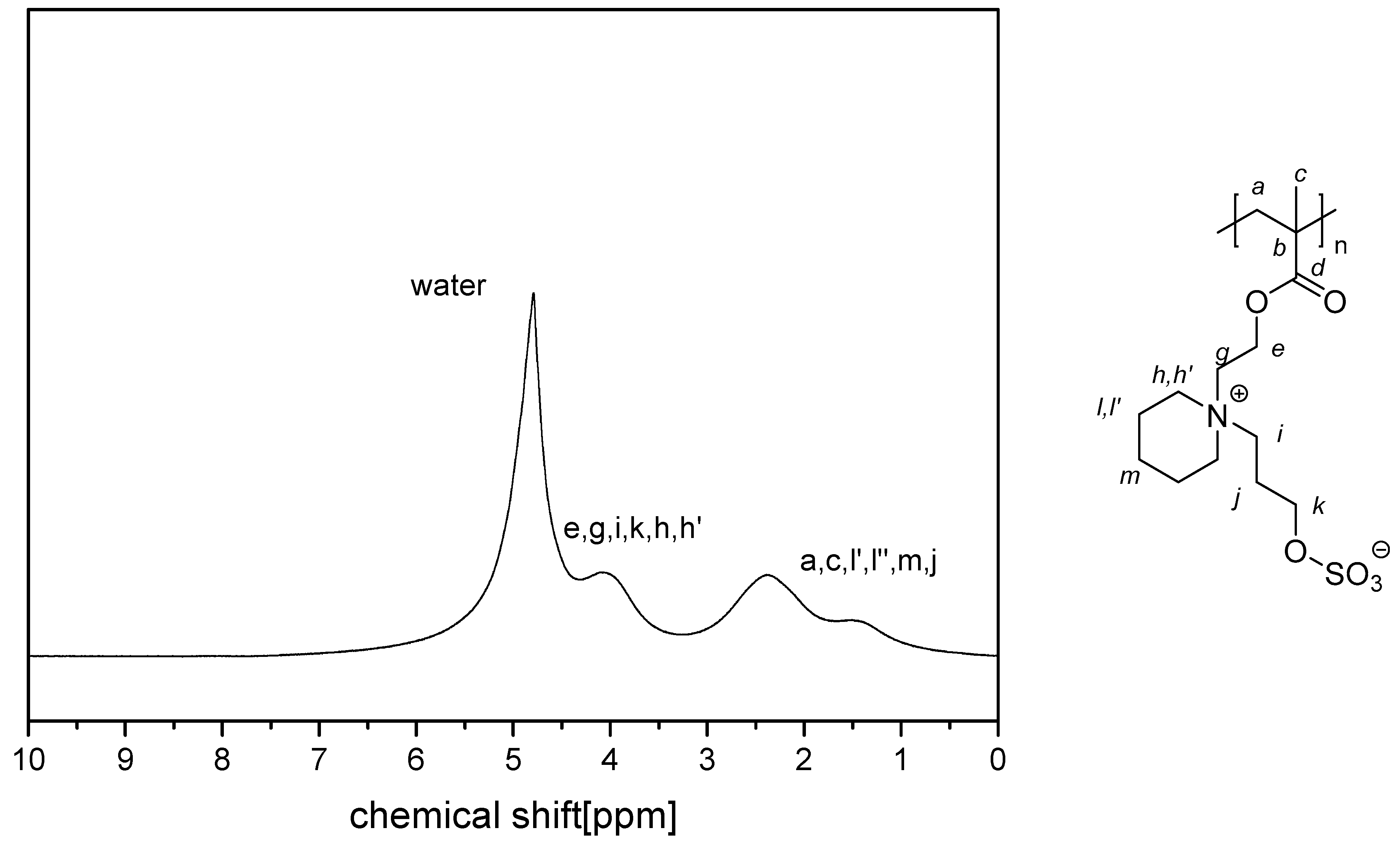
- P−x5c:Insufficient solubility in D2O, D2O saturated with NaCl, or standard deuterated solvents for taking 1H NMR spectra.Elemental analysis (C12H21NO7S, MCRU = 323.10 g∙mol−1): Calculated: C = 44.57%, H = 6.55%, N = 4.33%, S = 9.91%, O = 34.63%; found: C = 41.90%, H = 7.04%, N = 4.09%, S = 9.62%.FT-IR (selected bands, cm−1): 2994, 2891, 1728, 1639, 1480, 1264, 1231, 1132, and 1026.
- P−x5d:Insufficient solubility in D2O, D2O saturated with NaCl, or standard deuterated solvents for taking 1H NMR spectra.Elemental analysis (C13H23NO7S, MCRU = 337.12 g∙mol−1): Calculated: C = 46.28%, H = 6.87%, N = 4.15%, S = 9.50%, O = 33.19%; found: C = 42.52%, H = 7.12%, N = 3.80%, S = 9.09%.FT-IR (selected bands, cm−1): 2969, 2897, 1728, 1638, 1481, 1259, 1226, 1129, 1065, 1024.
2.3. Instrumentation and Methods
2.4. Film Preparation and Protein Adsorption Studies
3. Results and Discussion
3.1. Monomer and Polymer Synthesis
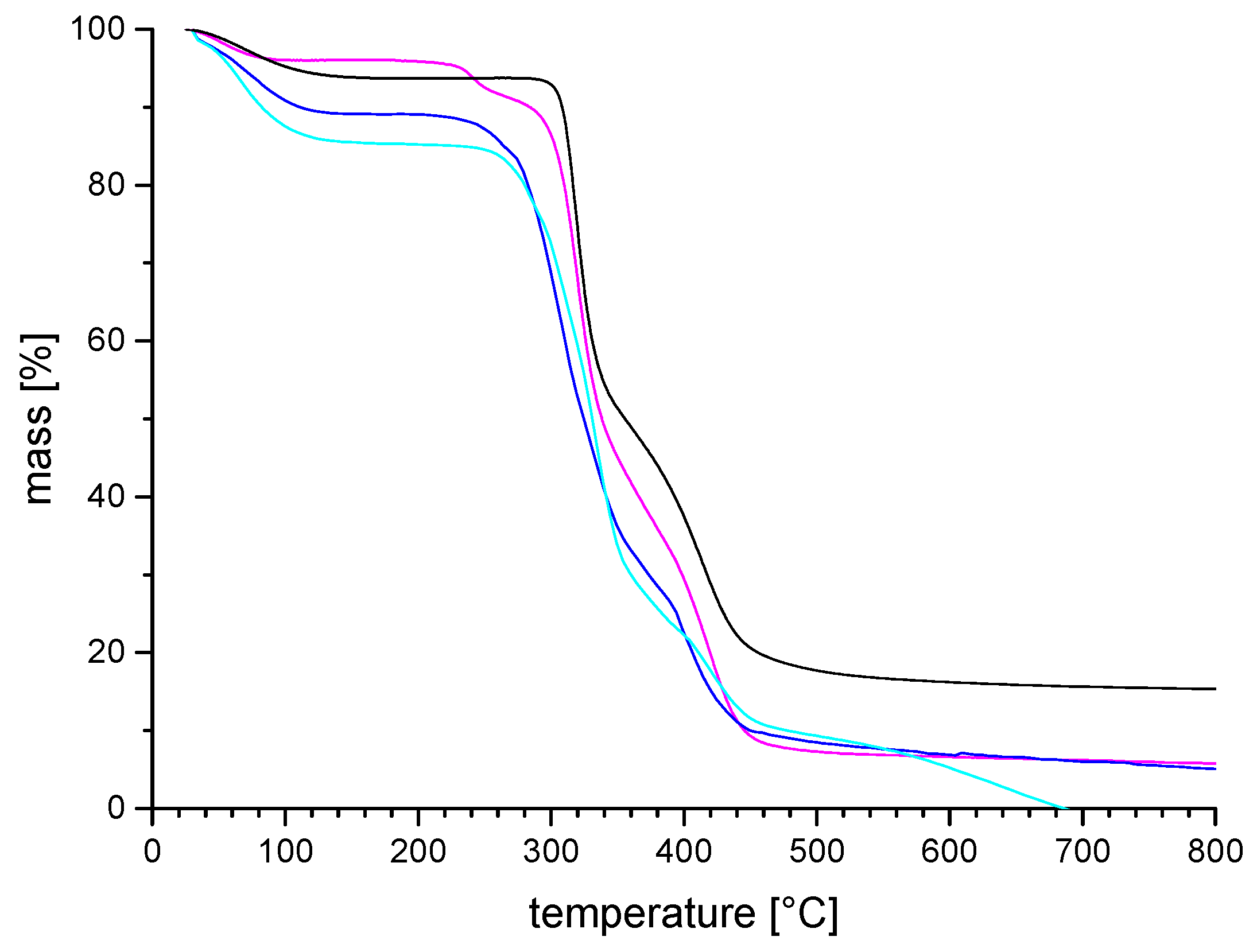
3.2. General Properties of the Homopolymers
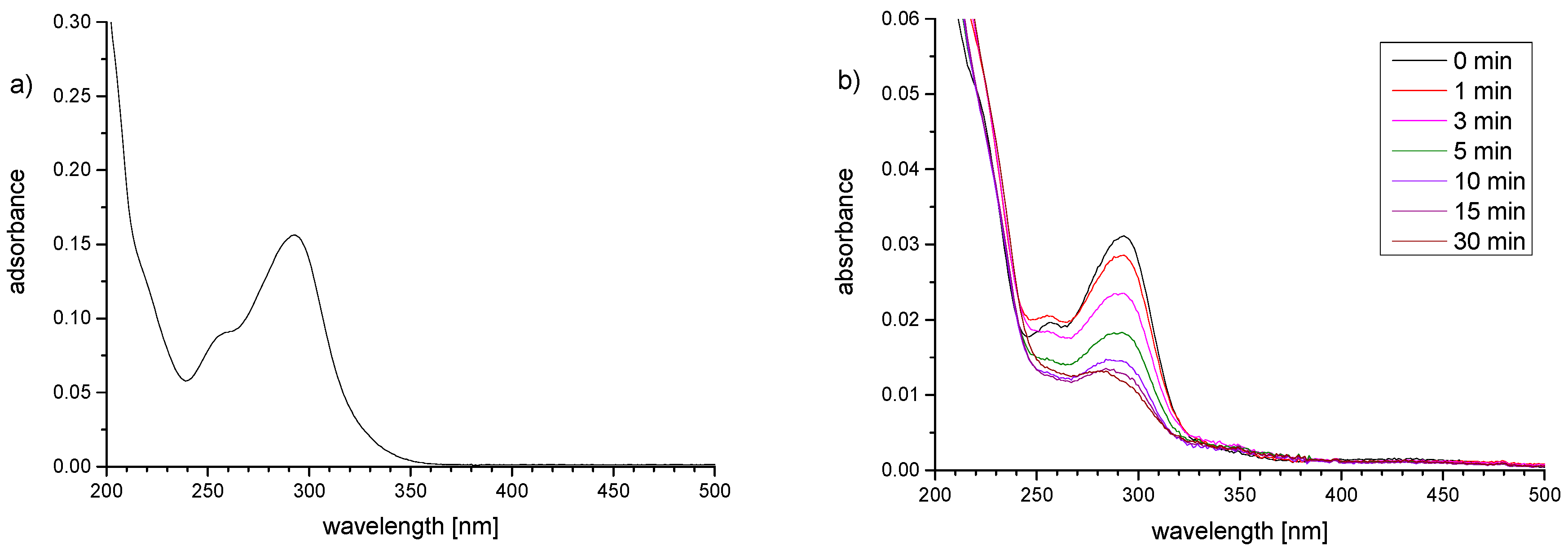
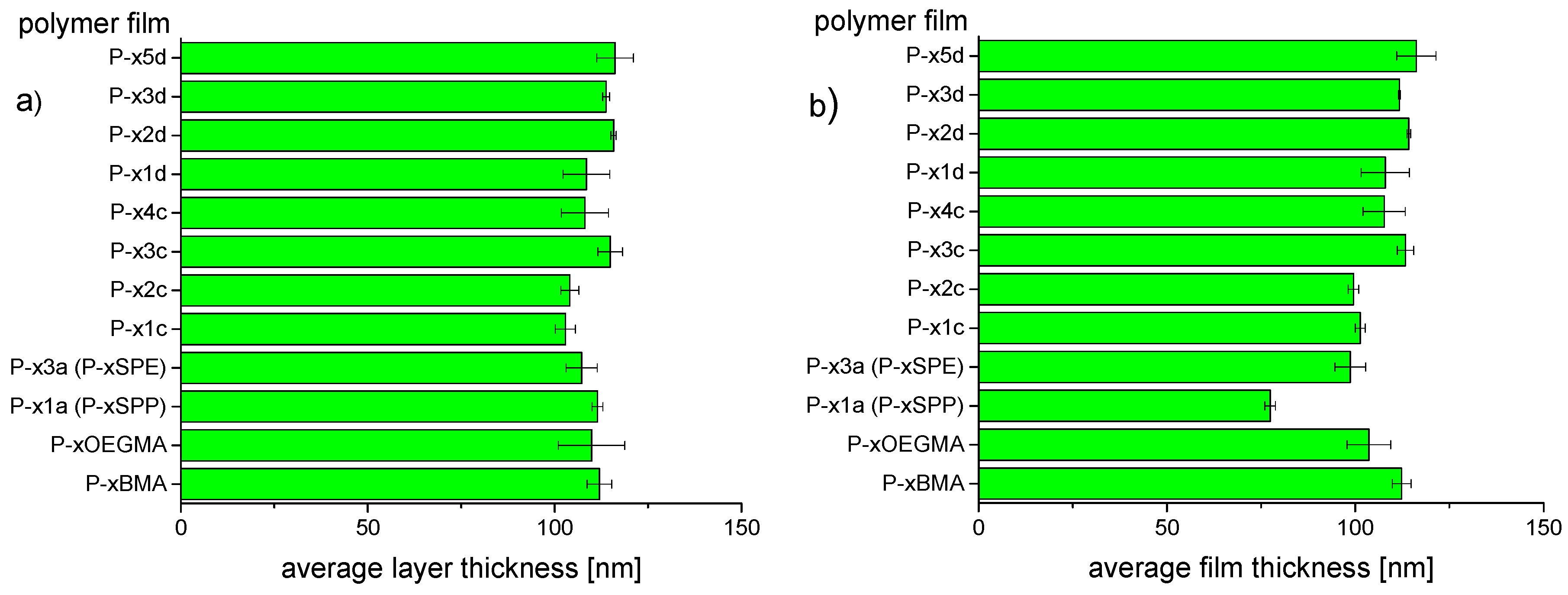
3.3. Preparation of Thin Hydrogel Coatings
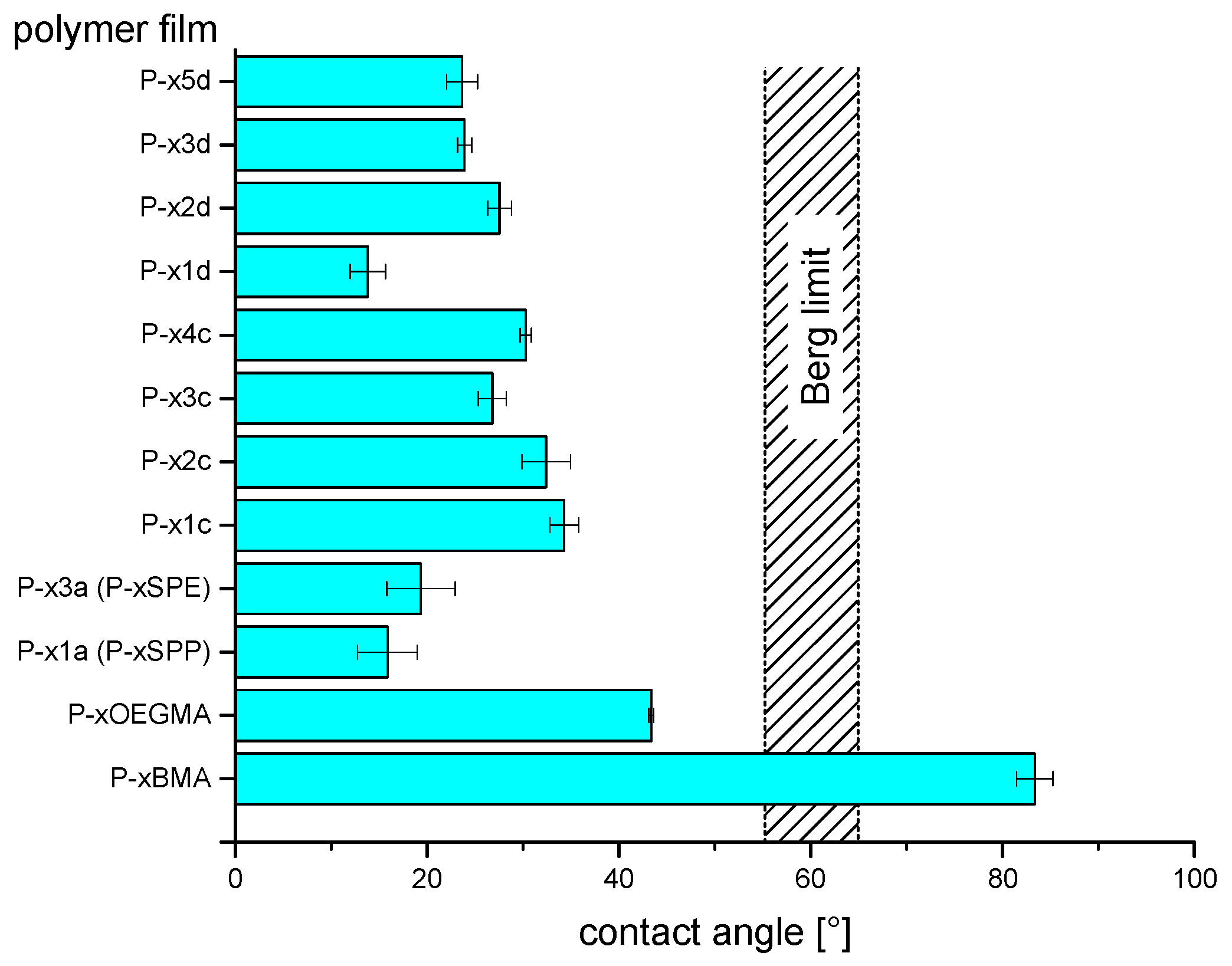
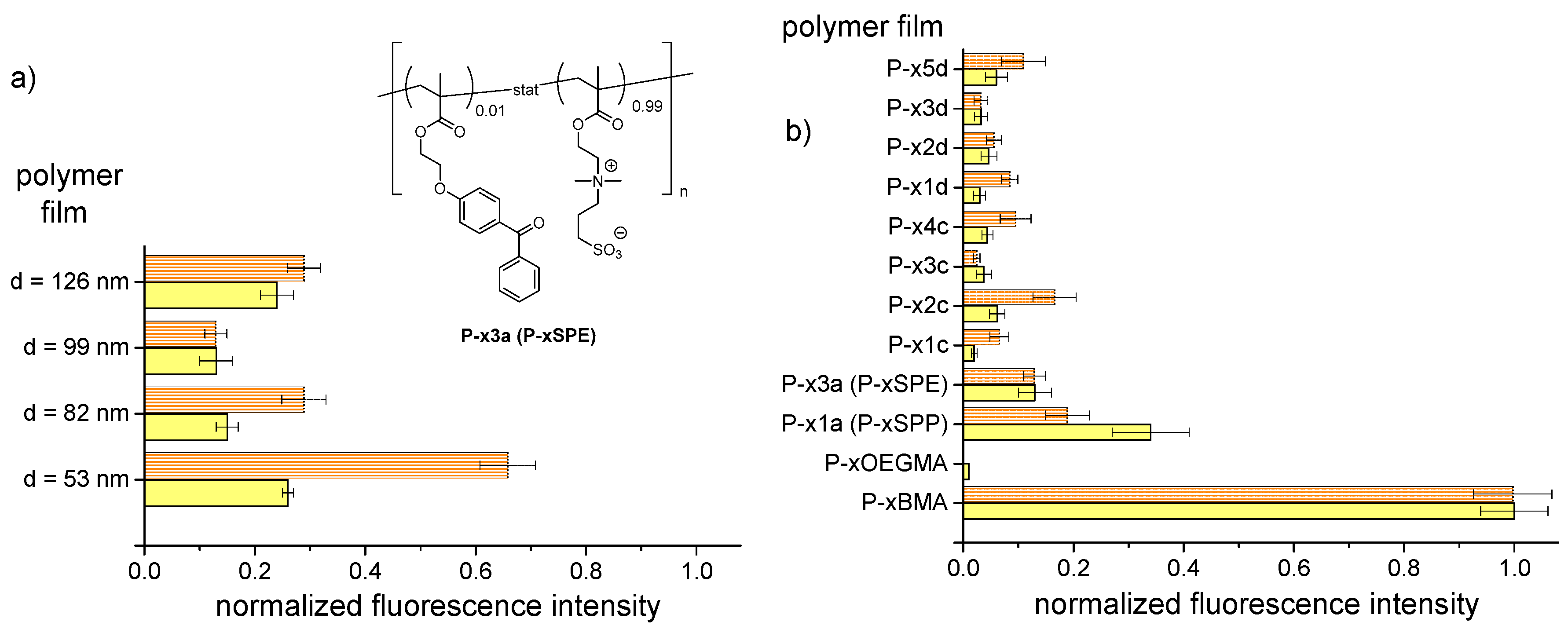
3.4. Resistance Against Unspecific Adsorption of Proteins (“Fouling”)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

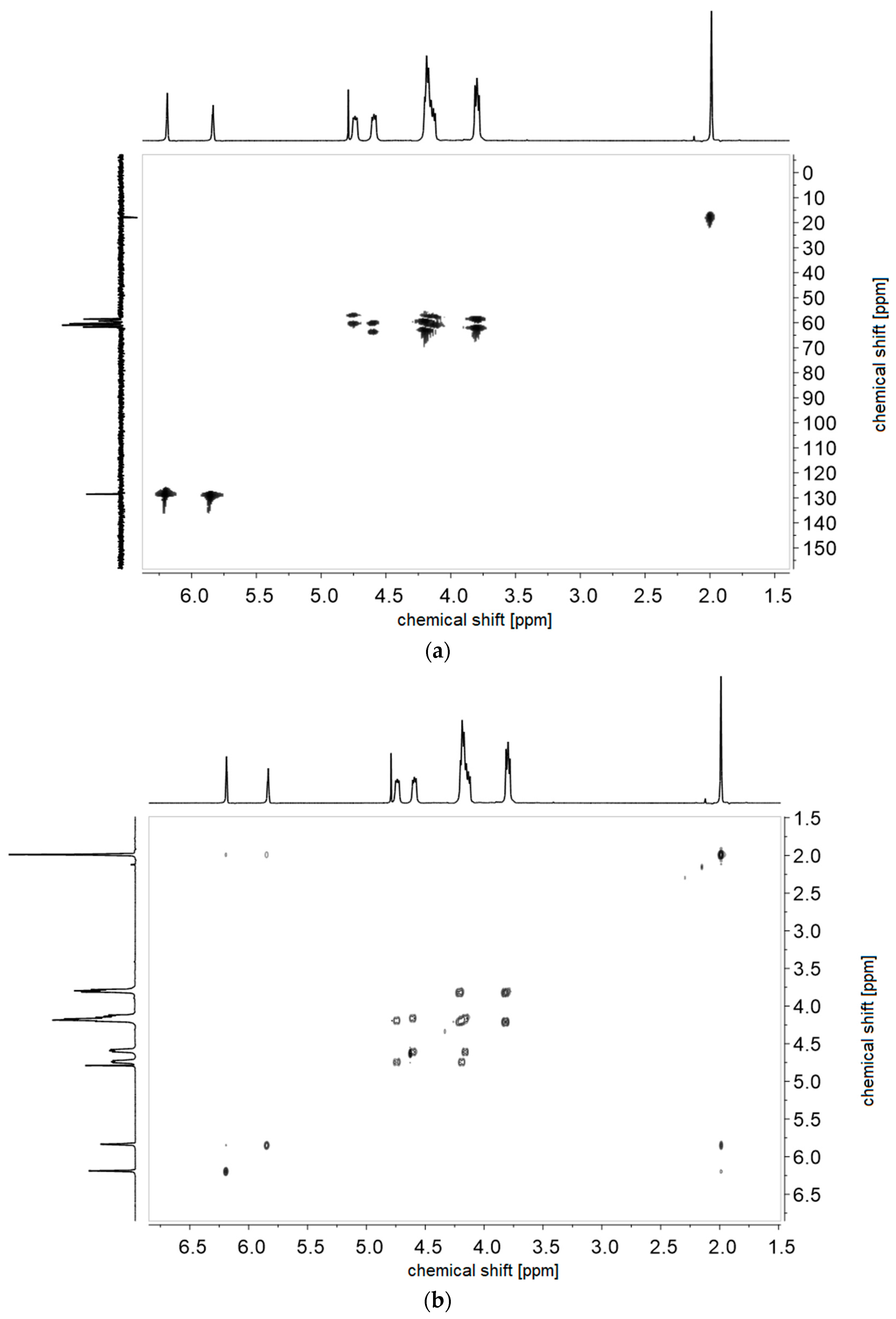
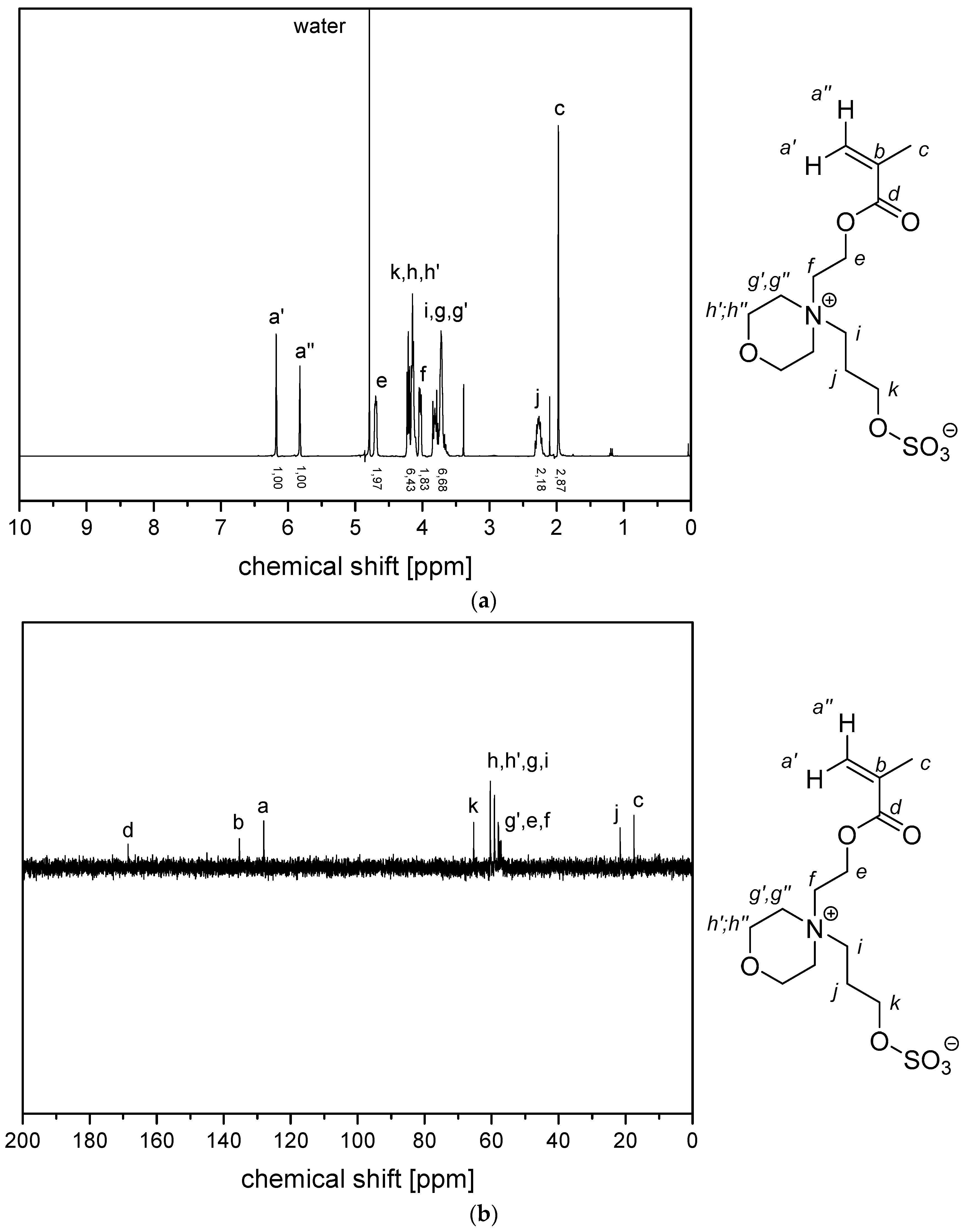
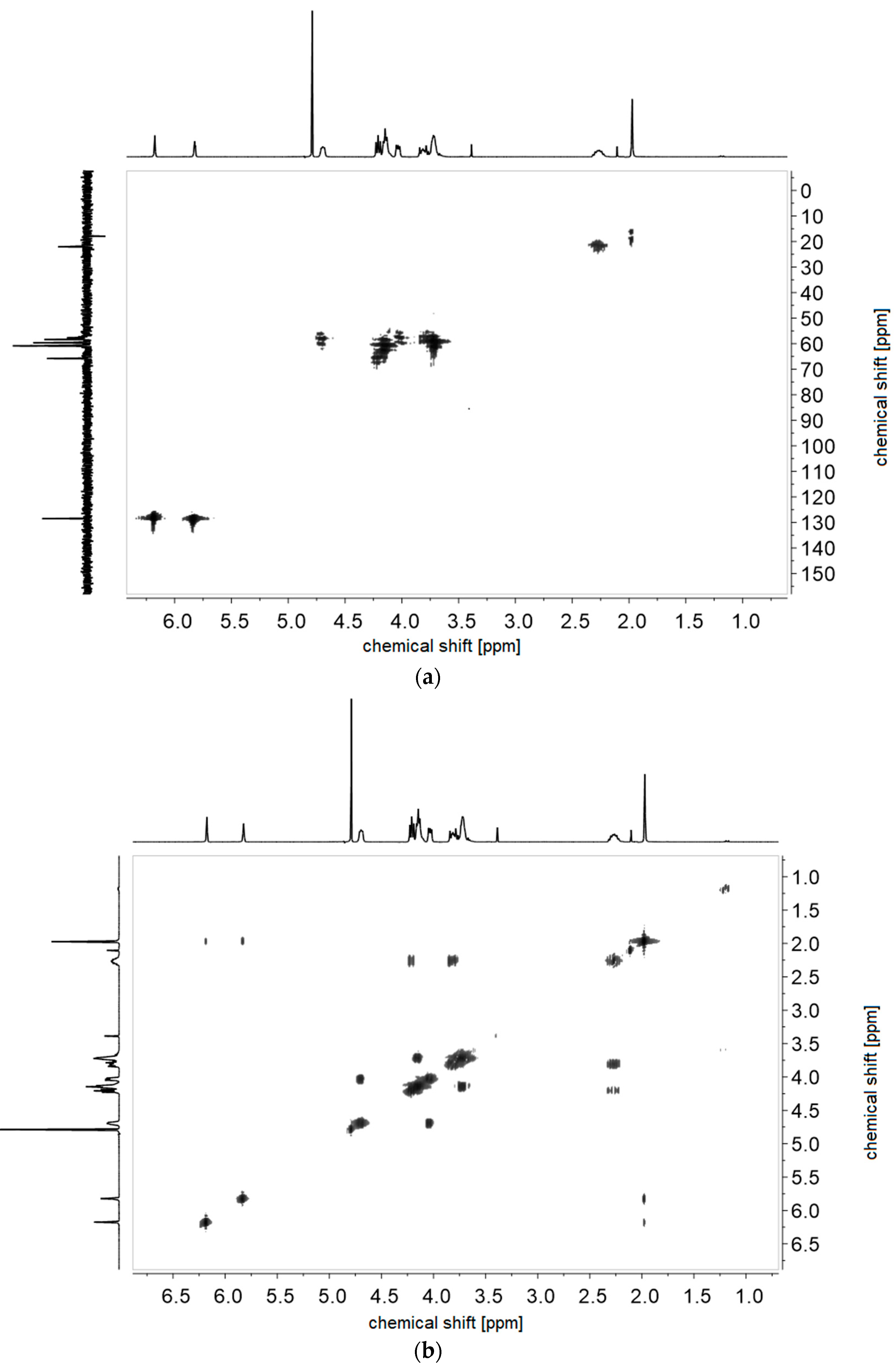
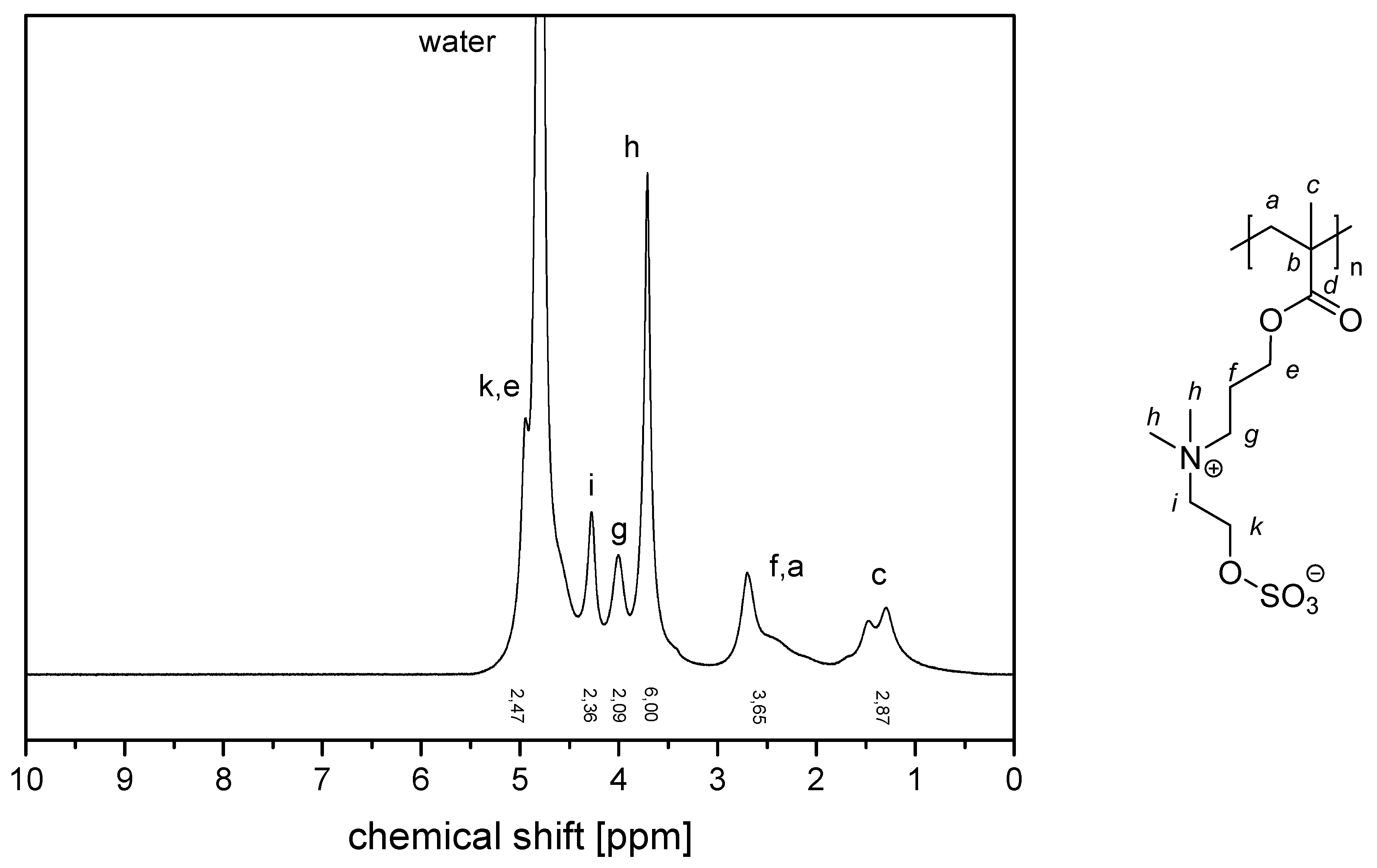
References
- Galin, J.-C. Polyzwitterions. In Polymer Materials Encyclopedia; Salamone, J.C., Ed.; CRC Press: Boca Raton, FL, USA, 1996; Volume 9, pp. 7189–7201. [Google Scholar]
- Lowe, A.B.; McCormick, C.L. Synthesis and solution properties of zwitterionic polymers. Chem. Rev. 2002, 102, 4177–4189. [Google Scholar] [CrossRef] [PubMed]
- Laschewsky, A. Structures and Synthesis of Zwitterionic Polymers. Polymers 2014, 6, 1544–1601. [Google Scholar] [CrossRef]
- Chapman, D. Biomembranes and new hemocompatible materials. Langmuir 1993, 9, 39–45. [Google Scholar] [CrossRef]
- Chen, M.; Briscoe, W.H.; Armes, S.P.; Klein, J. Lubrication at physiological pressures by polyzwitterionic brushes. Science 2009, 323, 1698–1701. [Google Scholar] [CrossRef]
- Schlenoff, J.B. Zwitteration: Coating Surfaces with Zwitterionic Functionality to Reduce Nonspecific Adsorption. Langmuir 2014, 30, 9625–9636. [Google Scholar] [CrossRef]
- Ilčíková, M.; Tkáč, J.; Kasák, P. Switchable Materials Containing Polyzwitterion Moieties. Polymers 2015, 7, 2344–2370. [Google Scholar] [CrossRef]
- He, M.; Gao, K.; Zhou, L.; Jiao, Z.; Wu, M.; Cao, J.; You, X.; Cai, Z.; Su, Y.; Jiang, Z. Zwitterionic materials for antifouling membrane surface construction. Acta Biomater. 2016, 40, 142–152. [Google Scholar] [CrossRef]
- Biehl, P.; von der Lühe, M.; Dutz, S.; Schacher, F.H. Synthesis, Characterization, and Applications of Magnetic Nanoparticles Featuring Polyzwitterionic Coatings. Polymers 2018, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K. Blood-Compatible Surfaces with Phosphorylcholine-Based Polymers for Cardiovascular Medical Devices. Langmuir 2019, 35, 1778–1787. [Google Scholar] [CrossRef]
- Baggerman, J.; Smulders, M.M.J.; Zuilhof, H. Romantic Surfaces: A Systematic Overview of Stable, Biospecific, and Antifouling Zwitterionic Surfaces. Langmuir 2019, 35, 1072–1084. [Google Scholar] [CrossRef]
- Laschewsky, A.; Rosenhahn, A. Molecular Design of Zwitterionic Polymer Interfaces: Searching for the Difference. Langmuir 2019, 35, 1056–1071. [Google Scholar] [CrossRef] [PubMed]
- Venault, A.; Chang, Y. Designs of Zwitterionic Interfaces and Membranes. Langmuir 2019, 35, 1714–1726. [Google Scholar] [CrossRef] [PubMed]
- Carr, L.R.; Zhou, Y.; Krause, J.E.; Xue, H.; Jiang, S. Uniform zwitterionic polymer hydrogels with a nonfouling and functionalizable crosslinker using photopolymerization. Biomaterials 2011, 32, 6893–6899. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Jang, H.; Stocker, R.; Gleason, K.K. Synergistic Prevention of Biofouling in Seawater Desalination by Zwitterionic Surfaces and Low-Level Chlorination. Adv. Mater. 2014, 26, 1711–1718. [Google Scholar] [CrossRef] [PubMed]
- Le, N.L.; Ulbricht, M.; Nunes, S.P. How Do Polyethylene Glycol and Poly(sulfobetaine) Hydrogel Layers on Ultrafiltration Membranes Minimize Fouling and Stay Stable in Cleaning Chemicals? Ind. Eng. Chem. Res. 2017, 56, 6785–6795. [Google Scholar] [CrossRef]
- Nakaya, T.; Li, Y.J. Phospholipid polymers. Prog. Polym. Sci. 1999, 24, 143–181. [Google Scholar] [CrossRef]
- Mueller, A.; O’Brien, D.F. Supramolecular Materials via Polymerization of Mesophases of Hydrated Amphiphiles. Chem. Rev. 2002, 102, 727–758. [Google Scholar] [CrossRef]
- Monge, S.; Canniccioni, B.; Graillot, A.; Robin, J.-J. Phosphorus-Containing Polymers: A Great Opportunity for the Biomedical Field. Biomacromolecules 2011, 12, 1973–1982. [Google Scholar] [CrossRef]
- Hu, G.; Emrick, T. Functional Choline Phosphate Polymers. J. Am. Chem. Soc. 2016, 138, 1828–1831. [Google Scholar] [CrossRef]
- Wielema, T.A.; Engberts, J.B.F.N. Zwitterionic polymers—II. Synthesis of a novel series of poly(vinylbetaines) and the effect of the polymeric structure on the solubility behaviour in water. Eur. Polym. J. 1990, 26, 415–421. [Google Scholar] [CrossRef]
- Bonte, N.; Laschewsky, A. Zwitterionic polymers with carbobetaine moieties. Polymer 1996, 37, 2011–2019. [Google Scholar] [CrossRef]
- Kathmann, E.E.; White, L.A.; McCormick, C.L. Water soluble polymers: 70. Effects of methylene versus propylene spacers in the pH and electrolyte responsiveness of zwitterionic copolymers incorporating carboxybetaine monomers. Polymer 1997, 38, 879–886. [Google Scholar] [CrossRef]
- Favresse, P.; Laschewsky, A. Synthesis and Investigation of New Amphiphilic Poly(carbobetaine)s Made from Diallylammonium Monomers. Polymer 2001, 42, 2755–2766. [Google Scholar] [CrossRef]
- Avci, D.; Lemopulo, K.; Mathias, L.J. Cyclocopolymerization of allyl-acrylate quaternary ammonium salts with diallyldimethylammonium chloride. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 640–649. [Google Scholar] [CrossRef]
- Cao, Z.; Mi, L.; Mendiola, J.; Ella-Menye, J.-R.; Zhang, L.; Xue, H.; Jiang, S. Reversibly Switching the Function of a Surface between Attacking and Defending against Bacteria. Angew. Chem. Int. Ed. 2012, 51, 2602–2605. [Google Scholar] [CrossRef] [PubMed]
- Colak, S.; Tew, G.N. Amphiphilic Polybetaines: The Effect of Side-Chain Hydrophobicity on Protein Adsorption. Biomacromolecules 2012, 13, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Rössel, C.; Billing, M.; Görls, H.; Festag, G.; Grube, M.; Bellstedt, P.; Nischang, I.; Schacher, F.H. Synthesis and modification of poly(ethyl 2-(imidazol-1-yl)acrylate) (PEImA). Polymer 2017, 127, 182–191. [Google Scholar] [CrossRef]
- Kurowska, M.; Eickenscheidt, A.; Al-Ahmad, A.; Lienkamp, K. Simultaneously Antimicrobial, Protein-Repellent, and Cell-Compatible Polyzwitterion Networks: More Insight on Bioactivity and Physical Properties. ACS Appl. Bio Mater. 2018, 1, 613–626. [Google Scholar] [CrossRef]
- Monroy Soto, V.M.; Galin, J.C. Poly(sulphopropylbetaines): 1. Synthesis and characterization. Polymer 1984, 25, 121–128. [Google Scholar] [CrossRef]
- Laschewsky, A.; Zerbe, I. Polymerizable and polymeric zwitterionic surfactants: 2. Surface activity and aggregation behaviour in aqueous systems. Polymer 1991, 32, 2081–2086. [Google Scholar] [CrossRef]
- Anton, P.; Laschewsky, A. Zwitterionic polysoaps with reduced density of surfactant side groups. Makromol. Chem. 1993, 194, 601–624. [Google Scholar] [CrossRef]
- Köberle, P.; Laschewsky, A. Hydrophobically modified zwitterionic polymers: Synthesis, bulk properties, and miscibility with inorganic salts. Macromolecules 1994, 27, 2165–2179. [Google Scholar] [CrossRef]
- Köberle, P.; Laschewsky, A.; Lomax, T.D. Interactions of a zwitterionic polysoap and its cationic analog with inorganic salts. Makromol. Chem. Rapid Commun. 1991, 12, 427–433. [Google Scholar] [CrossRef]
- Willcock, H.; Lu, A.; Hansell, C.F.; Chapman, E.; Collins, I.R.; O’Reilly, R.K. One-pot synthesis of responsive sulfobetaine nanoparticles by RAFT polymerisation: The effect of branching on the UCST cloud point. Polym. Chem. 2014, 5, 1023–1030. [Google Scholar] [CrossRef]
- Woodfield, P.A.; Zhu, Y.; Pei, Y.; Roth, P.J. Hydrophobically Modified Sulfobetaine Copolymers with Tunable Aqueous UCST through Postpolymerization Modification of Poly(pentafluorophenyl acrylate). Macromolecules 2014, 47, 750–762. [Google Scholar] [CrossRef]
- Chang, C.-C.; Letteri, R.; Hayward, R.C.; Emrick, T. Functional Sulfobetaine Polymers: Synthesis and Salt-Responsive Stabilization of Oil-in-Water Droplets. Macromolecules 2015, 48, 7843–7850. [Google Scholar] [CrossRef]
- Hildebrand, V.; Laschewsky, A.; Päch, M.; Müller-Buschbaum, P.; Papadakis, C.M. Effect of the Zwitterion Structure on the Thermo-responsive Behaviour of Poly(Sulfobetaine Methacrylate)s. Polym. Chem. 2017, 8, 310–322. [Google Scholar] [CrossRef]
- Ghoussoub, Y.E.; Fares, H.M.; Delgado, J.D.; Keller, L.R.; Schlenoff, J.B. Antifouling Ion-Exchange Resins. ACS Appl. Mater. Interfaces 2018, 10, 41747–41756. [Google Scholar] [CrossRef] [PubMed]
- Armitage, B.A.; Bennett, D.E.; Lamparski, H.G.; O’Brien, D.F. Polymerization and Domain Formation in Lipid Assemblies. Adv. Polym. Sci. 1996, 126, 54–84. [Google Scholar] [CrossRef]
- Puri, A.; Blumenthal, R. Polymeric Lipid Assemblies as Novel Theranostic Tools. Acc. Chem. Res. 2011, 44, 1071–1079. [Google Scholar] [CrossRef]
- Ishihara, K.; Mu, M.; Konno, T.; Inoue, Y.; Fukazawa, K. The unique hydration state of poly(2-methacryloyloxyethyl phosphorylcholine). J. Biomater. Sci. Polym. Ed. 2017, 28, 884–899. [Google Scholar] [CrossRef] [PubMed]
- Vasantha, V.A.; Jana, S.; Parthiban, A.; Vancso, J.G. Halophilic polysulfabetaines - synthesis and study of gelation and thermoresponsive behavior. RSC Adv. 2014, 4, 22596–22600. [Google Scholar] [CrossRef]
- Wen, J.; Weinhart, M.; Lai, B.; Kizhakkedathu, J.; Brooks, D.E. Reversible hemostatic properties of sulfabetaine/quaternary ammonium modified hyperbranched polyglycerol. Biomaterials 2016, 86, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Gal, Y.-S.; Jin, S.-H.; Park, J.W.; Lim, K.T. Conjugated organic polymer from the uncatalyzed polymerization of 2-ethynylpyridine via the ring-opening of 1,3-propandiol cyclic sulfate. Synth. Met. 2013, 174, 19–23. [Google Scholar] [CrossRef]
- Shao, Q.; Jiang, S. Influence of Charged Groups on the Properties of Zwitterionic Moieties: A Molecular Simulation Study. J. Phys. Chem. B 2014, 118, 7630–7637. [Google Scholar] [CrossRef] [PubMed]
- Vasantha, V.A.; Jana, S.; Lee, S.S.-C.; Lim, C.-S.; Teo, S.L.-M.; Parthiban, A.; Vancso, J.G. Dual hydrophilic and salt responsive schizophrenic block copolymers—Synthesis and study of self-assembly behavior. Polym. Chem. 2015, 6, 599–606. [Google Scholar] [CrossRef]
- Vasantha, V.A.; Zainul Rahim, S.Z.; Jayaraman, S.; Junyuan, G.H.; Puniredd, S.R.; Ramakrishna, S.; Teo, S.L.-M.; Parthiban, A. Antibacterial, electrospun nanofibers of novel poly(sulfobetaine) and poly(sulfabetaine)s. J. Mater. Chem. B 2016, 4, 2731–2738. [Google Scholar] [CrossRef]
- Arjunan Vasantha, V.; Junhui, C.; Ying, T.B.; Parthiban, A. Salt-Responsive Polysulfabetaines from Acrylate and Acrylamide Precursors: Robust Stabilization of Metal Nanoparticles in Hyposalinity and Hypersalinity. Langmuir 2015, 31, 11124–11134. [Google Scholar] [CrossRef]
- Schönemann, E.; Laschewsky, A.; Rosenhahn, A. Exploring the Long-Term Hydrolytic Behavior of Zwitterionic Polymethacrylates and Polymethacrylamides. Polymers 2018, 10, 639. [Google Scholar] [CrossRef]
- Nizardo, N.; Schanzenbach, D.; Schönemann, E.; Laschewsky, A. Exploring Poly(ethylene glycol)-Polyzwitterion Diblock Copolymers as Biocompatible Smart Macrosurfactants Featuring UCST-Phase Behavior in Normal Saline Solution. Polymers 2018, 10, 325. [Google Scholar] [CrossRef]
- Koc, J.; Schönemann, E.; Amuthalingam, A.; Clarke, J.; Finlay, J.A.; Clare, A.S.; Laschewsky, A.; Rosenhahn, A. Low Fouling Thin Hydrogel Coatings Made of Photo-crosslinked Polyzwitterions. Langmuir 2019, 35, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Buller, J.; Laschewsky, A.; Wischerhoff, E. Photoreactive Oligoethylene Glycol Polymers—Versatile Compounds for Surface Modification by Thin Hydrogel Films. Soft Matter 2013, 9, 929–937. [Google Scholar] [CrossRef]
- Terayama, Y.; Kikuchi, M.; Kobayashi, M.; Takahara, A. Well-Defined Poly(sulfobetaine) Brushes Prepared by Surface-Initiated ATRP Using a Fluoroalcohol and Ionic Liquids as the Solvents. Macromolecules 2011, 44, 104–111. [Google Scholar] [CrossRef]
- Galin, M.; Marchal, E.; Mathis, A.; Meurer, B.; Monroy Soto, Y.M.; Galin, J.C. Poly(sulphopropylbetaines): 3. Bulk properties. Polymer 1987, 28, 1937–1944. [Google Scholar] [CrossRef]
- Lowe, A.B.; Billingham, N.C.; Armes, S.P. Synthesis and Properties of Low-Polydispersity Poly(sulfopropylbetaine)s and Their Block Copolymers. Macromolecules 1999, 32, 2141–2148. [Google Scholar] [CrossRef]
- Hildebrand, V.; Laschewsky, A.; Zehm, D. On the hydrophilicity of polyzwitterion poly (N,N-dimethyl-N-(3-(methacrylamido)propyl)ammoniopropane sulfonate) in water, deuterated water, and aqueous salt solutions. J. Biomater. Sci. Polym. Ed. 2014, 25, 1602–1618. [Google Scholar] [CrossRef] [PubMed]
- Salamone, J.C.; Volksen, W.; Olson, A.P.; Israel, S.C. Aqueous solution properties of a poly(vinyl imidazolium sulphobetaine). Polymer 1978, 19, 1157–1162. [Google Scholar] [CrossRef]
- Monroy Soto, V.M.; Galin, J.C. Poly(sulphopropylbetaines): 2. Dilute solution properties. Polymer 1984, 25, 254–262. [Google Scholar] [CrossRef]
- Wielema, T.A.; Engberts, J.B.F.N. Zwitterionic polymers—III. Salt effects on the solubility of poly(vinyl sulphobetaines) and poly(vinyl betaines) in aqueous solution. Eur. Polym. J. 1990, 26, 639–642. [Google Scholar] [CrossRef]
- Kathmann, E.E.L.; White, L.A.; McCormick, C.L. Water-Soluble Polymers. 73. Electrolyte- and pH-Responsive Zwitterionic Copolymers of 4-[(2-Acrylamido-2-methylpropyl)- dimethylammonio]butanoate with 3-[(2-Acrylamido-2-methyl- propyl)dimethylammonio]propanesulfonate. Macromolecules 1997, 30, 5297–5304. [Google Scholar] [CrossRef]
- Delgado, J.D.; Schlenoff, J.B. Static and Dynamic Solution Behavior of a Polyzwitterion Using a Hofmeister Salt Series. Macromolecules 2017, 50, 4454–4464. [Google Scholar] [CrossRef]
- Wang, T.; Kou, R.; Liu, H.; Liu, L.; Zhang, G.; Liu, G. Anion Specificity of Polyzwitterionic Brushes with Different Carbon Spacer Lengths and Its Application for Controlling Protein Adsorption. Langmuir 2016, 32, 2698–2707. [Google Scholar] [CrossRef] [PubMed]
- Shao, Q.; Jiang, S. Molecular Understanding and Design of Zwitterionic Materials. Adv. Mater. 2015, 27, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Prucker, O.; Naumann, C.A.; Rühe, J.; Knoll, W.; Frank, C.W. Photochemical Attachment of Polymer Films to Solid Surfaces via Monolayers of Benzophenone Derivatives. J. Am. Chem. Soc. 1999, 121, 8766–8770. [Google Scholar] [CrossRef]
- Beines, P.W.; Klosterkamp, I.; Menges, B.; Jonas, U.; Knoll, W. Responsive Thin Hydrogel Layers from Photo-Cross-Linkable Poly(N-isopropylacrylamide) Terpolymers. Langmuir 2007, 23, 2231–2238. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Fukazawa, K.; Ishihara, K. Photoreactive Polymers Bearing a Zwitterionic Phosphorylcholine Group for Surface Modification of Biomaterials. ACS Appl. Mater. Interfaces 2015, 7, 17489–17498. [Google Scholar] [CrossRef] [PubMed]
- Hartleb, W.; Saar, J.S.; Zou, P.; Lienkamp, K. Just Antimicrobial is not Enough: Toward Bifunctional Polymer Surfaces with Dual Antimicrobial and Protein-Repellent Functionality. Macromol. Chem. Phys. 2016, 217, 225–231. [Google Scholar] [CrossRef]
- Martinez, J.S.; Kelly, K.D.; Ghoussoub, Y.E.; Delgado, J.D.; Keller, T.C.S., III; Schlenoff, J.B. Cell resistant zwitterionic polyelectrolyte coating promotes bacterial attachment: An adhesion contradiction. Biomater. Sci. 2016, 4, 689–698. [Google Scholar] [CrossRef]
- Liu, Q.; Locklin, J. Transparent Grafted Zwitterionic Copolymer Coatings That Exhibit Both Antifogging and Self-Cleaning Properties. ACS Omega 2018, 3, 17743–17750. [Google Scholar] [CrossRef]
- Prucker, O.; Brandstetter, T.; Rühe, J. Surface-attached hydrogel coatings via C,H-insertion crosslinking for biomedical and bioanalytical applications (Review). Biointerphases 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Laschewsky, A.; Rekai, E.D.; Wischerhoff, E. Tailoring of Stimuli-Responsive Water-Soluble Acrylamide and Methacrylamide Polymers. Macromol. Chem. Phys. 2001, 202, 276–286. [Google Scholar] [CrossRef]
- Kim, M.; Ondrusek, B.A.; Lee, C.; Douglas, W.G.; Chung, H. Synthesis of lightly crosslinked zwitterionic polymer-based bioinspired adhesives for intestinal tissue sealing. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1564–1573. [Google Scholar] [CrossRef]
- Chou, Y.-N.; Sun, F.; Hung, H.-C.; Jain, P.; Sinclair, A.; Zhang, P.; Bai, T.; Chang, Y.; Wen, T.-C.; Yu, Q.; et al. Ultra-low fouling and high antibody loading zwitterionic hydrogel coatings for sensing and detection in complex media. Acta Biomater. 2016, 40, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Liu, Y.; Zhang, Y.; Ren, B.; Xu, J.; Zheng, J. Synthesis and Characterization of Ultralow Fouling Poly(N-acryloyl-glycinamide) Brushes. Langmuir 2017, 33, 13964–13972. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, S.; Cheng, G.; Vaisocherová, H.; Xue, H.; Li, W.; Zhang, J.; Jiang, S. Film Thickness Dependence of Protein Adsorption from Blood Serum and Plasma onto Poly(sulfobetaine)-Grafted Surfaces. Langmuir 2008, 24, 9211–9214. [Google Scholar] [CrossRef]
- Yang, W.; Xue, H.; Li, W.; Zhang, J.; Jiang, S. Pursuing “Zero” Protein Adsorption of Poly(carboxybetaine) from Undiluted Blood Serum and Plasma. Langmuir 2009, 25, 11911–11916. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Li, L.; Wang, Q.; Yu, Q.; Zheng, J. Effect of Film Thickness on the Antifouling Performance of Poly(hydroxy-functional methacrylates) Grafted Surfaces. Langmuir 2011, 27, 4906–4913. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Singh, A.; Liu, L. Amino Acid-Based Zwitterionic Poly(serine methacrylate) as an Antifouling Material. Biomacromolecules 2013, 14, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Onodera, Y.; Ishihara, K. Preparation of a thick polymer brush layer composed of poly(2-methacryloyloxyethyl phosphorylcholine) by surface-initiated atom transfer radical polymerization and analysis of protein adsorption resistance. Colloids Surf. B 2016, 141, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Utrata-Wesołek, A.; Wałach, W.; Anioł, J.; Sieroń, A.L.; Dworak, A. Multiple and terminal grafting of linear polyglycidol for surfaces of reduced protein adsorption. Polymer 2016, 97, 44–54. [Google Scholar] [CrossRef]
- Yuan, L.; Qu, B.; Li, J.; Lv, H.; Yang, X. Photoreactive benzophenone as anchor of modifier to construct durable anti-platelets polymer surface. Eur. Polym. J. 2019, 111, 114–122. [Google Scholar] [CrossRef]
- Vogler, E.A. Structure and reactivity of water at biomaterial surfaces. Adv. Colloid Interface Sci. 1998, 74, 69–117. [Google Scholar] [CrossRef]
- Herrwerth, S.; Eck, W.; Reinhardt, S.; Grunze, M. Factors that Determine the Protein Resistance of Oligoether Self-Assembled Monolayers—Internal Hydrophilicity, Terminal Hydrophilicity, and Lateral Packing Density. J. Am. Chem. Soc. 2003, 125, 9359–9366. [Google Scholar] [CrossRef] [PubMed]
- Rosenhahn, A.; Schilp, S.; Kreuzer, H.J.; Grunze, M. The role of “inert” surface chemistry in marine biofouling prevention. Phys. Chem. Chem. Phys. 2010, 12, 4275–4286. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Somorjai, G.A. Molecular Packing of Lysozyme, Fibrinogen, and Bovine Serum Albumin on Hydrophilic and Hydrophobic Surfaces Studied by Infrared−Visible Sum Frequency Generation and Fluorescence Microscopy. J. Am. Chem. Soc. 2003, 125, 3150–3158. [Google Scholar] [CrossRef] [PubMed]
- Bratek-Skicki, A.; Eloy, P.; Morga, M.; Dupont-Gillain, C. Reversible Protein Adsorption on Mixed PEO/PAA Polymer Brushes: Role of Ionic Strength and PEO Content. Langmuir 2018, 34, 3037–3048. [Google Scholar] [CrossRef] [PubMed]

| Polymer | Monomer (mg) | Monomer (mmol) | Initiator (mg) | Initiator (mmol) | Crosslinker (mg) | Crosslinker (mmol) |
|---|---|---|---|---|---|---|
| P−x1c | 1180 | 4.00 | 3.3 | 0.02 | 12.4 | 0.04 |
| P−2c | 300 | 1.00 | 0.8 | 0.005 | 0 | - |
| P−x2c | 1770 | 6.00 | 4.9 | 0.03 | 18.6 | 0.06 |
| P−x3c | 1130 | 4.00 | 3.3 | 0.02 | 12.4 | 0.04 |
| P−4c | 670 | 2.00 | 1.6 | 0.01 | 0 | - |
| P−x4c | 3210 | 10.0 | 8.2 | 0.05 | 31.0 | 0.1 |
| P−5c | 650 | 2.00 | 1.6 | 0.01 | 0 | - |
| P−x5c | 1620 | 5.00 | 4.1 | 0.025 | 15.5 | 0.05 |
| P−x6c | 1140 | 4.00 | 3.3 | 0.02 | 12.4 | 0.04 |
| P−2d | 620 | 2.00 | 1.6 | 0.01 | 0 | - |
| P−x2d | 2470 | 8.00 | 6.6 | 0.04 | 24.8 | 0.08 |
| P−x3d | 5910 | 20.00 | 16.4 | 0.1 | 62.0 | 0.2 |
| P−4d | 670 | 2.00 | 1.6 | 0.01 | 0 | - |
| P−x4d | 3350 | 10.00 | 8.2 | 0.05 | 31.0 | 0.1 |
| P−5d | 640 | 2.00 | 1.6 | 0.01 | 0 | - |
| P−x5d | 640 | 2.00 | 1.6 | 0.01 | 6.2 | 0.02 |
| P−x6d | 1200 | 4.00 | 3.3 | 0.02 | 12.4 | 0.04 |
| Polymer | Yield [%] | Mnapp [kg mol−1] (a) | Dispersity Ð (a) | Tdecomp [°C] (b) | CP in PBS [°C] (c) | CP in Brine [(°C] (d) |
|---|---|---|---|---|---|---|
| P−1c | 36 | - (e) | - (e) | 290 | - (f) | <0 |
| P−1d | 34 | 220 | 2.3 | 290 | - (f) | <0 |
| P−2c | 87 | - (e) | - (e) | 290 | - (f) | sw (g) |
| P−2d | 46 | 180 | 3.3 | 290 | - (f) | <0 |
| P−3c | 82 | 400 | 1.8 | 280 | - (f) | <0 |
| P−3d | 85 | 420 | 1.6 | 290 | - (f) | <0 |
| P−4c | 89 | 220 | 3.2 | 290 | - (f) | <0 |
| P−4d | 89 | - (e) | - (e) | 270 | - (f) | sw (g) |
| P−5c | 92 | - (e) | - (e) | 270 | - (f) | sw (g) |
| P−5d | 75 | - (e) | - (e) | 270 | - (f) | sw (g) |
| P−6c | quant. | - (e) | - (e) | 290 | - (f) | sw (g) |
| P−6d | quant. | - (e) | - (e) | 290 | - (f) | sw (g) |
| Polymer | Yield [%] | Mnapp [kg mol−1] (a) | Dispersity Ð (a) | Content of BPEMA [mol %] b) | Soluble in TFE |
|---|---|---|---|---|---|
| P−x1c | 45 | - (c) | - (c) | 1.5 ± 0.5 | yes |
| P−x1d | 79 | 190 | 3.6 | 1.1 ± 0.5 | yes |
| P−x2c | 54 | 130 | 3.7 | 1.1 ± 0.5 | yes |
| P−x2d | 76 | 130 | 3.8 | 1.4 ± 0.5 | yes |
| P−x3c | 90 | >240 (d) | >2.8 (d) | 1.1 ± 0.5 | yes |
| P−x3d | 91 | >230 (d) | >3.0 (d) | 0.8 ± 0.5 | yes |
| P−x4c | quantitative | 140 | 4.1 | 0.8 ± 0.5 | yes |
| P−x4d | quantitative | - (c) | - (c) | 0.9 ± 0.5 | sw (f) |
| P−x5c | 89 | - (c) | - (c) | 1.0 ± 0.5 | sw (f) |
| P−x5d | 76 | 110 | 4.4 | 1.3 ± 0.5 | yes |
| P−x6c | quantitative | - (c) | - (c) | n.d. (e) | sw (f) |
| P−x6d | quantitative | - (c) | - (c) | n.d (e) | sw (f) |
| P−x1a(P–xSPP) | 56 | 120 | 5.0 | 1.2 ± 0.5 | yes |
| P−x3a(P–xSPE) | 63 | 90 | 5.4 | 1.1 ± 0.5 | yes |
| P−xOEGMA | 82 | 130 | 3.7 | 1.0 ± 0.5 | yes |
| P−xBMA | 53 | 50 | 2.8 | 1.3 ± 0.5 | yes |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schönemann, E.; Laschewsky, A.; Wischerhoff, E.; Koc, J.; Rosenhahn, A. Surface Modification by Polyzwitterions of the Sulfabetaine-Type, and Their Resistance to Biofouling. Polymers 2019, 11, 1014. https://doi.org/10.3390/polym11061014
Schönemann E, Laschewsky A, Wischerhoff E, Koc J, Rosenhahn A. Surface Modification by Polyzwitterions of the Sulfabetaine-Type, and Their Resistance to Biofouling. Polymers. 2019; 11(6):1014. https://doi.org/10.3390/polym11061014
Chicago/Turabian StyleSchönemann, Eric, André Laschewsky, Erik Wischerhoff, Julian Koc, and Axel Rosenhahn. 2019. "Surface Modification by Polyzwitterions of the Sulfabetaine-Type, and Their Resistance to Biofouling" Polymers 11, no. 6: 1014. https://doi.org/10.3390/polym11061014
APA StyleSchönemann, E., Laschewsky, A., Wischerhoff, E., Koc, J., & Rosenhahn, A. (2019). Surface Modification by Polyzwitterions of the Sulfabetaine-Type, and Their Resistance to Biofouling. Polymers, 11(6), 1014. https://doi.org/10.3390/polym11061014






