1. Introduction
In recent years, environmental problems have become a big concern for the tire industry; thus, silica is being used instead of carbon black as a filler to manufacture eco-friendly tires with excellent rolling resistance (RR) performance [
1,
2]. To improve the RR performance of tires, silica must be well dispersed in a rubber matrix. However, in natural rubber (NR), butadiene rubber (BR), and styrene butadiene rubber (SBR), which are the typically used raw materials for tire treads, there is no polar group and the affinity with silica is poor [
3]. There are two main ways to solve this problem: (i) Hydrophobation of the silica surface using a coupling agent and then chemical bonding between the rubber molecules and coupling agents; (ii) facilitating the chemical bonding between rubber and silica by introducing a silica-friendly functional group to the rubber molecules. To improve RR, SSBR is the most widely used rubber in the manufacturing of silica compounds for passenger car tire treads [
4]. This is because silica-affinity functional groups can be easily introduced at chain ends due to the nature of anionic polymerization. However, because SSBR is polymerized using an organic solvent, volatile organic compounds (VOCs) are discharged. Furthermore, compared with emulsion SBR (ESBR), which is polymerized through radical mechanism, the molecular weight of SSBR is low and its mechanical properties are poor. Furthermore, ESBR is more eco-friendly than SSBR because it uses water as a solvent. However, chain-end modification in ESBR is difficult because of the characteristics of radical polymerization [
5].
To overcome this problem, Thielen [
6] introduced a third monomer with a silica-affinity functional group in the polymerization of ESBR. The third monomer used in the polymerization is piperylene, isoprene, vinyl pyridine, hydroxypropyl methacrylate, and acrylonitrile. These third monomers resulted in improved filler-rubber interaction by hydrogen bonding. Therefore, dynamic properties were improved. One of the most effective methods was to polymerize functionalized ESBR by introducing glycidyl methacrylate (GMA). This is because GMA causes improved filler-rubber interaction by chemical reaction with silica. Studies on GMA-ESBR [
5,
7] suggest that the affinity between rubber and silica was improved by GMA, resulting in improved mechanical properties, dynamic characteristics, and abrasion resistance compared with conventional ESBR. Kim et al. [
7] attempted to solve the processability problem of the previous study by controlling the amount of GMA during GMA-ESBR polymerization. Qiao et al. [
8] investigated the effect of GMA in GMA-ESBR silica compound without a coupling agent; the GMA-ESBR silica compound showed better physical properties than ESBR silica compound with bis (3-(triethoxysilyl) propyl) tetrasulfide (TESPT), even though TESPT is a silica coupling agent.
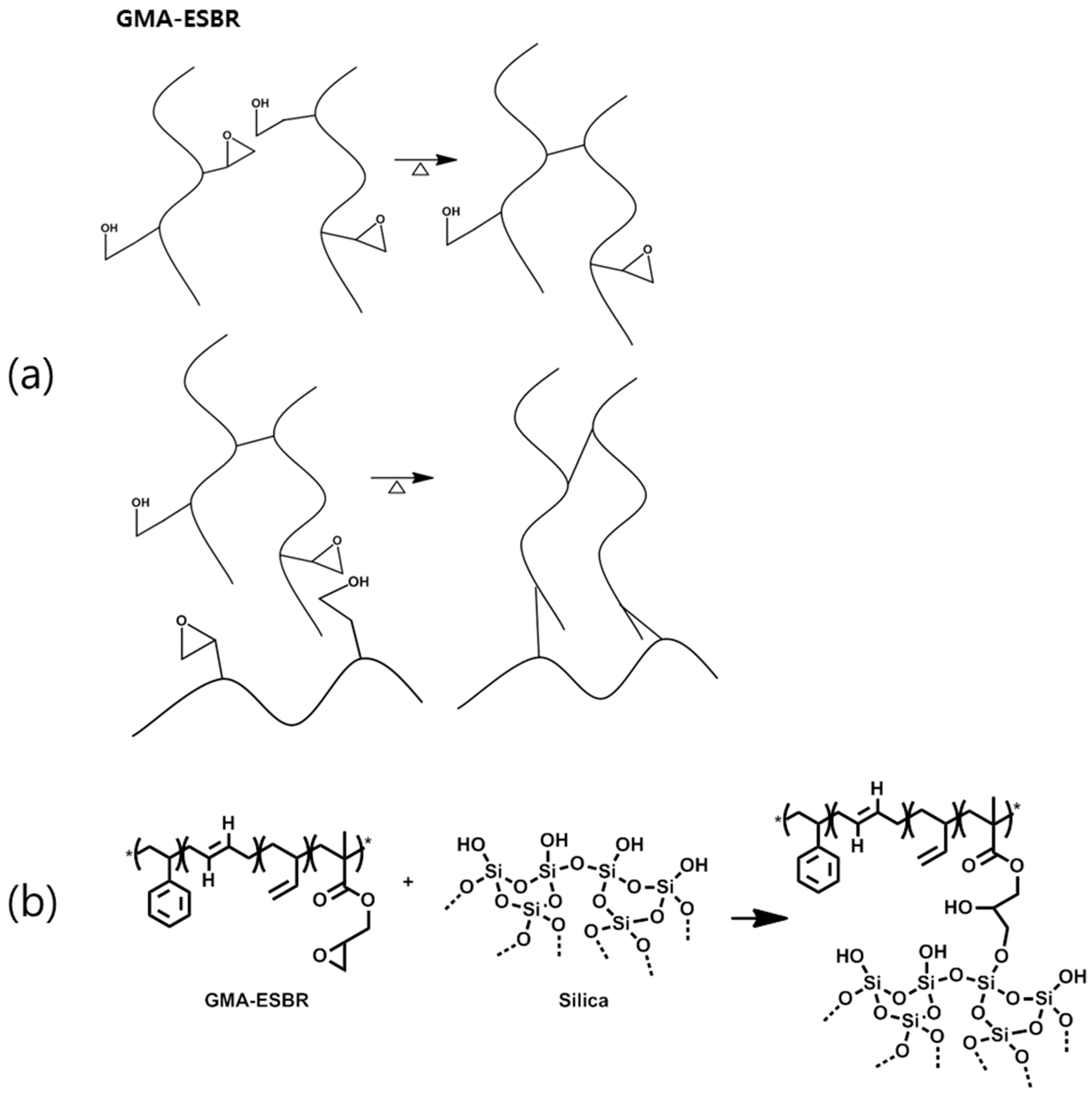
GMA-ESBR can be used as a tire tread silica compound for PCR with excellent physical properties, however, it is not used because of its unfavorable processability. Therefore, if the problem of processability can be solved, an excellent tire tread can be manufactured. However, no studies have been tried to solve this problem. In our study, ESBR was also polymerized as a comparative polymer. The silica compounds of GMA-ESBR and ESBR were manufactured through dry masterbatch (DMB) technology, which is a conventional compounding method. To solve the problems, in this study, the silica compound was also manufactured by applying the wet masterbatch (WMB) technique. According to Lightsey et al. [
9], WMB is a new method for dispersing silica in emulsion polymers. In addition, modified silica was used for the silica WMB used modified silica, so the silanization reaction is unnecessary in the compounding step; therefore, the temperature can be lowered during compounding [
10]. Thus, by using the WMB technology, silica can be dispersed in a rubber matrix without exposure of the functional group of GMA-ESBR to high mixing temperature. This process will prevent the gelation of GMA-ESBR molecules. The physical properties of DMB and WMB compounds were evaluated.
2. Experimental
2.1. Materials
2.1.1. Materials for Emulsion Polymerization
1,3-Butadiene, surfactant fatty acid, and rosin soap were supplied by Kumho Petrochemical Co. and used without further purification. Styrene and GMA were purchased from Samchun Chemicals CO., Seoul, Korea. A chain transfer agent; tert-dodecyl mercaptan, an initiator; p-menthane hydroperoxide (PMH, 98%), catalysts; sodium formaldehyde sulfoxylate (SFS, 86%), ferrous sulfate (FES, 99%), and ethylenediaminetetracetic acid, and a shortstop agent; diethylhydroxylamine (98%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Calcium dichloride (CaCl2, Daejung Chemicals & Metals Co., Ltd., Siheung, Korea) was used as a coagulant to obtain the rubber.
2.1.2. Materials for the Silica Compounds
In the preparation of the silica compound, used modified silica (7000GR, Evonik, Essen, Germany), which was modified with 10 wt % of bis [3-(triethoxysilyl) propyl] tetrasulfide (TESPT, Evonik, Essen, Germany). As the additives, ZnO, stearic acid (CH3 (CH2)16COOH), N-(1,3-dimethyl-butyl)-N’-phenyl-p-phenylenediamine (6PPD) were used. 1,3-Diphenyl-guanidine (DPG) and N-cychlohexyl-2-benzothiazylsulfenamide (CBS) were used as accelerators in the sulfur vulcanization. Additives, DPG, CBS, and sulfur were purchased from Sigma-Aldrich.
2.1.3. Materials for Analysis of Vulcanizates
Tetrahydrofuran (THF) and n-hexane were used to remove organic substances in the vulcanizates before the swelling experiment, and toluene was used to confirm the crosslink density. All these materials were obtained from Daejung Chemicals & Metals Co., Ltd., Siheung, Korea.
2.2. Polymerization of ESBR and GMA-ESBR
Two types of ESBR were synthesized by low-temperature emulsion polymerization by using the formulation shown in
Table 1. A high-pressure agitating reactor was made of stainless steel to feed gaseous 1,3-butadiene. The polymerization temperature was controlled by connecting a circulator to the reactor. First, styrene, GMA, water, surfactant, and catalyst were added in the high-pressure reactor; its cap was closed; and air was substituted with nitrogen for 30 min. Then, the initiator was introduced into the reactor by using a syringe, and the gaseous 1,3-butadiene was metered using a chamber and introduced into the reactor by using nitrogen pressure. The temperature of the reactor was maintained at 9 °C, and the total solid contents for the conversion determination were measured using a water drier (MB45, OHAUS, Parsippany, NJ, USA). When the conversion reached 65%, a shortstop agent was added to stop the polymerization.
2.3. Conversion Measurement and Latex Coagulation
During the reaction, the polymer was sampled at intervals of 2 h, dried using a water drier, and then the weight ratio was measured and conversion was confirmed by calculating the dry content. A 2 wt % CaCl2 aqueous solution was slowly dropped on the prepared latex and coagulated. Then, the coagulated ESBR was dried in a circulating hot air dryer at 50 °C for 24 h.
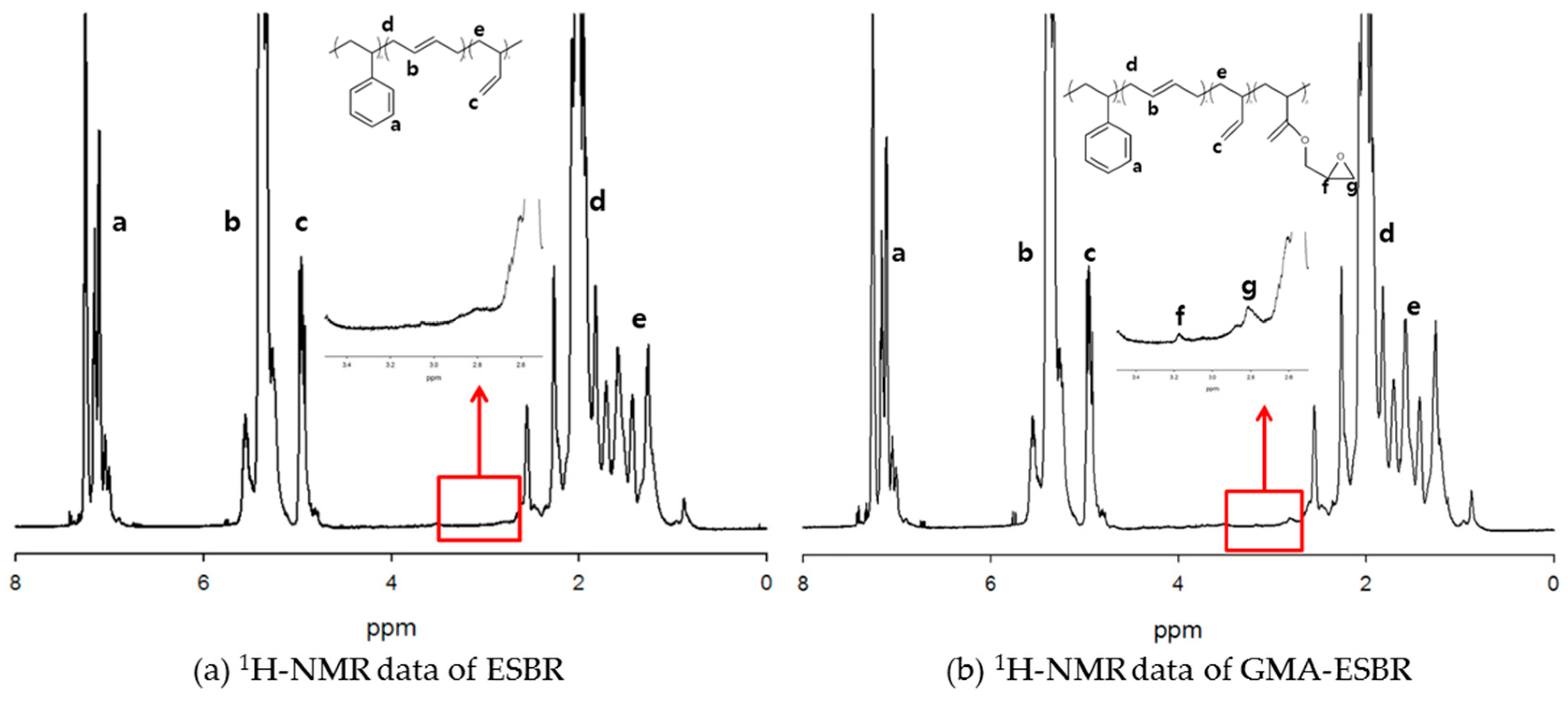
2.4. Characterization of ESBR
The contents of styrene, GMA, butadiene, and vinyl in butadiene were determined by using a nuclear magnetic resonance spectrometer (1H-NMR; Varian, Unity Plus 300 spectrometer, Garden State Scientific, Morristown, NJ, USA).
The molecular weight and its distribution were measured using gel permeation chromatography (GPC, Shimadzu, Kyoto, Japan), which consists of a solvent delivery unit, a reflex index detector, and three Stryagel columns (HT 6E, 10 μm, 7.8 mm × 300 mm; HMW 7, 15–20 μm, 7.8 mm × 300 mm; HMW 6E, 15–20 μm, 7.8 mm × 300 mm). Molecular weight was calibrated using polystyrene standard samples. The Mooney viscosity of raw polymers was measured according to ASTM D 1646 using a Mooney viscometer (Vluchem IND Co., Seoul, Korea).
2.5. Thermal Stability of ESBR and GMA-ESBR
To determine the thermal stability of GMA-ESBR, ESBR and GMA-ESBR samples were heated for 5 min at 100 °C using a Mooney viscometer (Vluchem IND Co., Seoul, Korea). After that, gel tests were conducted for the virgin samples and heated samples according to ASTM D3616 to determine the increase of gel content caused by the applied heat.
2.6. Manufacture of GMA-ESBR/Silica WMB
The surface-modified silica with 10 wt % TESPT was added to distilled water and stirred at 50 °C for 15 min to make silica slurry. Then, the mixture was mixed with GMA-ESBR latex heated at 50 °C and further stirred for 30 min. The WMB was then coagulated with 2 wt % CaCl2 aqueous solution, washed once, and dried at 50 °C for 24 h.
2.7. Manufacture of Compounds and Vulcanizates
Two-stage kneading was performed by applying the formulation shown in
Table 2. The first mixing was performed using a mixer (Kneader, 300cc, Mirae SI Co., Gwangju, Korea) with a fill factor of 0.7. The start temperature was 110 °C, and the end temperature was controlled to 150–155 °C. In the second mixing, sulfur and crosslinking accelerator were added and mixed for 2 min, and sheet-forming was performed by using a two-roll mill (rotor speed ratio of 1:1.1). After the second mixing, the specimens were crosslinked during the optimum vulcanization time (
t90) in a 160 °C with 3.5 bar pressure by using a hydraulic high-temperature press. The detailed mixing procedure is described in
Table 3.
2.8. Experimental Methods
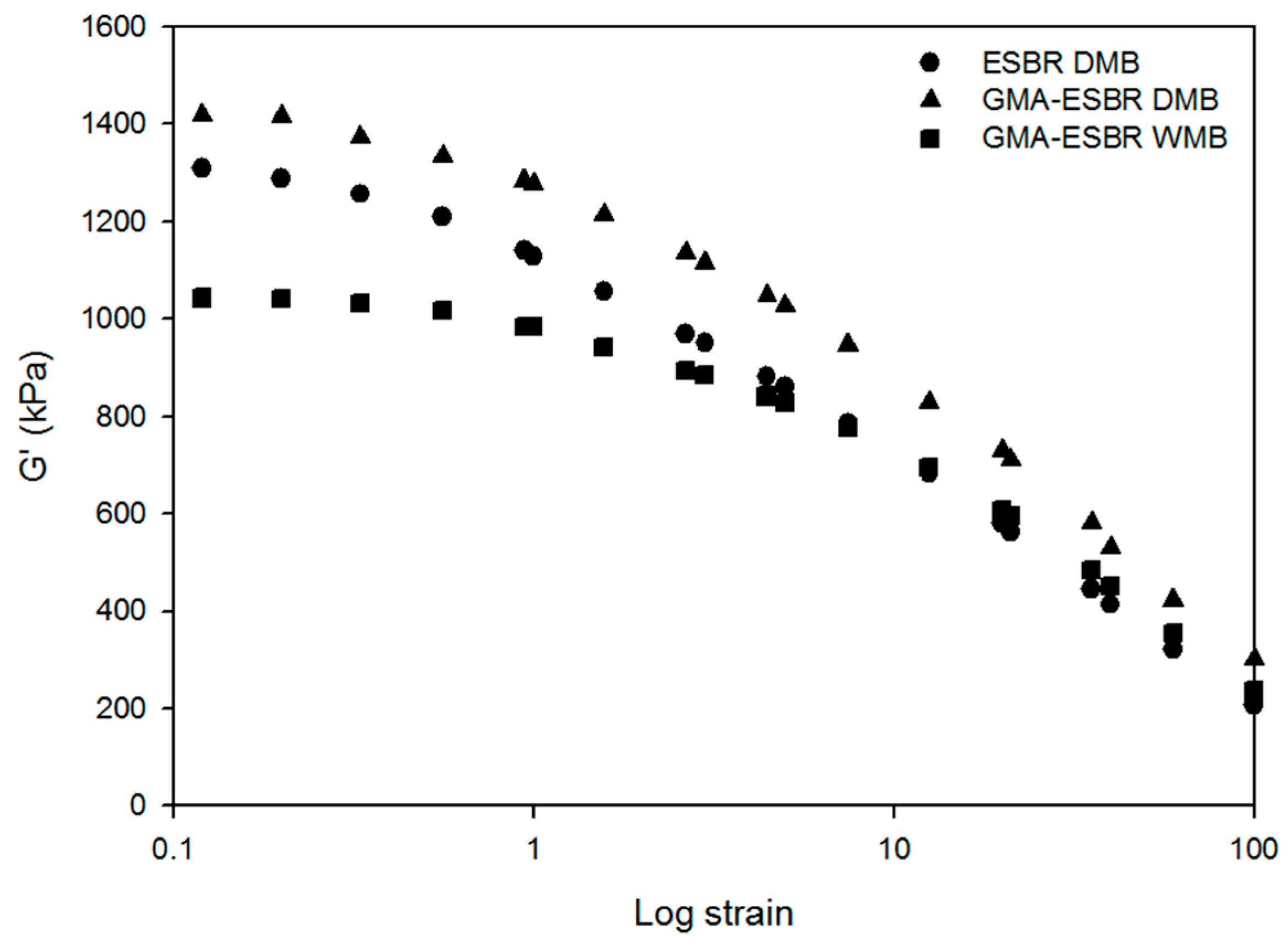
2.8.1. Payne effect
The Payne effect was measured to confirm the silica dispersion. Rubber process analyzer (RPA 2000, Alpha Technologies, Hudson, OH, USA) was used according to ASTM D 8059.
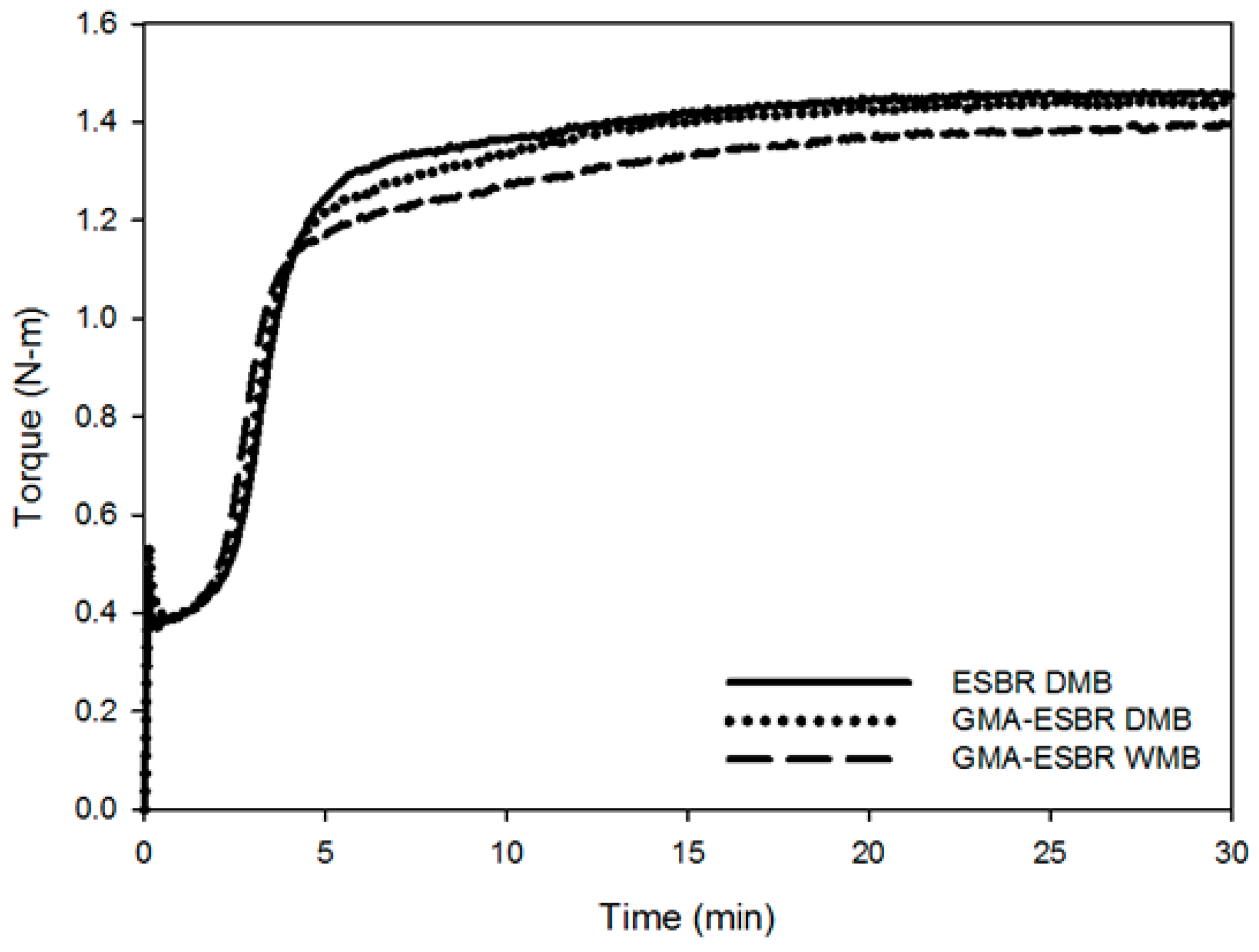
2.8.2. Cure Characteristics
Cure characteristics, such as the minimum and maximum torque values and the optimum vulcanization time (t90), were determined by using a moving die rheometer (MDR, Myung Ji Co., Seoul, Korea). The measurement was performed using a silica compound with a vibration angle of ± 1° and a temperature of 160 °C for 30 min. By using the optimum vulcanization time, vulcanization was performed in a 160 °C press.
2.8.3. Analysis of the Vulcanizate Structure
The crosslinked specimen was cut into a size of 10 mm × 10 mm, and the organic additives in the specimen were removed by immersing the specimen in THF and n-hexane as a solvent for 2 days and 1 day, respectively, followed by drying for 1 day at room temperature. The specimen from which the additives were removed was swollen with toluene for 1 day and then weighed; the following equation was used to calculate crosslink density
v (mol/g):
where
MC is the average molecular weight between the crosslink points (g/mol),
V1 is the volume fraction of rubber in the swollen gel at equilibrium,
V0 is the molar volume of solvent (cm
3/mol),
ρr is the density of the rubber sample (g/cm
3), and
χ is the polymer–solvent interaction parameter.
2.8.4. Mechanical Properties
A universal testing machine (UTM, Model: KSU-05M-C, KSU Co., Ansan, Korea) was used to measure the modulus, tensile strength, and elongation at break. The dumbbell specimens were prepared according to ASTM D412. The measurement speed was 500 mm/min.
2.8.5. Abrasion Loss
The specimens were prepared according to DIN 53516 for wear testing. After the initial weight of the specimen was measured, the specimen was abraded for 40 m at a speed of 40 rpm with 5N of load using a Deutsche Industrie Normen (DIN) abrasion tester, and the specimen weight was measured again to measure weight loss.
2.8.6. Dynamic Viscoelastic Properties
By using a dynamic mechanical thermal analyzer (DMTA, EPLEXOR 500N, GABO, Ahlden, Germany), the three vulcanizates were measured at a strain of 30 µm and a frequency of 10 Hz. The measurement was conducted in the tension mode, at a temperature increase of 3 °C/min, and a temperature range of –80 to 70 °C.
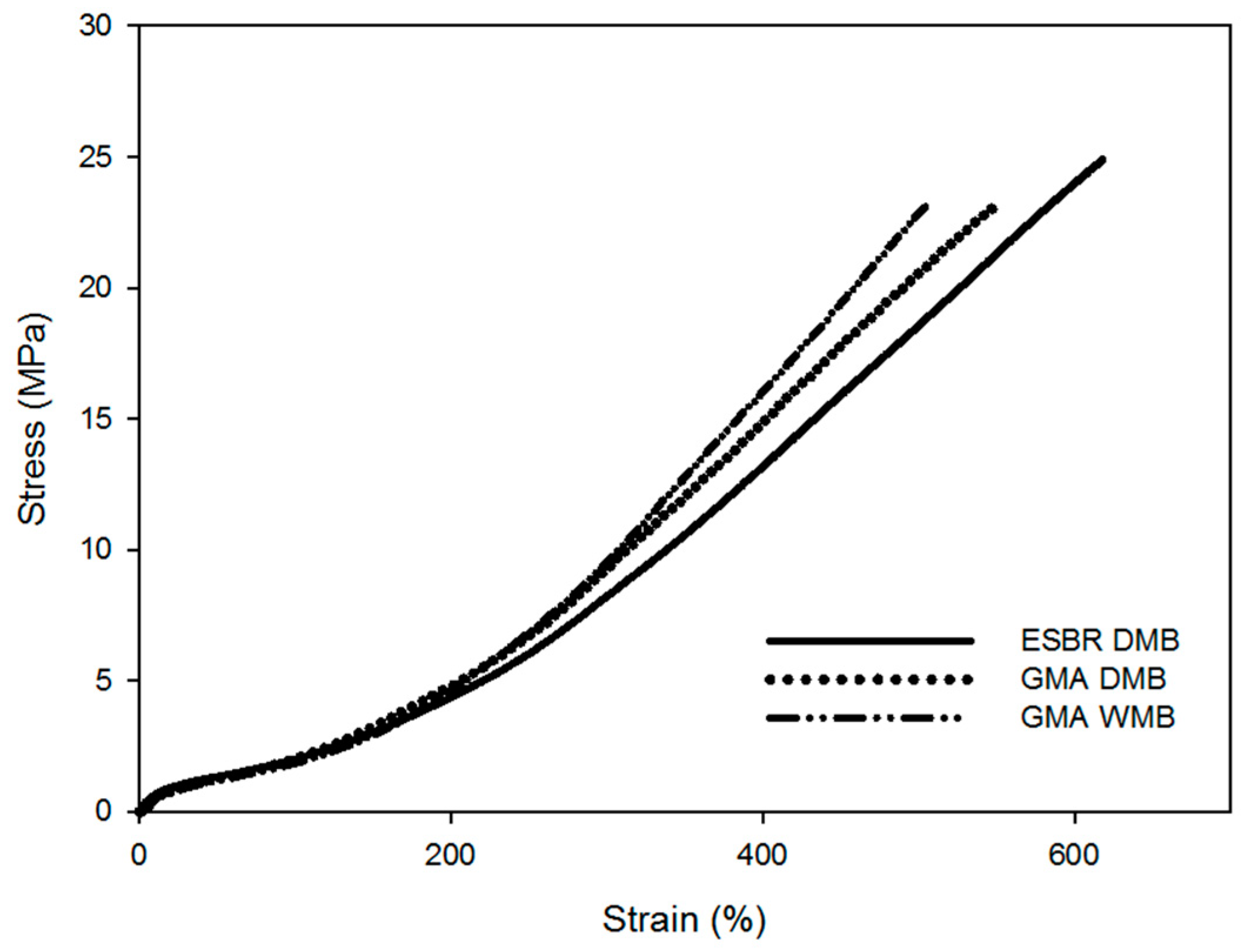
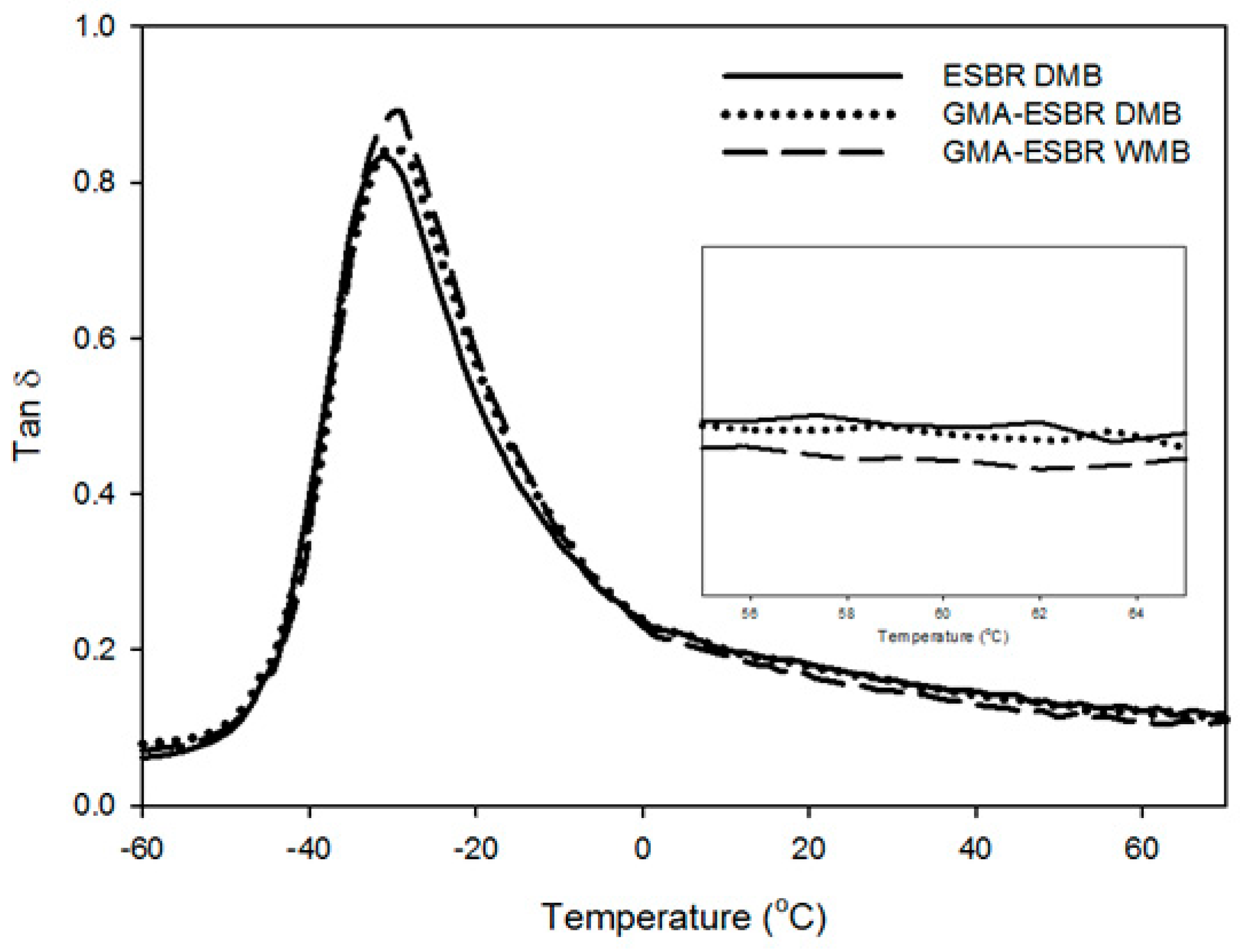
4. Conclusions
This study confirmed that GMA-ESBR can form a gel when heated, resulting in higher compound viscosity and lower processability. GMA-ESBR was predicted to have a better filler–rubber interaction if reacted with silica rather than GMA when the epoxy ring is opened. Therefore, a silica compound was prepared by applying WMB technology, which can coagulate the rubber latex and silica through a relatively uniform dispersion in the rubber latex at low temperatures. Furthermore, the silica compound was manufactured by existing DMB technology, and the properties were compared and evaluated. The results confirmed that the GMA-ESBR silica compound exhibits superior physical properties to the ESBR silica compound, and the GMA-ESBR WMB compound shows better physical properties than the GMA-ESBR DMB compound. These results show that the thermal gelation problem of GMA-ESBR can be solved by reacting an epoxy group with a silanol group on the silica surface using WMB technology. If GMA-ESBR, which is a functionalized ESBR, is used in the tread compound for passenger cars, it will be able to produce tires with excellent abrasion and rolling resistance.