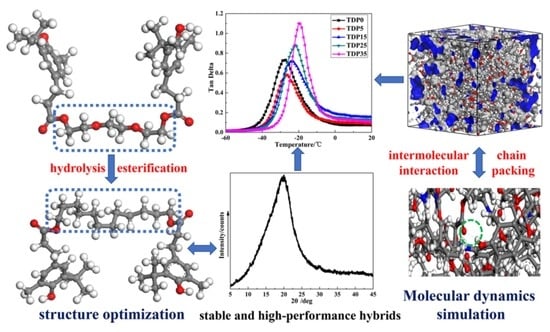
Towards a Stable and High-Performance Hindered Phenol/Polymer-Based Damping Material Through Structure Optimization and Damping Mechanism Revelation
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
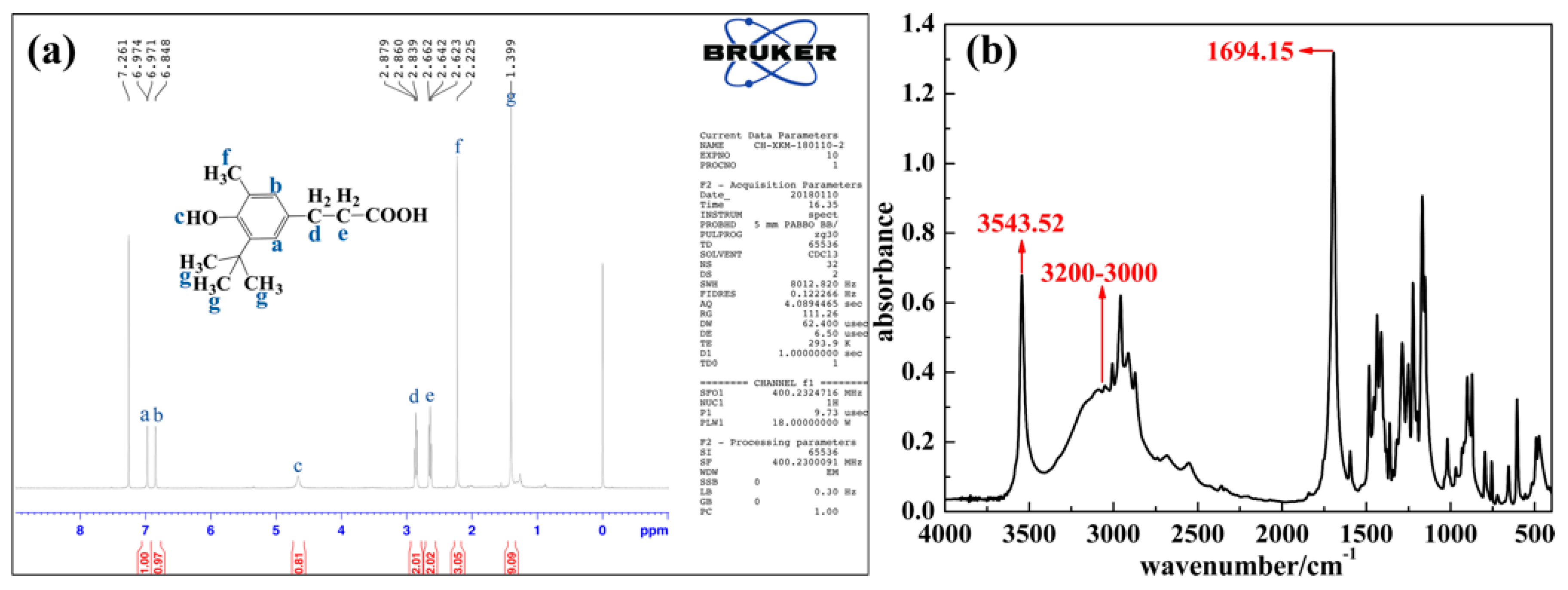
2.2. Synthesis of the Linear Hindered Phenol: Dodecane-1,12-diyl bis 3-(3-tertbutyl-4-hydroxy-5-methylphenyl)propionate (DP)
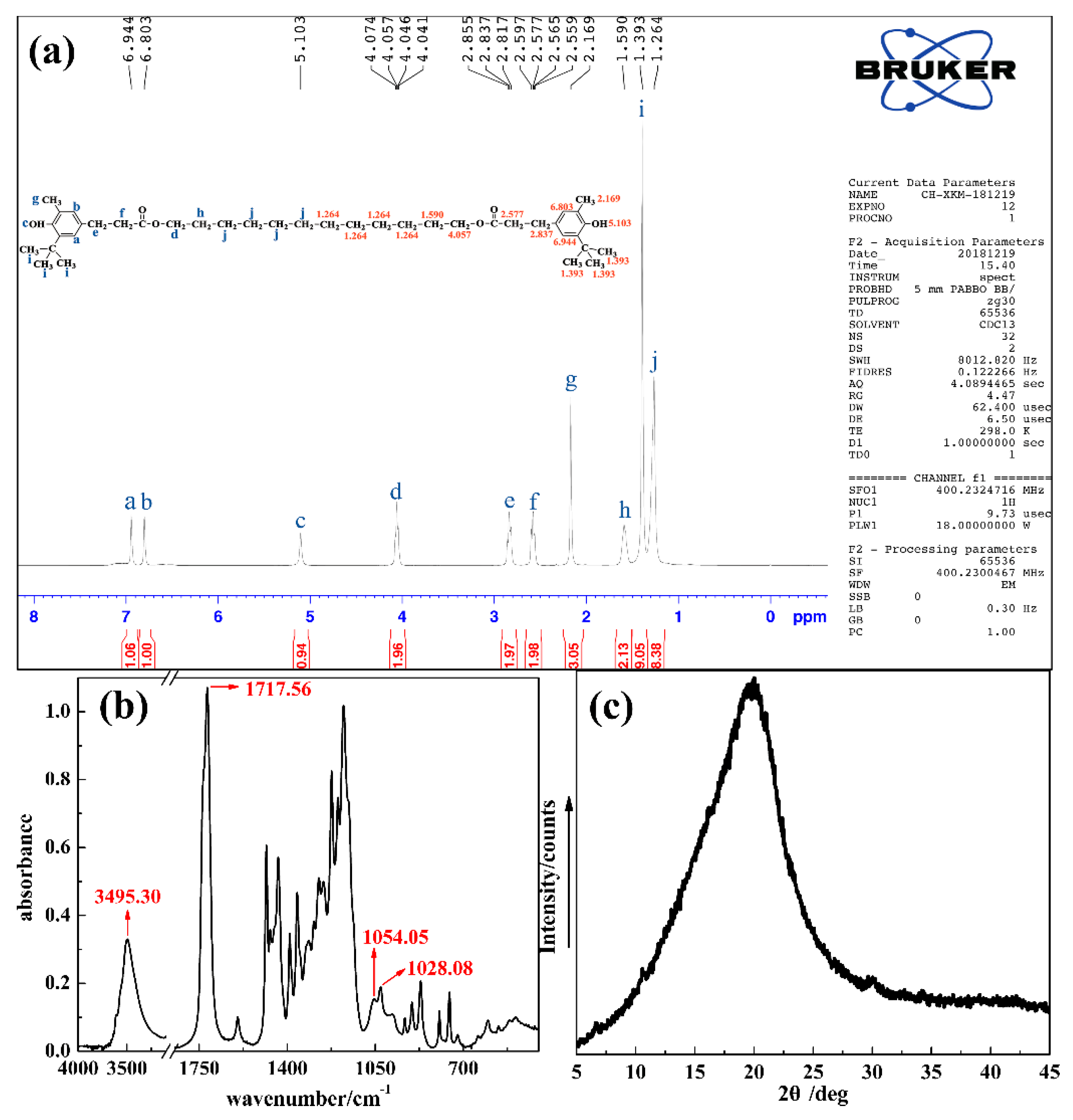
2.3. Preparation of TPU/DP Hybrids
2.4. Characterization
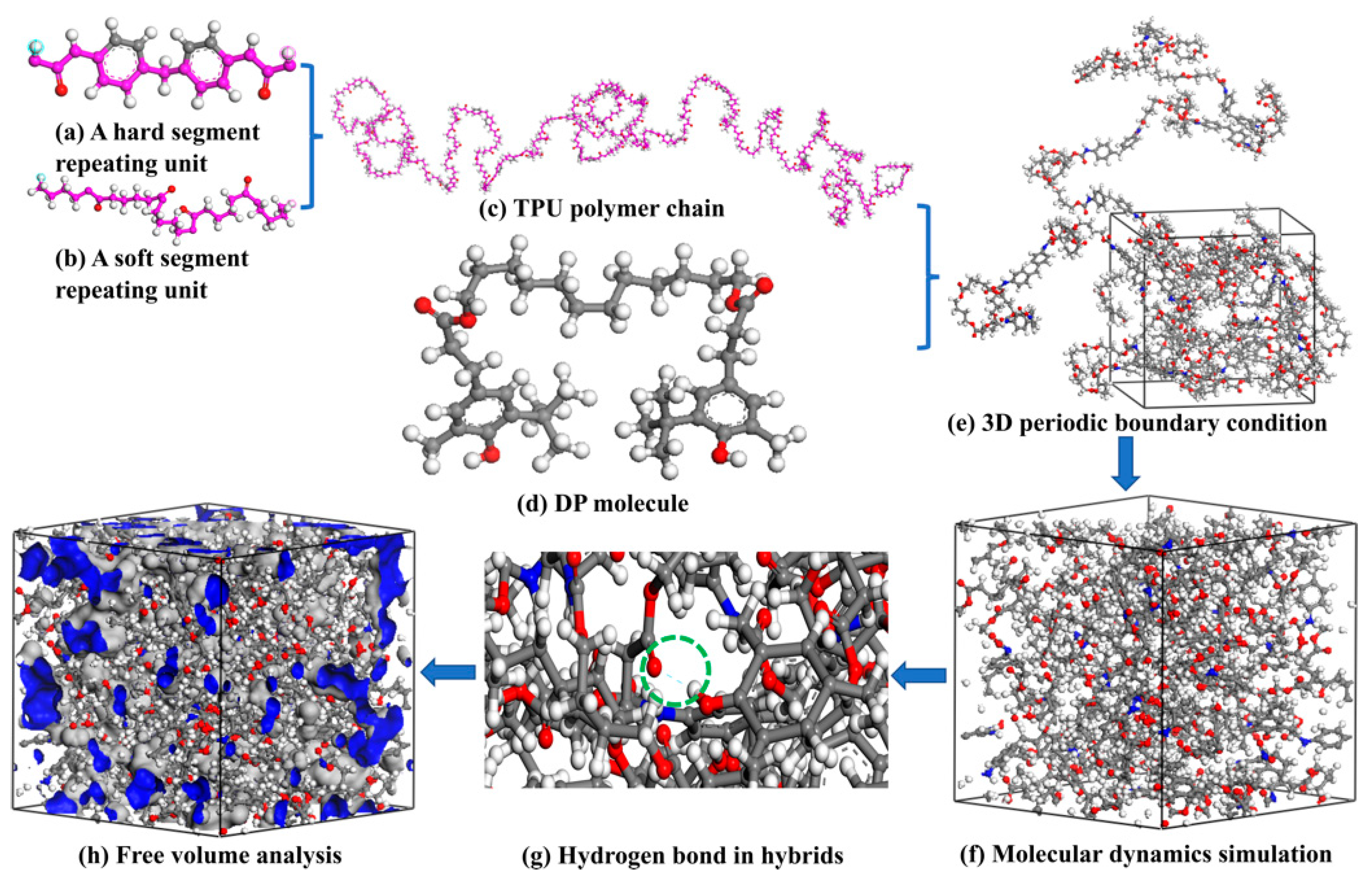
2.5. MD Simulation for TPU/DP Hybrids
3. Results and Discussion
3.1. Structure of AO and DP
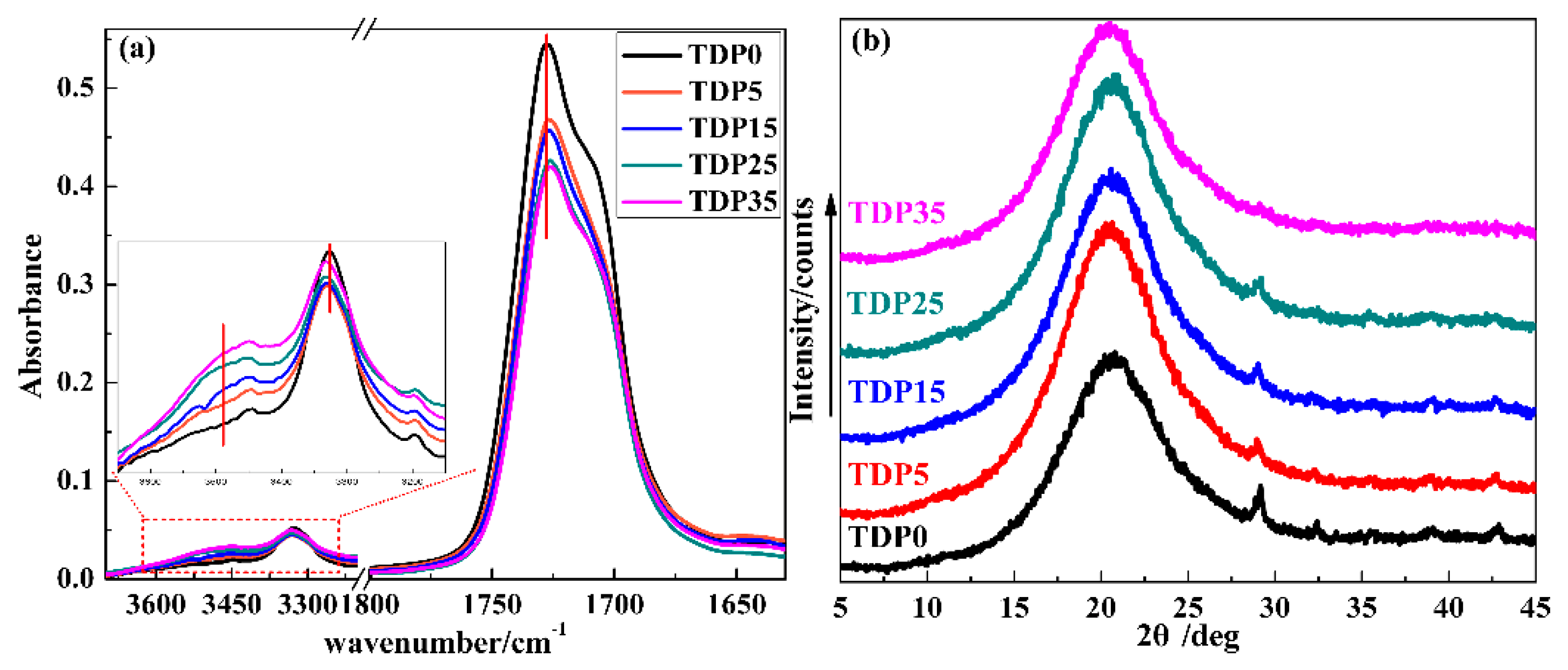
3.2. The Character of TDP Hybrids
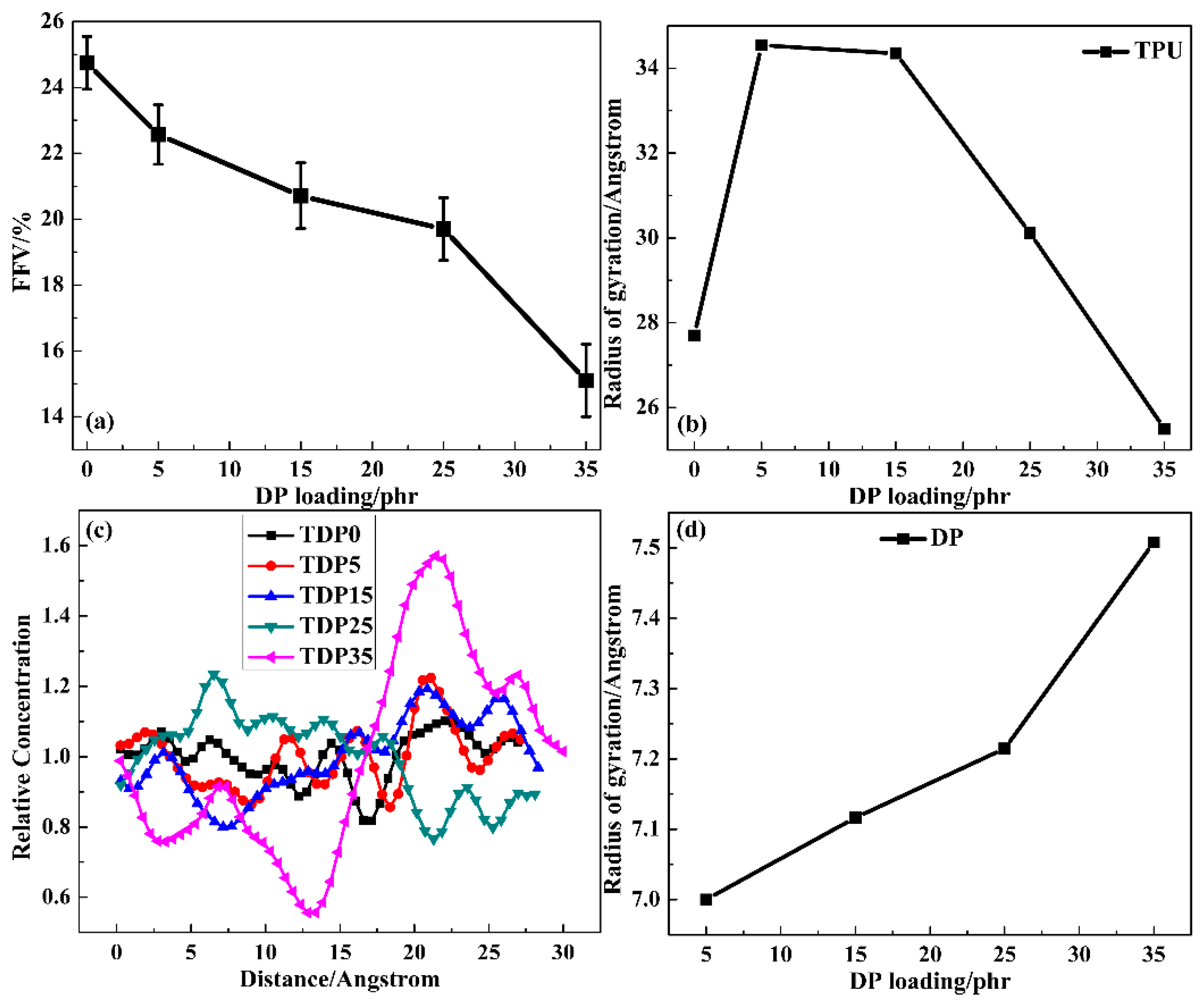
3.3. MD Simulation of TDP Hybrids
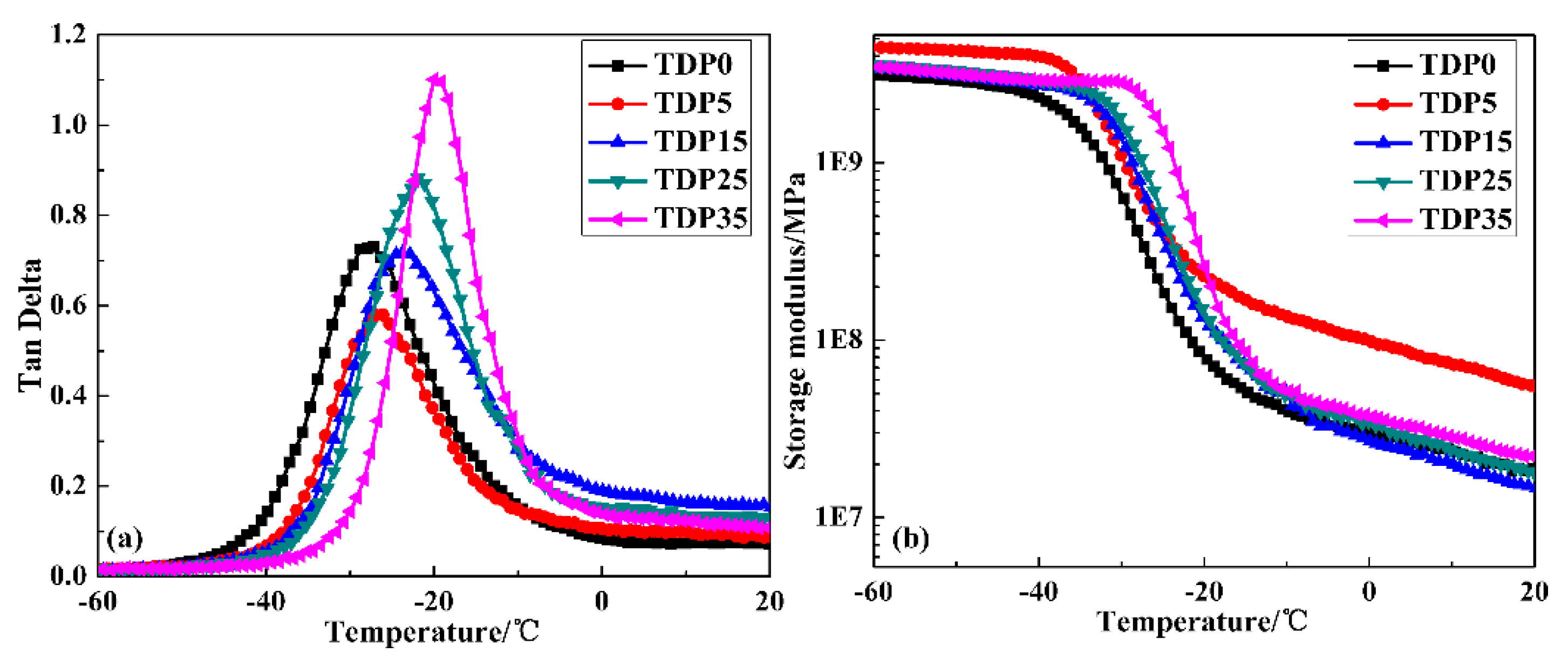
3.4. Damping Property and Mechanism of TDP Hybrids
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lakes, R.-S.; Lee, T.; Bersie, A.; Wang, Y. Extreme damping in composite materials with negative-stiffness inclusions. Nature 2001, 410, 565–567. [Google Scholar] [CrossRef]
- Adams, J.-D.; Erickson, B.-W.; Grossenbacher, J.; Brugger, J.; Nievergelt, A.; Fantner, G.-E. Harnessing the damping properties of materials for high-speed atomic force microscopy. Nat. Nanotechnol. 2016, 11, 147. [Google Scholar] [CrossRef]
- Eichler, A.; Moser, J.; Chaste, J.; Zdrojek, M.; Wilson-Rae, I.; Bachtold, A. Nonlinear damping in mechanical resonators made from carbon nanotubes and graphene. Nat. Nanotechnol. 2011, 6, 339–342. [Google Scholar] [CrossRef]
- Sun, T.-L.; Kurokawa, T.; Kuroda, S.; Bin Ihsan, A.; Akasaki, T.; Sato, K.; Haque, M.-A.; Nakajima, T.; Gong, J.-P. Physical hydrogels composed of polyampholytes demonstrate high toughness and viscoelasticity. Nat. Mater. 2013, 12, 932–937. [Google Scholar] [CrossRef]
- Sun, L.; Gibson, R.-F.; Gordaninejad, F.; Suhr, J. Energy absorption capability of nanocomposites: A. review. Compos. Sci. Technol. 2009, 69, 2392–2409. [Google Scholar] [CrossRef]
- Liu, Q.-L.; Li, M.; Gu, Y.-Z.; Wang, S.-K.; Zhang, Y.-Y.; Li, Q.-W.; Gao, L.-M.; Zhang, Z.-G. Interlocked CNT networks with high damping and storage modulus. Carbon 2015, 86, 46–53. [Google Scholar] [CrossRef]
- Wu, J.-H.; Li, C.-H.; Wu, Y.-T.; Leu, M.-T.; Tsai, Y. Thermal resistance and dynamic damping properties of poly (styrene–butadiene–styrene)/thermoplastic polyurethane composites elastomer material. Compos. Sci. Technol. 2010, 70, 1258–1264. [Google Scholar] [CrossRef]
- Yamazaki, H.; Takeda, M.; Kohno, Y.; Ando, H.; Urayama, K.; Takigawa, T. Dynamic viscoelasticity of poly(butyl acrylate) elastomers containing dangling chains with controlled lengths. Macromolecules 2011, 44, 8829–8834. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, D.; Du, M.; Zheng, Q. Role of graded length side chains up to 18 carbons in length on the damping behavior of polyurethane/epoxy interpenetrating polymer networks. Eur. Polym. J. 2013, 49, 1731–1741. [Google Scholar] [CrossRef]
- Ward, I.-M.; Sweeney, J. Mechanical Properties of Solid Polymers; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Gao, Y.; Wang, X.-P.; Liu, M.-J.; Xi, X.; Zhang, X.; Jia, D.-M. Effect of montmorillonite on carboxylated styrene butadiene rubber/hindered phenol damping material with improved extraction resistance. Mater. Des. 2014, 58, 316–323. [Google Scholar] [CrossRef]
- Roland, C.-M. Viscoelastic Behavior of Rubbery Materials; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Treviso, A.; Van Genechten, B.; Mundo, D.; Tournour, M. Damping in composite materials: Properties and models. Compos. Part B Eng. 2015, 78, 144–152. [Google Scholar] [CrossRef]
- Wu, C.-F.; Yamagishi, T.-A.; Nakamoto, Y.; Ishida, S.; Nitta, K.-H.; Kubota, S. Organic hybrid of chlorinated polyethylene and hindered phenol. I. Dynamic mechanical properties. J. Polym. Sci. Pol. Phys. 2000, 38, 2285–2295. [Google Scholar] [CrossRef]
- Wu, C.-F.; Yamagishi, T.-A.; Nakamoto, Y.; Ishida, S.; Kubota, S.; Nitta, K.-H. Organic hybrid of chlorinated polyethylene and hindered phenol. II. Influence of the chemical structure of small molecules on viscoelastic properties. J. Polym. Sci. Pol. Phys. 2000, 38, 1496–1503. [Google Scholar] [CrossRef]
- Wu, C.-F.; Yamagishi, T.-A.; Nakamoto, Y.; Ishida, S.; Nitta, K.-H. Organic hybrid of chlorinated polyethylene and hindered phenol. III. Influence of the molecular weight and chlorine content of the polymer on the viscoelastic properties. J. Polym. Sci. Pol. Phys. 2000, 38, 2943–2953. [Google Scholar] [CrossRef]
- Wu, C.-F. Organic hybrid of chlorinated polyethylene and hindered phenol. IV. Modification on dynamic mechanical properties by chlorinated paraffin. J. Polym. Sci. Pol. Phys. 2001, 39, 23–31. [Google Scholar] [CrossRef]
- Song, M.; Zhao, X.-Y.; Li, Y.; Chan, T.-W.; Zhang, L.-Q.; Wu, S.-Z. Effect of acrylonitrile content on compatibility and damping properties of hindered phenol AO-60/nitrile-butadiene rubber composites: Molecular dynamics simulation. RSC Adv. 2014, 4, 48472–48479. [Google Scholar] [CrossRef]
- Song, M.; Zhao, X.-Y.; Li, Y.; Hu, S.-K.; Zhang, L.-Q.; Wu, S.-Z. Molecular dynamics simulations and microscopic analysis of the damping performance of hindered phenol AO-60/nitrile-butadiene rubber composites. RSC Adv. 2014, 4, 6719–6729. [Google Scholar] [CrossRef]
- Xu, K.-M.; Zhang, F.-S.; Zhang, X.-L.; Hu, Q.-M.; Wu, H.; Guo, S.-Y. Molecular insights into hydrogen bonds in polyurethane/hindered phenol hybrids: Evolution and relationship with damping properties. J. Mater. Chem. A 2014, 2, 8545–8556. [Google Scholar] [CrossRef]
- Xu, K.-M.; Zhang, F.-S.; Zhang, X.-L.; Guo, J.-W.; Wu, H.; Guo, S.-Y. Molecular insights into the damping mechanism of poly (vinyl acetate)/hindered phenol hybrids by a combination of experiment and molecular dynamics simulation. RSC Adv. 2015, 5, 4200–4209. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Xiang, P.; Tian, M.; Fong, H.; Jin, R.-J.; Zhang, L.-Q. Nitrile butadiene rubber/hindered phenol nanocomposites with improved strength and high damping performance. Polymer 2007, 48, 6056–6063. [Google Scholar] [CrossRef]
- Qiao, B.; Zhao, X.-Y.; Yue, D.-M.; Zhang, L.-Q.; Wu, S.-Z. A combined experiment and molecular dynamics simulation study of hydrogen bonds and free volume in nitrile-butadiene rubber/hindered phenol damping mixtures. J. Mater. Chem. 2012, 22, 12339–12348. [Google Scholar] [CrossRef]
- Yang, D.-W.; Zhao, X.-Y.; Chan, T.; Zhang, L.-Q.; Wu, S.-Z. Investigation of the damping properties of hindered phenol AO-80/polyacrylate hybrids using molecular dynamics simulations in combination with experimental methods. J. Mater. Sci. 2016, 51, 5760–5774. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Zhang, G.; Lu, F.; Zhang, L.-Q.; Wu, S.-Z. Molecular-level insight of hindered phenol AO-70/nitrile-butadiene rubber damping composites through a combination of a molecular dynamics simulation and experimental method. RSC Adv. 2016, 6, 85994–86005. [Google Scholar] [CrossRef]
- Xiao, D.-L.; Zhao, X.-Y.; Feng, Y.-P.; Xiang, P.; Zhang, L.-Q.; Wang, W.-M. The structure and dynamic properties of thermoplastic polyurethane elastomer/hindered phenol hybrids. J. Appl. Polym. Sci. 2010, 116, 2143–2150. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Lu, Y.-L.; Xiao, D.-L.; Wu, S.-Z.; Zhang, L.-Q. Thermoplastic Ternary Hybrids of Polyurethane, Hindered Phenol and Hindered Amine with Selective Two-Phase Dispersion. Macromol. Mater. Eng. 2009, 294, 345–351. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Cao, Y.-J.; Zou, H.; Li, J.; Zhang, L.-Q. Structure and dynamic properties of nitrilebutadiene rubber/hindered phenol composites. J. Appl. Polym. Sci. 2012, 123, 3696–3702. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, X.-Y.; Liu, L.; Yang, R.-N.; Song, M.; Wu, S.-Z. Thermodynamic analyses of the hydrogen bond dissociation reaction and their effects on damping and compatibility capacities of polar small molecule/nitrile-butadiene rubber systems: Molecular simulation and experimental study. Polymer 2018, 155, 152–167. [Google Scholar] [CrossRef]
- Wu, C.-F.; Kuriyama, T.; Inoue, T. Crystalline structure and morphology of a hindered phenol in a chlorinated polyethylene matrix. J. Mater. Sci. 2004, 39, 1249–1254. [Google Scholar] [CrossRef]
- Lin, T.-F.; Tang, Z.-H.; Guo, B.-C. New Design Strategy for Reversible Plasticity Shape Memory Polymers with Deformable Glassy Aggregates. ACS Appl. Mater. Interfaces 2014, 6, 21060–21068. [Google Scholar] [CrossRef]
- Wu, C.-F.; Saburo, A. Crystallization of a vitrified hindered phenol within chlorinated polyethylene and its effects on dynamic mechanical properties. J. Polym. Sci. Pol. Phys. 2004, 42, 209–215. [Google Scholar] [CrossRef]
- Wu, C.-F. Microstructural development of a vitrified hindered phenol compound during thermal annealing. Polymer 2003, 44, 1697–1703. [Google Scholar] [CrossRef]
- Wu, C.-F.; Mori, K.; Otani, Y.; Namiki, N.; Emi, H. Effects of molecule aggregation state on dynamic mechanical properties of chlorinated polyethylene/hindered phenol blends. Polymer 2001, 42, 8289–8295. [Google Scholar] [CrossRef]
- Li, C.; Xu, S.-A.; Xiao, F.-Y.; Wu, C.-F. Dynamic mechanical properties of chlorinated butyl rubber blends. Eur. Polym. J. 2006, 42, 2507–2514. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Wang, L.-F.; Zhao, Y.-F. Fabrication of novel hindered phenol/phenol resin/nitrile butadiene rubber hybrids and their long–period damping properties. Polym. Compos. 2012, 33, 2125–2133. [Google Scholar] [CrossRef]
- Shi, G.-P.; Yin, X.-T.; Wu, G.-Z. Thermodynamic phase analysis of acrylic polymer/hindered phenol hybrids: Effects of hydrogen bonding strength. Polymer 2018, 153, 317–324. [Google Scholar] [CrossRef]
- Zhang, G.; Li, H.-X.; Antenseniner, M.; Chung, T.-C.-M. Synthesis of functional polypropylene containing hindered phenol stabilizers and applications in metallized polymer film capacitors. Macromolecules 2015, 48, 2925–2934. [Google Scholar] [CrossRef]
- Kim, T.-H.; Oh, D.-R. Melt grafting of maleimides having hindered phenol antioxidant onto low molecular weight polyethylene. Polym. Degrad. Stab. 2004, 84, 499–503. [Google Scholar] [CrossRef]
- Shi, X.-M.; Wang, J.-D.; Jiang, B.-B.; Yang, Y.-R. Hindered phenol grafted carbon nanotubes for enhanced thermal oxidative stability of polyethylene. Polymer 2013, 54, 1167–1176. [Google Scholar] [CrossRef]
- Bergenudd, H.; Eriksson, P.; DeArmitt, C.; Stenberg, B.; Jonsson, E.-M. Synthesis and evaluation of hyperbranched phenolic antioxidants of three different generations. Polym. Degrad. Stab. 2002, 76, 503–509. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.-F.; Zhan, M.-S.; Wang, J.-W.; Zhao, C.; Liu, X.-Y.; Zhang, J.-H. Nitrile rubber/hindered phenol exterminated hyperbranched polyester materials with improved damping and mechanical performance. J. Appl. Polym. Sci. 2015, 132, 42605. [Google Scholar] [CrossRef]
- Inada, Y.; Orita, H. Efficiency of numerical basis sets for predicting the binding energies of hydrogen bonded complexes: Evidence of small basis set superposition error compared to Gaussian basis sets. J. Comput. Chem. 2008, 29, 225–232. [Google Scholar] [CrossRef]
- Sun, H. COMPASS: An ab initio force-field optimized for condensed-phase applications overview with details on alkane and benzene compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Habasaki, J.; Ueda, A. Molecular dynamics study of one component soft-core system-Analytical expression of non-equilibrium relaxation in constant pressure conditions. J. Non-Cryst. Solids 2016, 447, 212–222. [Google Scholar] [CrossRef]
- Bian, C.; Wang, S.; Liu, Y.; Su, K.; Jing, X. Role of Nonbond Interactions in the Glass Transition of Novolac-Type Phenolic Resin: A Molecular Dynamics Study. Ind. Eng. Chem. Res. 2016, 55, 9440–9451. [Google Scholar] [CrossRef]
- Fuhai, C.; Jincheng, W. Preparation and characterization of hyperbranched polymer modified montmorillonite/chlorinated butyl rubber damping composites. J. Appl. Polym. Sci. 2016, 133, 43645. [Google Scholar] [CrossRef]
- Domnina, N.-S.; Sergeeva, O.-Y.; Komarova, E.-A.; Mikhailova, M.-E.; Vol’eva, V.-B.; Belostotskaya, I.-S.; Komissarova, N.-L. Indicator properties of oligoethylene glycol hybrids with sterically hindered phenols. Russ. J. Org. Chem. 2014, 50, 371–375. [Google Scholar] [CrossRef]
- Seuring, J.; Reiss, P.; Koert, U.; Agarwal, S. Synthesis, characterization and properties of a new polymerisable surfactant: 12-Methacryloyl dodecylphosphocholine. Chem. Phys. Lipids 2010, 163, 367–372. [Google Scholar] [CrossRef]
- Barbiroli, G.; Lorenzetti, C.; Berti, C.; Fiorini, M.; Manaresi, P. Polyethylene like polymers. Aliphatic polyesters of dodecanedioic acid: 1. Synthesis and properties. Eur. Polym. J. 2003, 39, 655–661. [Google Scholar] [CrossRef]
- Rodrigues, P.-C.; Cantão, M.-P.; Janissek, P.; Scarpa, P.-C.; Mathias, A.-L.; Ramos, L.-P.; Gomes, M.-A. Polyaniline/lignin blends: FTIR, MEV and electrochemical characterization. Eur. Polym. J. 2002, 38, 2213–2217. [Google Scholar] [CrossRef]
- Bates, S.; Zografi, G.; Engers, D.; Morris, K.; Crowley, K.; Newman, A. Analysis of amorphous and nanocrystalline solids from their X-ray diffraction patterns. Pharm. Res. 2006, 23, 2333–2349. [Google Scholar] [CrossRef]
- Zia, K.-M.; Bhatti, I.-A.; Barikani, M.; Zuber, M.; Bhatti, H.-N. XRD studies of polyurethane elastomers based on chitin/1, 4-butane diol blends. Carbohydr. Polym. 2009, 76, 183–187. [Google Scholar] [CrossRef]
- Yin, X.; Liu, C.; Lin, Y.; Guan, A.; Wu, G. Influence of hydrogen bonding interaction on the damping properties of poly(n-butyl methacrylate)/small molecule hybrids. J. Appl. Polym. Sci. 2015, 132, 41594–41605. [Google Scholar] [CrossRef]
- Mohammad, S.; Alkorta, I. Competition between nonclassical hydrogen-bonded acceptor sites in complexes of neutral AH2 radicals (A = B, Al, and Ga): A theoretical investigation. J. Phys. Chem. A 2006, 110, 10817–10821. [Google Scholar]
- Wu, C.-F. Cooperative behavior of poly (vinyl alcohol) and water as revealed by molecular dynamics simulations. Polymer 2010, 51, 4452–4460. [Google Scholar] [CrossRef]

| Sample Name | Etotal/kcal mol−1 | EDP/kcal mol−1 | Ebinding/kcal mol−1 |
|---|---|---|---|
| TDP5 | −3260.71 | −255.07 | 20.27 |
| TDP15 | −3532.09 | −461.37 | 86.09 |
| TDP25 | −3748.53 | −607.34 | 155.82 |
| TDP35 | −4096.18 | −821.52 | 289.29 |
| Sample Name | Tanδmax | Tanδ > 0.3 | ||||
|---|---|---|---|---|---|---|
| Tg/°C | Value | Tbegin | Tend | ΔT/°C | TA | |
| TDP0 | −27.14 | 0.73 | −35.65 | −17.02 | 18.63 | 10.22 |
| TDP5 | −26.11 | 0.58 | −32.91 | −18.55 | 14.36 | 7.24 |
| TDP15 | −23.74 | 0.72 | −31.90 | −10.88 | 21.02 | 11.63 |
| TDP25 | −21.90 | 0.88 | −30.24 | −10.21 | 20.03 | 12.17 |
| TDP35 | −19.98 | 1.10 | −26.65 | −9.79 | 16.86 | 12.35 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, K.; Hu, Q.; Wang, J.; Zhou, H.; Chen, J. Towards a Stable and High-Performance Hindered Phenol/Polymer-Based Damping Material Through Structure Optimization and Damping Mechanism Revelation. Polymers 2019, 11, 884. https://doi.org/10.3390/polym11050884
Xu K, Hu Q, Wang J, Zhou H, Chen J. Towards a Stable and High-Performance Hindered Phenol/Polymer-Based Damping Material Through Structure Optimization and Damping Mechanism Revelation. Polymers. 2019; 11(5):884. https://doi.org/10.3390/polym11050884
Chicago/Turabian StyleXu, Kangming, Qiaoman Hu, Junhui Wang, Hongdi Zhou, and Jinlei Chen. 2019. "Towards a Stable and High-Performance Hindered Phenol/Polymer-Based Damping Material Through Structure Optimization and Damping Mechanism Revelation" Polymers 11, no. 5: 884. https://doi.org/10.3390/polym11050884
APA StyleXu, K., Hu, Q., Wang, J., Zhou, H., & Chen, J. (2019). Towards a Stable and High-Performance Hindered Phenol/Polymer-Based Damping Material Through Structure Optimization and Damping Mechanism Revelation. Polymers, 11(5), 884. https://doi.org/10.3390/polym11050884