Development and Properties of Polymeric Nanocomposite Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
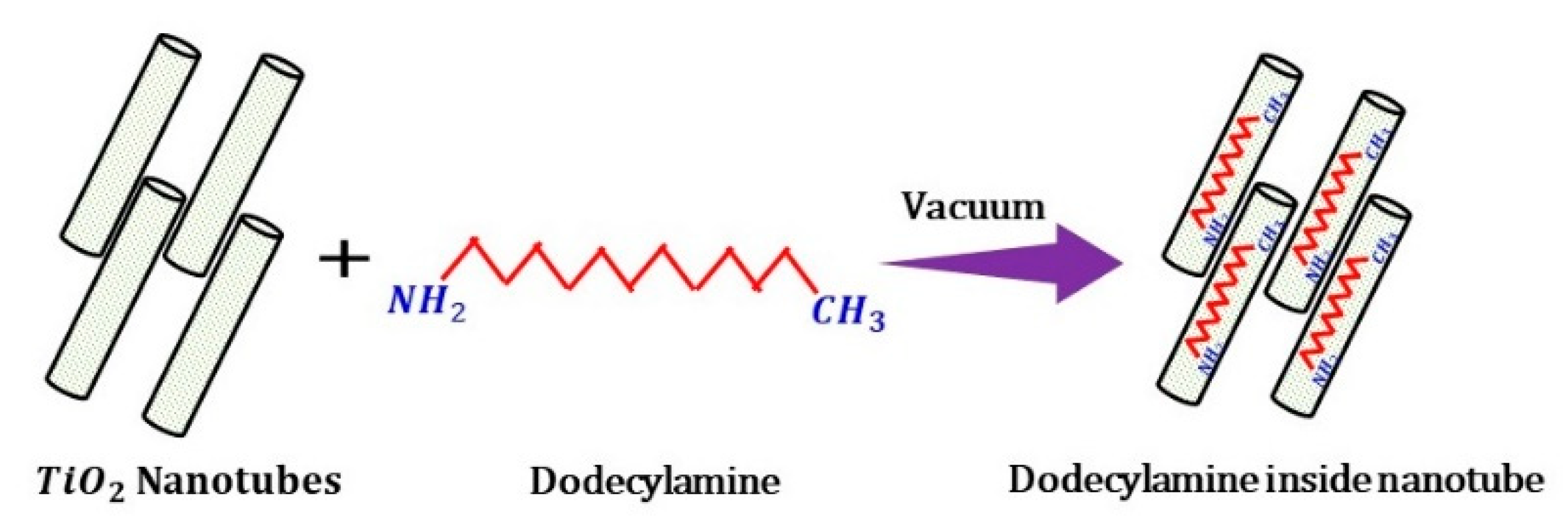
2.2. Synthesis of TiO2 Nanotubes (TNTs)-Nanocontainers
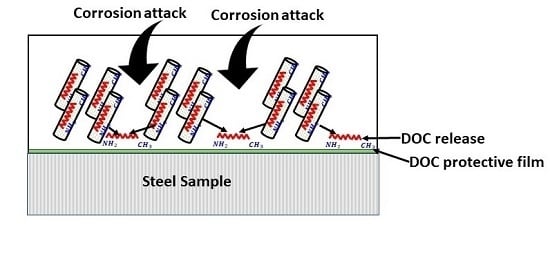
2.3. Loading of TNTs with an Inhibitor
2.4. Preparation of Nanocomposite Coatings
2.5. Characterization
3. Results and Discussions
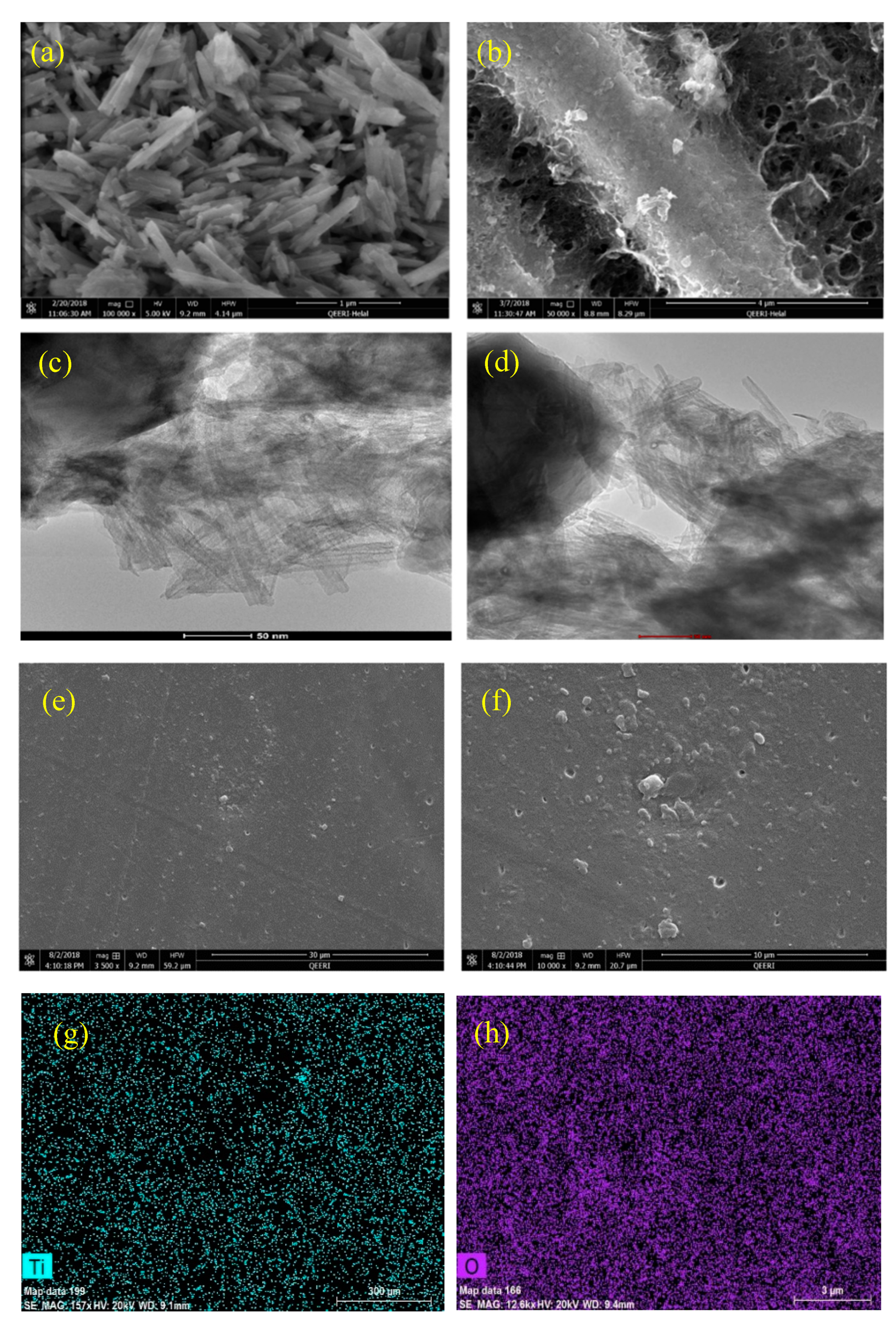
3.1. Morphological Analysis
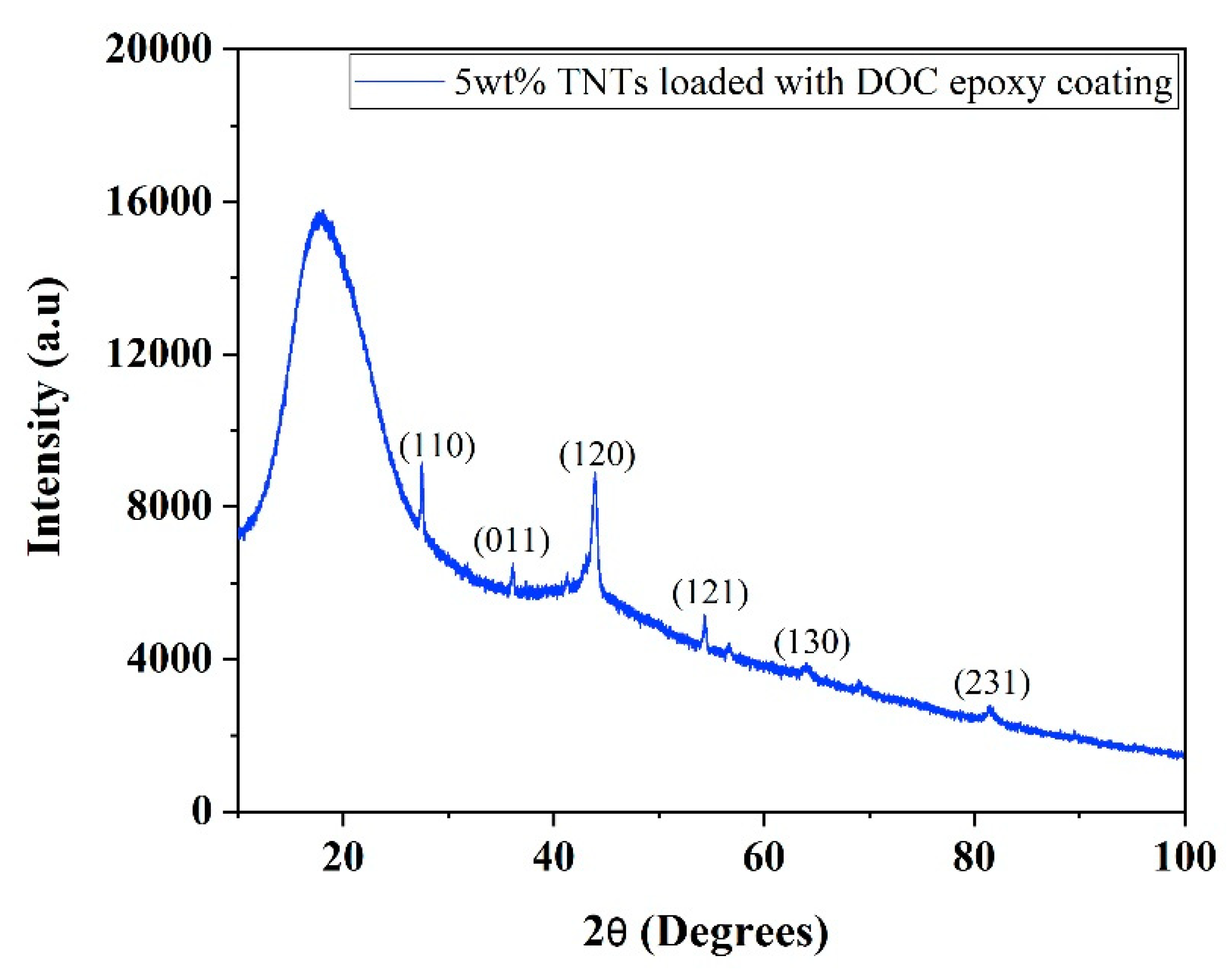
3.2. Structural Analysis
3.3. FTIR Analysis
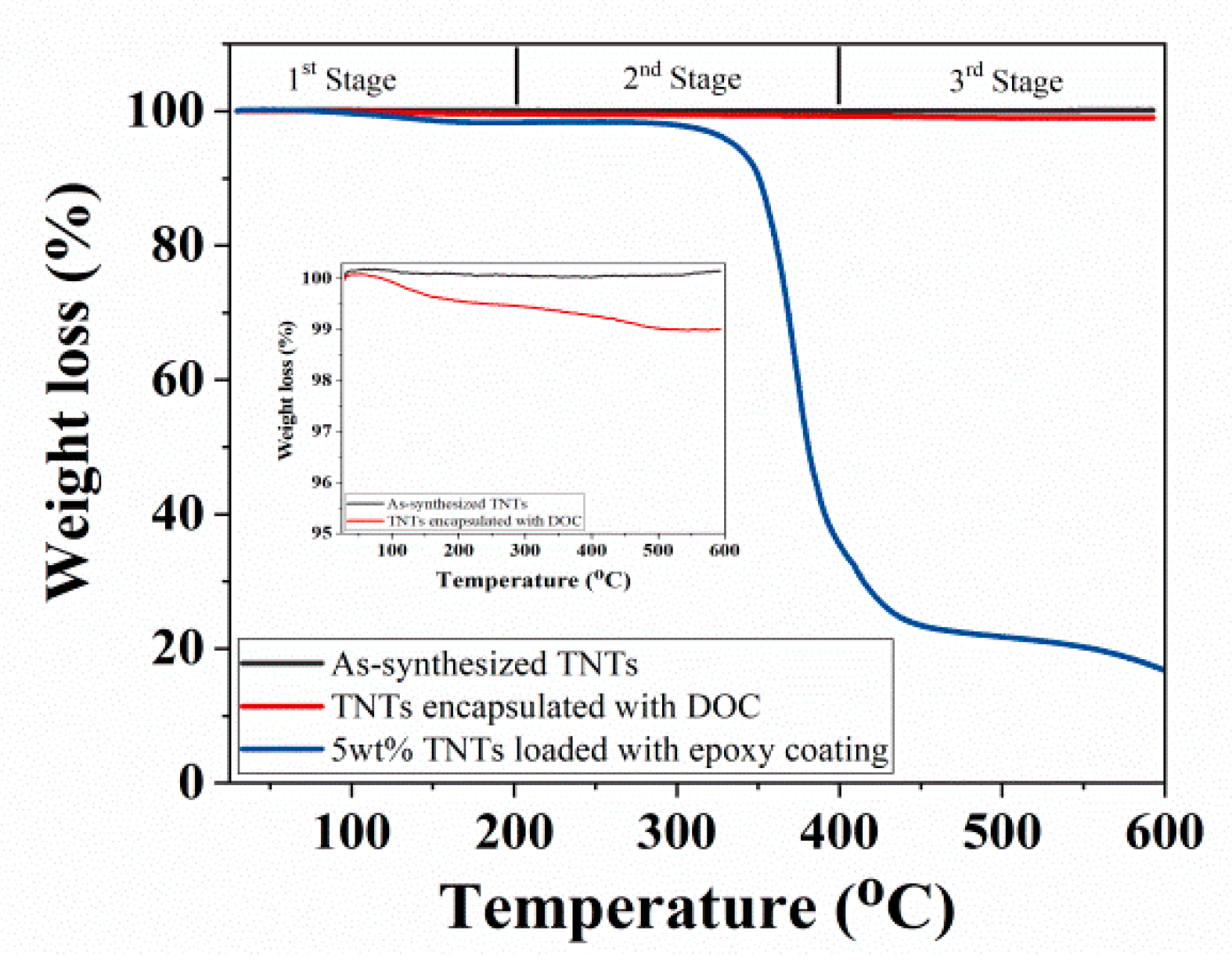
3.4. Thermal Stability
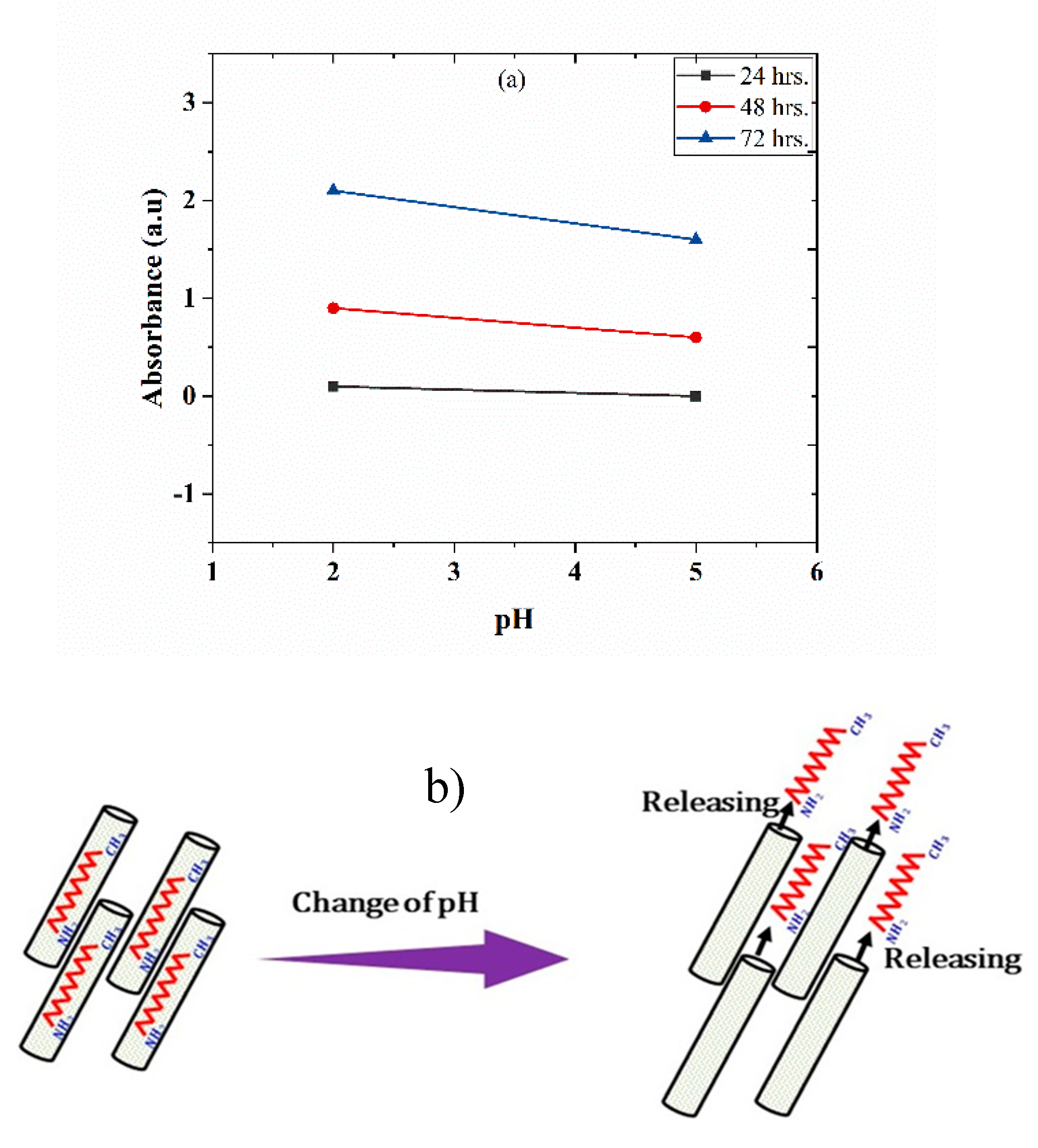
3.5. UV-vis Spectroscopy
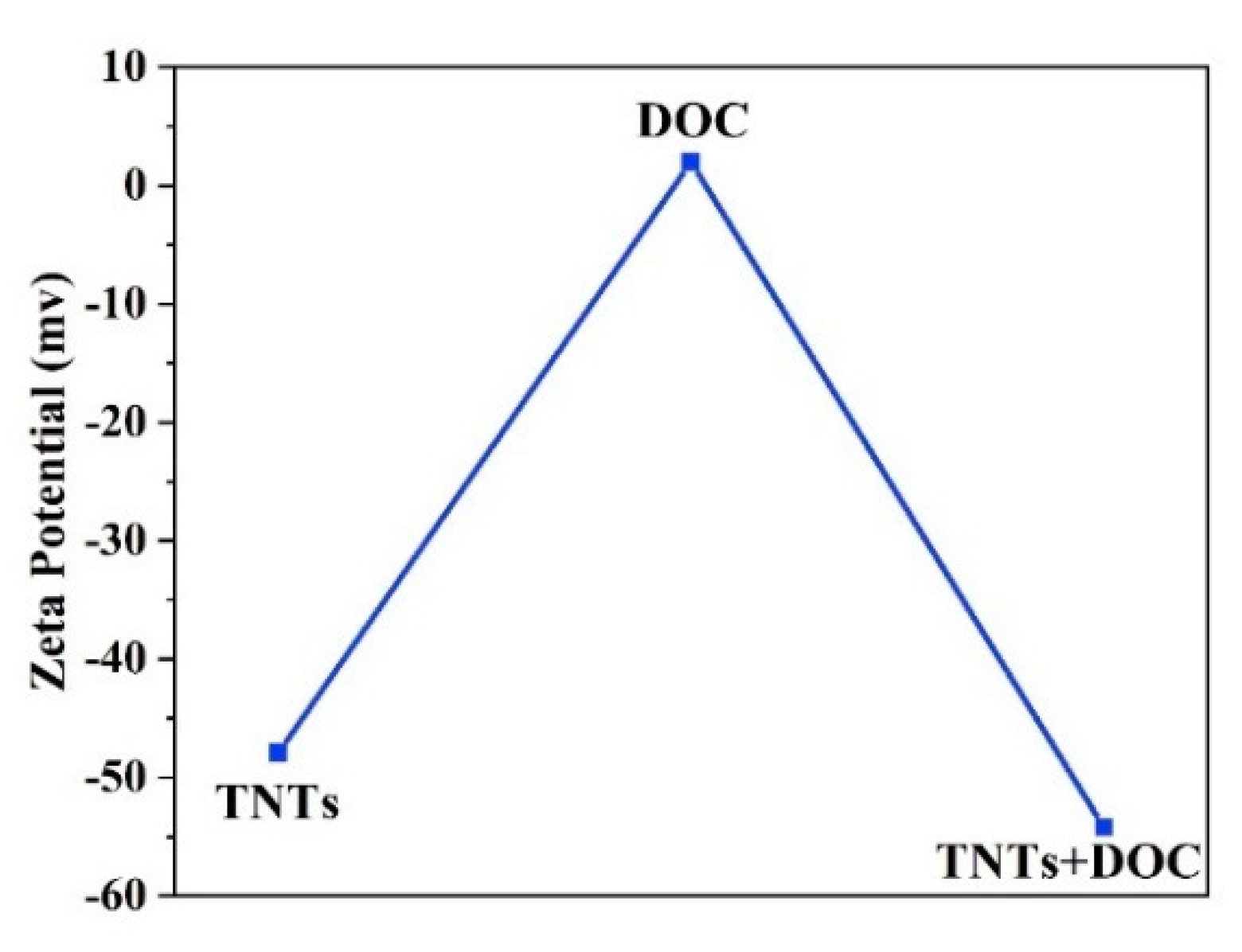
3.6. Zeta Potential Analysis
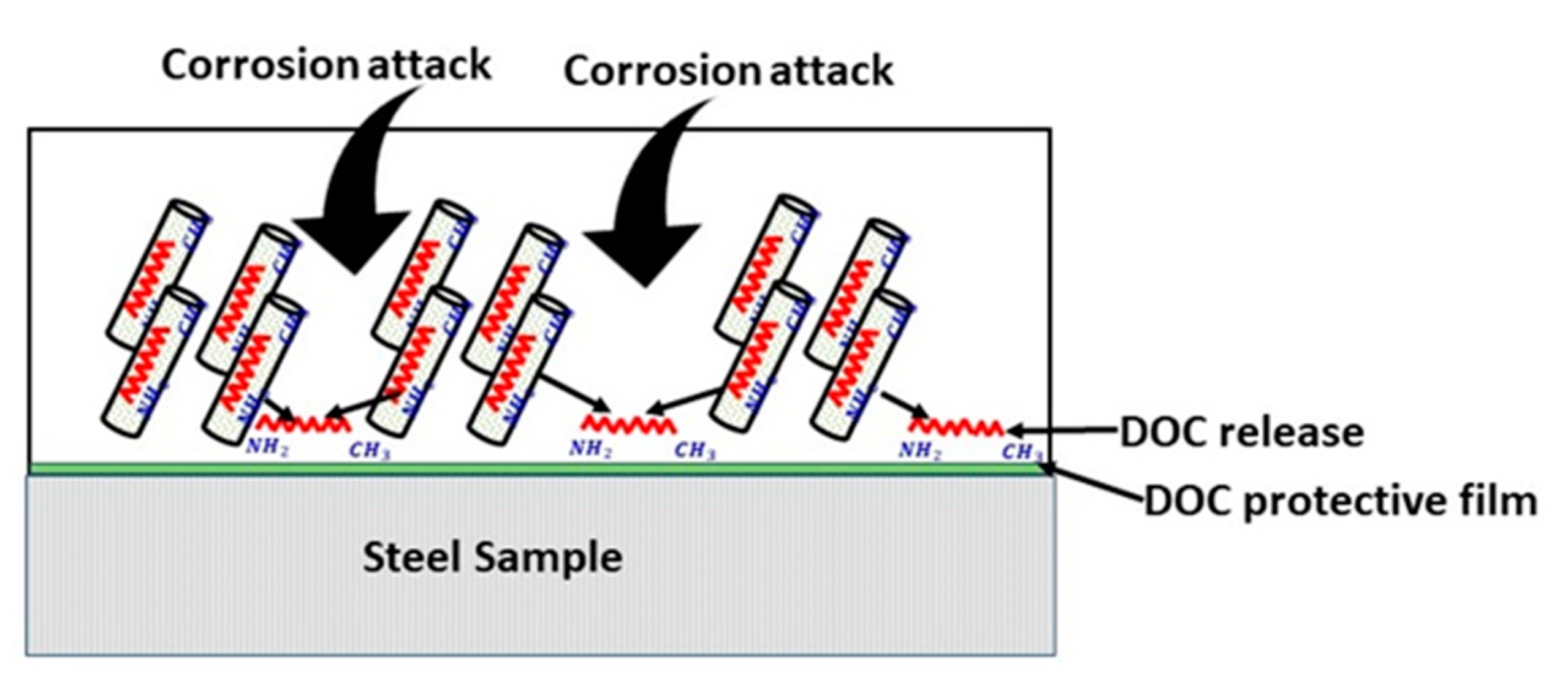
3.7. Electrochemical Impedance Spectroscopy (EIS)
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Krakowiak, S.; Darowicki, K.; Ślepski, P. Impedance of metastable pitting corrosion. J. Electroanal. Chem. 2005, 575, 33–38. [Google Scholar] [CrossRef]
- Rasouli, S.; Danaee, I. Effect of preparation method on the anti-corrosive properties of nanocrystalline Zn-CoO ceramic pigments. Mater. Corros. 2011, 62, 405–410. [Google Scholar] [CrossRef]
- Samiento-Bustos, E.; Rodriguez, J.G.G.; Uruchurtu, J.; Dominguez-Patiño, G.; Salinas-Bravo, V.M. Effect of inorganic inhibitors on the corrosion behavior of 1018 carbon steel in the LiBr + ethylene glycol + H2O mixture. Corros. Sci. 2008, 50, 2296–2303. [Google Scholar] [CrossRef]
- Cheng, F. Monitor safety of aged fuel pipelines. Nature 2016, 529, 156. [Google Scholar] [CrossRef]
- Shirehjini, F.T.; Danaee, I.; Eskandari, H.; Zarei, D. Effect of nano clay on corrosion protection of Zinc-rich epoxy coatings on steel 37. J. Mater. Sci. Technol. 2016, 32, 1152–1160. [Google Scholar] [CrossRef]
- Liu, S.; Gu, L.; Zhao, H.; Chen, J.; Yu, H. Corrosion resistance of graphene-reinforced waterborne epoxy coatings. J. Mater. Sci. Technol. 2016, 32, 425–431. [Google Scholar] [CrossRef]
- Hou, J.; Zhu, G.; Xu, J.; Liu, H. Anticorrosion performance of epoxy coatings containing small amount ofinherently conducting PEDOT/PSS on hull steel in seawater. J. Mater. Sci. Technol. 2013, 29, 678–684. [Google Scholar] [CrossRef]
- Beidokhti, B.; Dolati, A.; Koukabi, A.H. Effects of alloying elements and microstructure on the susceptibility of the welded HSLA steel to hydrogen-induced cracking and sulfide stress cracking. Mater. Sci. Eng. A 2009, 507, 167–173. [Google Scholar] [CrossRef]
- Bahgat Radwan, A.; Sliem, M.H.; Okonkwo, P.C.; Shibl, M.F.; Abdullah, A.M. Corrosion inhibition of API X120 steel in a highly aggressive medium using stearamidopropyl dimethylamine. J. Mol. Liq. 2017, 236, 220–231. [Google Scholar] [CrossRef]
- Hernández-Espejel, A.; Domínguez-Crespo, M.A.; Cabrera-Sierra, R.; Rodríguez-Meneses, C.; Arce-Estrada, E.M. Investigations of corrosion films formed on API-X52 pipeline steel in acid sour media. Corros. Sci. 2010, 52, 2258–2267. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Vishal, Y.; Quraishi, M.A. Quraishi Dendrimers: A new class of corrosion inhibitors for mild steel in 1 M HCl: Experimental and quantum chemical studies. J. Mol. Liq. 2016, 224, 1282–1293. [Google Scholar] [CrossRef]
- Mourya, P.; Banerjee, S.; Singh, M.M. Corrosion inhibition of mild steel in acidic solution by Tagetes erecta (Marigold flower) extract as a green inhibitor. Corros. Sci. 2014, 85, 352–363. [Google Scholar] [CrossRef]
- Finšgar, M.; Jackson, J. Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: A review. Corros. Sci. 2014, 86, 17–41. [Google Scholar] [CrossRef]
- De Damborenea, J.; Bastidas, J.M.; Vázquez, A.J. Adsorption and inhibitive properties of four primary aliphatic amines on mild steel in 2 M hydrochloric acid. Electrochim. Acta 1997, 42, 455–459. [Google Scholar] [CrossRef]
- Bastidas, J.M.; Polo, J.L.; Cano, E. Substitutional inhibition mechanism of mild steel hydrochloric acid corrosion by hexylamine and dodecylamine. J. Appl. Electrochem. 2000, 30, 1173–1177. [Google Scholar] [CrossRef]
- Feng, Y.; Cheng, Y.F. An intelligent coating doped with inhibitor-encapsulated nanocontainers for corrosion protection of pipeline steel. Chem. Eng. J. 2017, 315, 537–551. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Möhwald, H. Smart nanocontainers as depot media for feedback active coatings. Chem. Commun. 2011, 47, 8730–8739. [Google Scholar] [CrossRef]
- Shi, H.; Liu, F.; Han, E.H. Surface-engineered microcapsules by layer-by-layer assembling for entrapment of corrosion inhibitor. J. Mater. Sci. Technol. 2015, 31, 512–516. [Google Scholar] [CrossRef]
- Moazeni, N.; Mohamad, Z.; Faisal, N.L.I.; Tehrani, M.A.; Dehbari, N. Anticorrosion epoxy coating enriched with hybrid nanozinc dust and halloysite nanotubes. J. Appl. Polym. Sci. 2013, 130, 955–960. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Tedim, J.; Ferreira, M.G.S. “Smart” coatings for active corrosion protection based on multi-functional micro and nanocontainers. Electrochim. Acta 2012, 82, 314–323. [Google Scholar] [CrossRef]
- Fix, D.; Andreeva, D.V.; Lvov, Y.M.; Shchukin, D.G.; Möhwald, H. Application of inhibitor-loaded halloysite nanotubes in active anti-corrosive coatings. Adv. Funct. Mater. 2009, 19, 1720–1727. [Google Scholar] [CrossRef]
- Zea, C.; Barranco-García, R.; Alcántara, J.; Simancas, J.; Morcillo, M.; de la Fuente, D. pH-dependent release of environmentally friendly corrosion inhibitor from mesoporous silica nanoreservoirs. Microporous Mesoporous Mater. 2018, 255, 166–173. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhang, S.; Xu, S.; Li, W. Experimental and theoretical studies on the corrosion inhibition of copper by two indazole derivatives in 3.0% NaCl solution. J. Colloid Interface Sci. 2016, 472, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, D.V.; Fix, D.; Möhwald, H.; Shchukin, D.G. Self-healing anticorrosion coatings based on pH-sensitive polyelectrolyte/inhibitor sandwichlike nanostructures. Adv. Mater. 2008, 20, 2789–2794. [Google Scholar] [CrossRef]
- Singh, H.K.; Yeole, K.V.; Mhaske, S.T. Synthesis and characterization of layer-by-layer assembled magnesium zinc molybdate nanocontainer for anticorrosive application. Chem. Eng. J. 2016, 295, 414–426. [Google Scholar] [CrossRef]
- Arunchandran, C.; Ramya, S.; George, R.P.; Kamachi Mudali, U. Corrosion inhibitor storage and release property of TiO2 nanotube powder synthesized by rapid breakdown anodization method. Mater. Res. Bull. 2013, 48, 635–639. [Google Scholar] [CrossRef]
- Poornima Vijayan, P.; Al-Maadeed, M.A.S.A. TiO2nanotubes and mesoporous silica as containers in self-healing epoxy coatings. Sci. Rep. 2016, 6, 1–9. [Google Scholar]
- Falcón, J.M.; Otubo, L.M.; Aoki, I.V. Highly ordered mesoporous silica loaded with dodecylamine for smart anticorrosion coatings. Surf. Coat. Technol. 2016, 303, 319–329. [Google Scholar] [CrossRef]
- Vijayan, P.P.; Hany El-Gawady, Y.M.; Al-Maadeed, M.A.S.A. Halloysite nanotube as multifunctional component in epoxy protective coating. Ind. Eng. Chem. Res. 2016, 55, 11186–11192. [Google Scholar] [CrossRef]
- Abdullayev, E.; Lvov, Y. Halloysite clay nanotubes for controlled release of protective agents delivered by ingenta. J. Nanosci. Nanotechnol. 2011, 11, 10007–10026. [Google Scholar] [CrossRef]
- Kartsonakis, I.A.; Balaskas, A.C.; Kordas, G.C. Influence of cerium molybdate containers on the corrosion performance of epoxy coated aluminium alloys 2024-T3. Corros. Sci. 2011, 53, 3771–3779. [Google Scholar] [CrossRef]
- Snihirova, D.; Lamaka, S.V.; Cardoso, M.M.; Condeço, J.A.D.; Ferreira, H.E.C.S.; De Fatima Montemor, M. PH-sensitive polymeric particles with increased inhibitor-loading capacity as smart additives for corrosion protective coatings for AA2024. Electrochim. Acta 2014, 145, 123–131. [Google Scholar] [CrossRef]
- Thi, D.; Phan, H.; Cuong, D.H.; Duc, L.M. Corrosion resistance and mechanical properties of TiO 2 nanotubes/epoxy coating on steel spcc-jisg 3141. Vietnam J. Sci. Technol. 2017, 55, 203–209. [Google Scholar]
- Imai, H.; Takei, Y.; Shimizu, K.; Matsuda, M.; Hirashima, H. Direct preparation of anatase TiO2 nanotubes in porous alumina membranes. J. Mater. Chem. 1999, 9, 2971–2972. [Google Scholar] [CrossRef]
- Yamamoto, R.; Sakai, K.; Takei, Y.; Yamasaki, N.Y.; Mitsuda, K. Performance of frequency division multiplexing readout system for AC-biased transition-edge sensor x-Ray microcalorimeters. J. Low Temp. Phys. 2014, 176, 453–458. [Google Scholar] [CrossRef]
- Gong, D.; Grimes, C.A.; Varghese, O.K.; Hu, W.; Singh, R.S.; Chen, Z.; Dickey, E.C. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 3331–3334. [Google Scholar] [CrossRef]
- Price, R.R.; Gaber, B.P.; Lvov, Y. In-vitro release characteristics of tetracycline HC1, khellin and nicotinamide adenine dineculeotide from halloysite; a cylindrical mineral. J. Microencapsul. 2001, 18, 713–722. [Google Scholar]
- Bavykin, D.V.; Milsom, E.V.; Marken, F.; Kim, D.H.; Marsh, D.H.; Riley, D.J.; Walsh, F.C.; El-Abiary, K.H.; Lapkin, A.A. A novel cation-binding TiO2 nanotube substrate for electro- and bioelectro-catalysis. Electrochem. Commun. 2005, 7, 1050–1058. [Google Scholar] [CrossRef]
- Chen, P.; Li, H.; Song, S.; Weng, X.; He, D.; Zhao, Y. Adsorption of dodecylamine hydrochloride on graphene oxide in water. Results Phys. 2017, 7, 2281–2288. [Google Scholar] [CrossRef]
- Chakraborty, S.; Kar, S.; Dasgupta, S.; Mukhopadhyay, R.; Bandyopadhyay, S.; Joshi, M.; Ameta, S.C. Study of the properties of in-situ sodium activated and organomodified bentonite clay—SBR rubber nanocomposites—Part I: Characterization and rheometric properties. Polym. Test. 2010, 29, 181–187. [Google Scholar] [CrossRef]
- Maity, P.; Kasisomayajula, S.V.; Parameswaran, V.; Basu, S.; Gupta, N. Improvement in surface degradation properties of polymer composites due to pre-processed nanometric alumina fillers. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 63–72. [Google Scholar] [CrossRef]
- Tantra, R.; Schulze, P.; Quincey, P. Effect of nanoparticle concentration on zeta-potential measurement results and reproducibility. Particuology 2010, 8, 279–285. [Google Scholar] [CrossRef]
- Falcón, J.M.; Batista, F.F.; Aoki, I.V. Encapsulation of dodecylamine corrosion inhibitor on silica nanoparticles. Electrochim. Acta 2014, 124, 109–118. [Google Scholar] [CrossRef]
- Yang, M.; Wu, J.; Fang, D.; Li, B.; Yang, Y. Corrosion protection of waterborne epoxy coatings containing mussel-inspired adhesive polymers based on polyaspartamide derivatives on carbon steel. J. Mater. Sci. Technol. 2018, 34, 2464–2471. [Google Scholar] [CrossRef]
- Falcón, J.M.; Sawczen, T.; Aoki, I.V. Dodecylamine-loaded halloysite nanocontainers for active anticorrosion coatings. Front. Mater. 2015, 2, 1–13. [Google Scholar] [CrossRef]

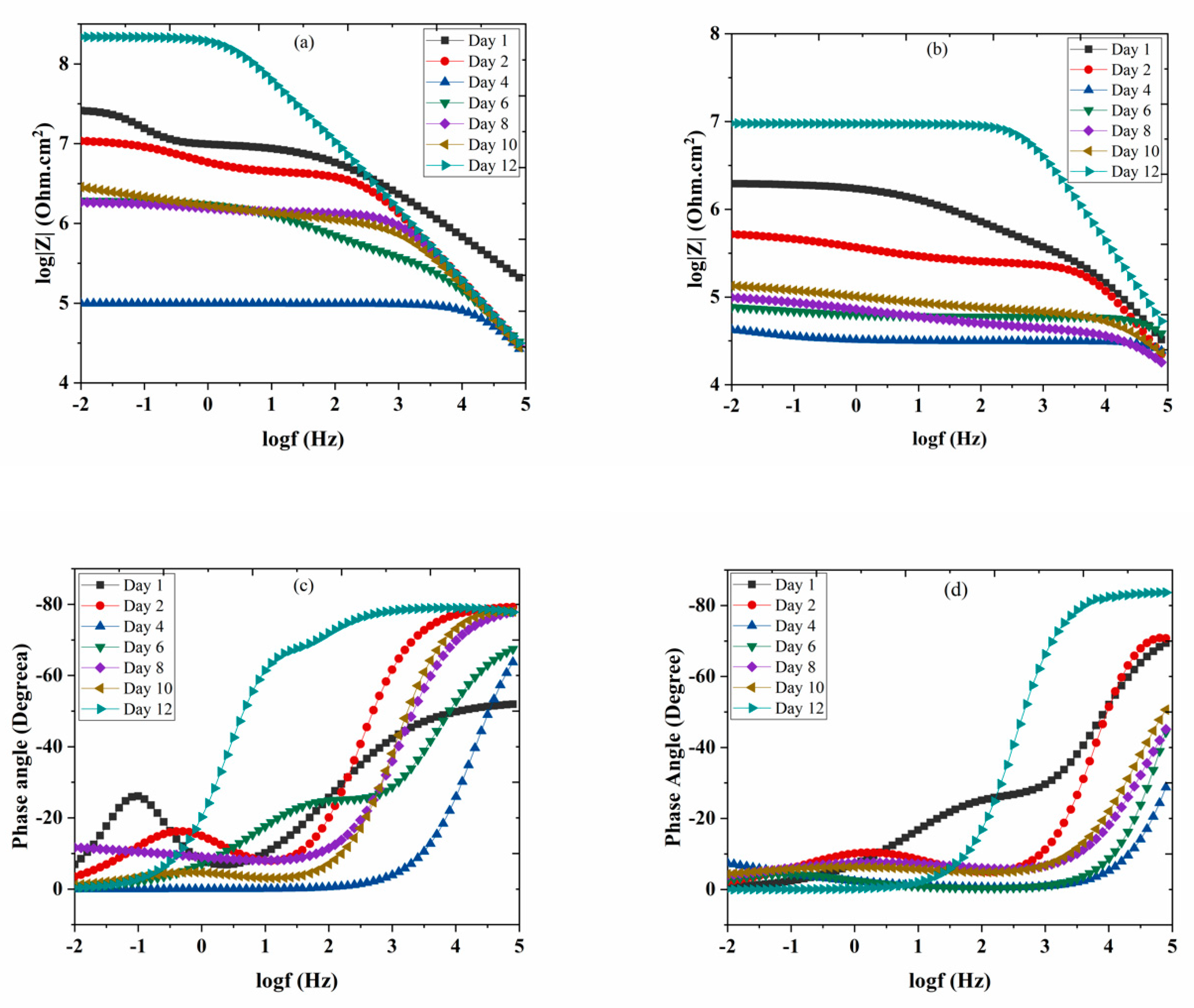
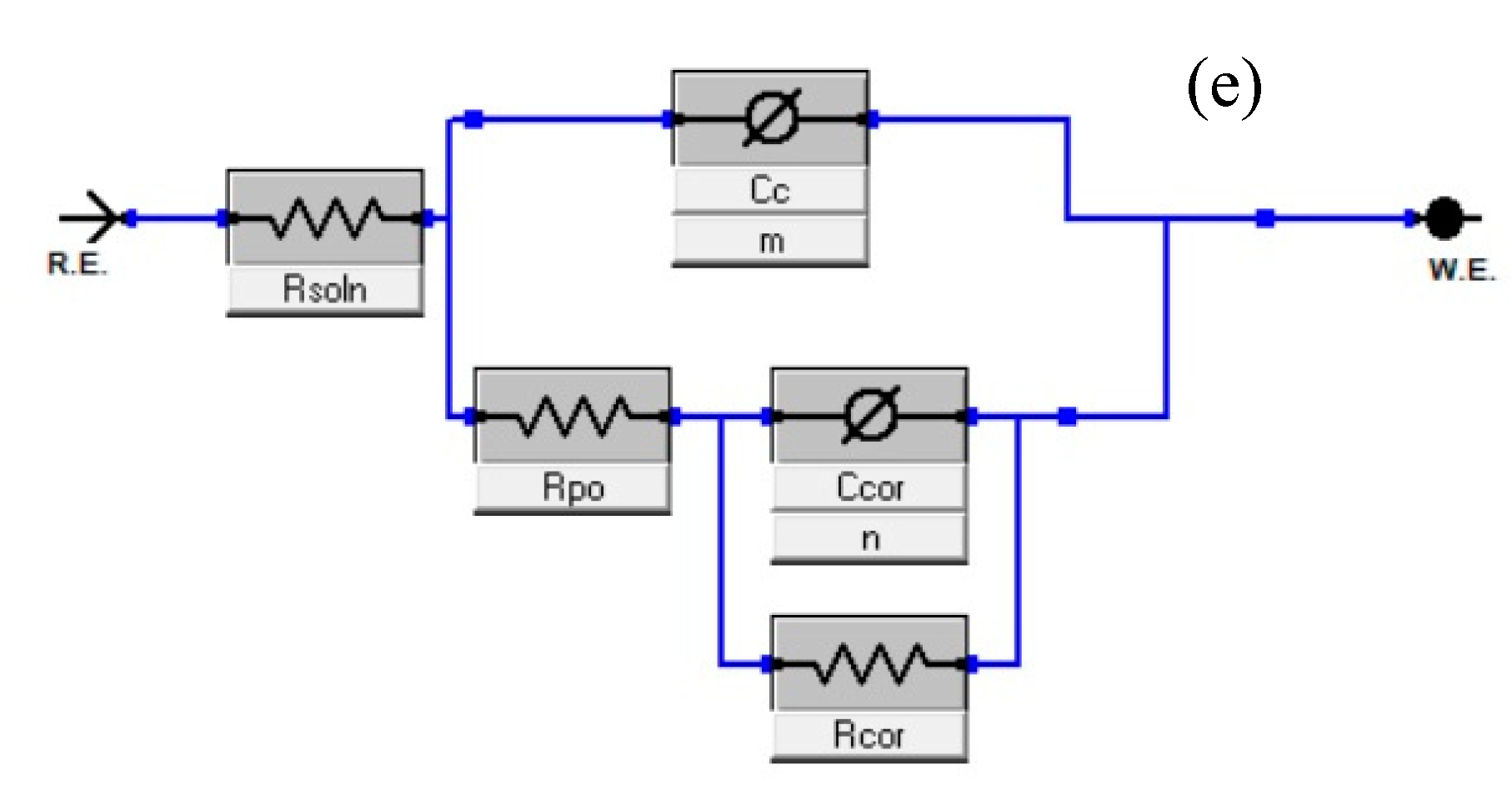
| Coating | Time (Day) | Rct (Ω·cm2) | CC (F/cm2) | RPO (Ω·cm2) | Ccor (F/cm2) |
|---|---|---|---|---|---|
| 5 wt.% TNTs loaded with DOC at pH 2 | 1 | 16.54 × 106 | 2.128 × 10−9 | 10.11 × 106 | 166.4 × 10−9 |
| 2 | 6.914 × 106 | 287.8 × 10−12 | 4.284 × 106 | 117.0 × 10−9 | |
| 4 | 136.2 × 103 | 319.5 × 10−12 | 98.79 × 103 | 101.9 × 10−6 | |
| 6 | 1.561 × 106 | 811.1 × 10−12 | 378.5 × 103 | 50.68 × 10−9 | |
| 8 | 28.79 × 106 | 325.4 × 10−12 | 801.7 × 103 | 763.4 × 10−9 | |
| 10 | 520.0 × 103 | 252.2 × 10−12 | 1.355 × 106 | 1.005 × 10−6 | |
| 12 | 148.2 × 106 | 301.1 × 10−12 | 69.95 × 106 | 110.1 × 10−12 | |
| 5 wt.% TNTs loaded with DOC at pH 5 | 1 | 2.651 × 106 | 423.2 × 10−12 | 265.4 × 103 | 34.41 × 10−9 |
| 2 | 328.9 × 103 | 289.6 × 10−12 | 231.9 × 103 | 1.986 × 10−6 | |
| 4 | 11.56 × 103 | 445.2 × 10−12 | 32.01 × 103 | 278.8 × 10−6 | |
| 6 | 23.11 × 103 | 76.82 × 10−12 | 59.49 × 103 | 77.51 × 10−6 | |
| 8 | 76.61 × 103 | 1.678 × 10−9 | 39.52 × 103 | 10.13 × 10−6 | |
| 10 | 91.31 × 103 | 1.624 × 10−9 | 65.25 × 103 | 9.279 × 10−6 | |
| 12 | 7.683 × 106 | 66.88 × 10−12 | 1.798 × 106 | 699.21 × 10−12 |
| Sr. No | Coatings | Immersion time | Rct | References |
|---|---|---|---|---|
| 1 | 1 wt.% of nanocontainers (MS) with encapsulated DOC at pH 2 | 1 hr. | 5.00 × 102 Ω·cm2 | [43] |
| 3 hrs. | 6.50 × 102 Ω·cm2 | |||
| 5 hrs. | 7.50 × 102 Ω·cm2 | |||
| 16 hrs. | 2.500 × 103 Ω·cm2 | |||
| 1 wt.% of nanocontainers (MS) with encapsulated DOC at pH 6.2 | 1 hr. | 1.500 × 103 Ω·cm2 | ||
| 3 hrs. | 1.750 × 103 Ω·cm2 | |||
| 5 hrs. | 2.000 × 103 Ω·cm2 | |||
| 16 hrs. | 3.000 × 103 Ω·cm2 | |||
| 2 | 1 wt.% Halloysite nanotubes with encapsulated DOC at pH 2 | 1,2,3,5 and 7 hrs. | 2.00 × 102 Ω·cm2 | [45] |
| 18 hrs. | 5.50 × 102 Ω·cm2 | |||
| 36 hrs. | 8.00 × 102 Ω·cm2 | |||
| 48 hrs. | 1.100 × 103 Ω·cm2 | |||
| 1 wt.% Halloysite nanotubes with encapsulated DOC at pH 6.2 | 1,2,3,5 and 7 hrs. | 1.700 × 103 Ω·cm2 | ||
| 18,36 and 48 hrs. | 2.000 × 103 Ω·cm2 | |||
| 3 | 1 wt.% of highly ordered Mesoporous silica with encapsulated DOC at pH 2 | 1,2,3,5 and 6 hrs. | 7.50 × 102 Ω·cm2 | [28] |
| 24, 26, 28 hrs. | 1.750 × 103 Ω·cm2 | |||
| 1 wt.% of highly ordered Mesoporous silica with encapsulated DOC at pH 6.2 | 1,2,3,5 and 6 hrs. | 2.250 × 103 Ω·cm2 | ||
| 24, 26, 28 hrs. | 3.250 × 103 Ω·cm2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawaz, M.; Yusuf, N.; Habib, S.; Shakoor, R.A.; Ubaid, F.; Ahmad, Z.; Kahraman, R.; Mansour, S.; Gao, W. Development and Properties of Polymeric Nanocomposite Coatings. Polymers 2019, 11, 852. https://doi.org/10.3390/polym11050852
Nawaz M, Yusuf N, Habib S, Shakoor RA, Ubaid F, Ahmad Z, Kahraman R, Mansour S, Gao W. Development and Properties of Polymeric Nanocomposite Coatings. Polymers. 2019; 11(5):852. https://doi.org/10.3390/polym11050852
Chicago/Turabian StyleNawaz, Muddasir, Noor Yusuf, Sehrish Habib, Rana Abdul Shakoor, Fareeha Ubaid, Zubair Ahmad, Ramazan Kahraman, Said Mansour, and Wei Gao. 2019. "Development and Properties of Polymeric Nanocomposite Coatings" Polymers 11, no. 5: 852. https://doi.org/10.3390/polym11050852
APA StyleNawaz, M., Yusuf, N., Habib, S., Shakoor, R. A., Ubaid, F., Ahmad, Z., Kahraman, R., Mansour, S., & Gao, W. (2019). Development and Properties of Polymeric Nanocomposite Coatings. Polymers, 11(5), 852. https://doi.org/10.3390/polym11050852