Optimizing the Extraction and Encapsulation of Mucilage from Brasenia Schreberi
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Extraction Optimization of BS Mucilage
2.2.1. Extraction of BS Mucilage
2.2.2. Experimental Design for Response Surface Methodology (RSM)
2.3. Preformulation Study
2.3.1. Influence Factor Test
2.3.2. Angle of Repose
2.3.3. Bulk Density and Tapped Density
2.4. Preparation of the Capsule with BS Mucilage
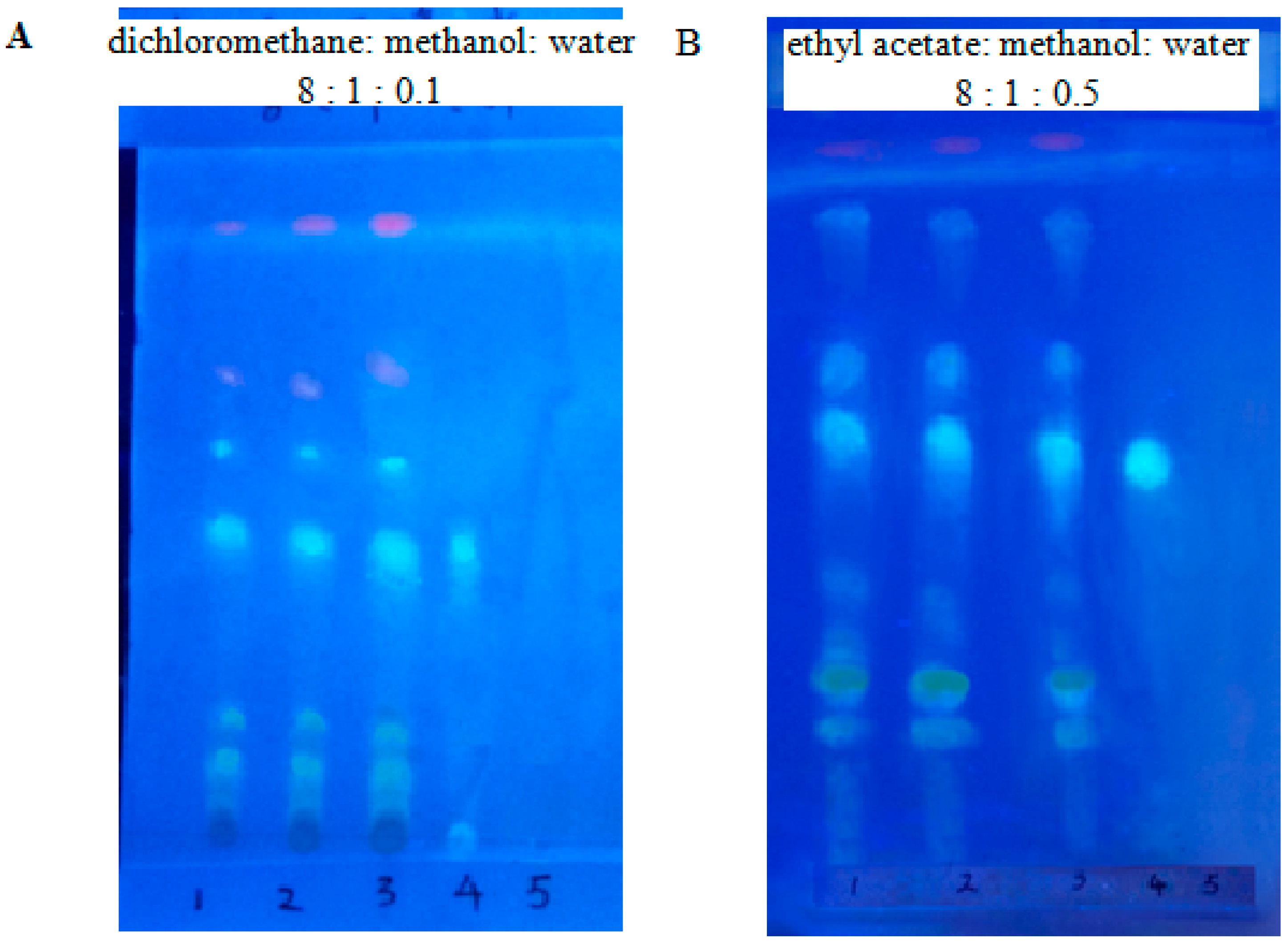
2.5. Identification by Thin-Layer Chromatography
2.6. Determination of Polysaccharide Content
2.6.1. Preparation of Glucose Standard Solution
2.6.2. Standard Curve Manufacture
2.7. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Extraction Conditions
3.2. Evaluation of the BS Mucilage
3.3. Preparation of Capsule by BS Mucilage
3.4. Evaluation of the Capsules
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Z.Z.; Gichira, A.W.; Wang, Q.F.; Chen, J.M. Genetic diversity and population structure of the endangered basal angiosperm Brasenia schreberi (Cabombaceae) in China. PeerJ 2018, 13, e5296. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Cai, X.; Fan, Y.; Luo, A. Antioxidant Activity of Water-soluble Polysaccharides from Brasenia schreberi. Pharmacogn. Mag. 2016, 12, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Wang, Q.; Shoemaker, C.F.; Zhong, F.; Bartley, G.E.; Yokoyama, W.H. Polysaccharide gel coating of the leaves of Brasenia schreberi lowers plasma cholesterol in hamsters. J. Tradit. Complement. Med. 2015, 5, 56–61. [Google Scholar] [CrossRef]
- Legault, J.; Perron, T.; Mshvildadze, V.; Girard Lalancette, K.; Perron, S.; Laprise, C.; Sirois, P.; Pichette, A. Antioxidant and anti-inflammatory activities of quercetin 7-O-β-d-glucopyranoside from the leaves of Brasenia schreberi. J. Med. Food 2011, 14, 1127–1134. [Google Scholar] [CrossRef]
- Kakuta, M.; Misaki, A. Polysaccharide of Junsai (Brasenia schreberi Jf Gmel) Mucilage-Constitution and Linkage Analysis. Agric. Biol. Chem. 1979, 43, 993–1005. [Google Scholar] [CrossRef][Green Version]
- Nie, Y.; Lin, Q.; Luo, F. Effects of Non-Starch Polysaccharides on Inflammatory Bowel Disease. Int. J. Mol. Sci. 2017, 18, 1372. [Google Scholar] [CrossRef]
- Liu, P.; Liu, Y.; Yang, Y.; Chen, Z.; Li, J.; Luo, J. Mechanism of Biological Liquid Superlubricity of Brasenia schreberi Mucilage. Langmuir 2014, 30, 3811–3816. [Google Scholar] [CrossRef]
- Hefny, A.F.; Ayad, A.Z.; Matev, N.; Bashir, M.O. Intestinal obstruction caused by a laxative drug (Psyllium): A case report and review of the literature. Int. J. Surg. Case Rep. 2018, 52, 59–62. [Google Scholar] [CrossRef]
- Zhao, X.; Qiao, L.; Wu, A.M. Effective extraction of Arabidopsis adherent seed mucilage by ultrasonic treatment. Sci. Rep. 2017, 7, 40672. [Google Scholar] [CrossRef]
- Nazir, S.; Wani, I.A.; Masoodi, F.A. Extraction optimization of mucilage from Basil (Ocimum basilicum L.) seeds using response surface methodology. J. Adv. Res. 2017, 8, 235–244. [Google Scholar] [CrossRef]
- Miart, F.; Fontaine, J.X.; Pineau, C.; Thomasset, B.; Wuytswinkel, O.V.; Pageau, K.; Mesnard, F. MuSeeQ, a novel supervised image analysis tool for the simultaneous phenotyping of the soluble mucilage and seed morphometric parameters. Plant Methods 2018, 14, 112. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Q.; Wang, H.; Jiang, X.; Cha, R. Preparation of green and gelatin-free nanocrystalline cellulose capsules. Carbohydr. Polym. 2017, 164, 358–363. [Google Scholar] [CrossRef]
- Nagai, T.; Suzuki, N.; Nagashima, T. Antioxidative activity of water extracts from the yam (Dioscorea opposita Thunb.) tuber mucilage tororo. Eur. J. Lipid Sci. Technol. 2006, 108, 526–531. [Google Scholar] [CrossRef]
- Shen, H.; Li, F.; Wang, D.; Yang, Z.; Yao, C.; Ye, Y.; Wang, X. Chitosan–alginate BSA-gel-capsules for local chemotherapy against drug-resistant breast cancer. Drug Des. Dev. Ther. 2018, 12, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, P.; Xu, X.; Ye, X.; Wang, J. Preparation of chitosan–sodium alginate microcapsules containing ZnS nanoparticles and its effect on the drug release. Mater. Sci. Eng. C Mater. Biol. Appl. 2009, 29, 2250–2253. [Google Scholar] [CrossRef]
- Ahmed, T.A. Preparation of finasteride capsules-loaded drug nanoparticles: Formulation, optimization, in vitro, and pharmacokinetic evaluation. Int. J. Nanomed. 2016, 11, 515–527. [Google Scholar] [CrossRef]
- Porter, N.T.; Canales, P.; Peterson, D.A.; Martens, E.C. A subset of polysaccharide capsules in the human symbiont Bacteroides thetaiotaomicron promote increased competitive fitness in the mouse gut. Cell Host Microbe 2017, 22, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Paini, M.; Aliakbarian, B.; Casazza, A.A.; Perego, P.; Ruggiero, C.; Pastorino, L. Chitosan/dextran multilayer microcapsules for polyphenol co-delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 46, 374–380. [Google Scholar] [CrossRef]
- Lei, H.; Hu, J.; Deng, W. Preparation and application of flavor and fragrance capsules. Polym. Chem. 2018, 9, 4926–4946. [Google Scholar] [CrossRef]
- Rebouh, S.; Lefnaoui, S.; Bouhedda, M.; Yahoum, M.M.; Hanini, S. Neuro-fuzzy modeling of ibuprofen-sustained release from tablets based on different cellulose derivatives. Drug Deliv. Trans. Res. 2019, 9, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Pawar, H.A.; Priscilla, M.D. Development and Evaluation of Herbal Laxative Granules. J. Chem. Pharm. Res. 2011, 3, 646–650. [Google Scholar] [CrossRef]
- Kim, J.J.; Kim, K.S.; Yu, B.J. Optimization of Antioxidant and Skin-Whitening Compounds Extraction Condition from Tenebrio molitor Larvae (Mealworm). Molecules 2018, 23, 2340. [Google Scholar] [CrossRef]
- Teboukeu, G.B.; Djikeng, F.T.; Klang, M.J.; Karuna, M.S.L.; Womeni, H.M. Optimization of the extraction of natural antioxidants from Coffea robusta leaves and evaluation of their ability to preserve palm olein from oxidation during accelerated storage. Food Sci. Nutr. 2018, 6, 1751–1761. [Google Scholar] [CrossRef]
- Liu, Y.J.; Mo, X.L.; Tang, X.Z.; Li, J.H.; Hu, M.B.; Yan, D.; Peng, W.; Wu, C.J. Extraction Optimization, Characterization, and Bioactivities of Polysaccharides from Pinelliae Rhizoma Praeparatum Cum Alumine Employing Ultrasound-Assisted Extraction. Molecules 2017, 22, 965. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.B.; Yan, H.L. The response of performance in grower and finisher pigs to diets formulated to different tryptophan to lysine ratios. Livest. Sci. 2019, 222, 25–30. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Extraction of polysaccharides by autohydrolysis of spent coffee grounds and evaluation of their antioxidant activity. Carbohydr. Polym. 2017, 157, 258–266. [Google Scholar] [CrossRef]
- Yan, H.L.; Zhang, L. Production phase affects the bioaerosol microbial composition and functional potential in swine confinement buildings. Animals 2019, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Li, G.Q.; Li, K.M.; Razmovski-Naumovski, V.; Chan, K. Optimisation of Pueraria isoflavonoids by response surface methodology using ultrasonic-assisted extraction. Food Chem. 2017, 231, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Cui, J.; Chen, A.H.; Zong, Z.M.; Wei, X.Y. Optimization of Ultrasonic-Microwave Assisted Extraction and Hepatoprotective Activities of Polysaccharides from Trametes orientalis. Molecules 2019, 24, 147. [Google Scholar] [CrossRef] [PubMed]
- USP Convention. U.S. Pharmacopeia National Formulary, Supplement ed.; United States Pharmacopeial: Rockville, MD, USA, 12 September 2016. [Google Scholar]
- Akseli, I.; Hilden, J.; Katz, J.M.; Kelly, R.C.; Kramer, T.T.; Mao, C.; Osei-Yeboah, F.; Strong, J.C. Reproducibility of the measurement of bulk/tapped density of pharmaceutical powders between pharmaceutical laboratories. J. Pharm. Sci. 2018, 108, 1081–1084. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Chen, X.G.; Liu, C.S.; Liu, C.G.; Xue, Y.P. Preparation of alginate-gelatin capsules and its properties. Front. Mater. Sci. China 2008, 2, 253–260. [Google Scholar] [CrossRef]
- Shisode, P.S.; Patil, C.B.; Mahulikar, P.P. Preparation and Characterization of Microcapsules Containing Soybean Oil and Their Application in Self-Healing Anticorrosive Coatings. Polym. Plast. Technol. 2017, 57, 1334–1343. [Google Scholar] [CrossRef]
- Amador Ríos, Z.; Ghaly, E.S. The Effect of Formulation Excipients and Thermal Treatment on the Release Properties of Lisinopril Spheres and Tablets. Biomed Res. Int. 2015, 2015, 423615. [Google Scholar] [CrossRef]
- Nemes, D.; Kovács, R.; Nagy, F.; Mező, M.; Poczok, N.; Ujhelyi, Z.; Pető, Á.; Fehér, P.; Fenyvesi, F.; Váradi, J.; et al. Interaction between Different Pharmaceutical Excipients in Liquid Dosage Forms—Assessment of Cytotoxicity and Antimicrobial Activity. Molecules 2018, 23, 1827. [Google Scholar] [CrossRef]
- Markl, D.; Zeitler, J.A. A Review of Disintegration Mechanisms and Measurement Techniques. Pharm. Res. 2017, 34, 890–917. [Google Scholar] [CrossRef]
- Ladola, M.K.; Gangurde, A.B. Development and Evaluation of Melt-in-Mouth Tablets of Metoclopramide Hydrochloride Using Novel Co-processed Superdisintegrants. Indian J. Pharm. Sci. 2014, 76, 423–429. [Google Scholar]
- Zheng, Y.; Fu, Z.; Li, D.; Wu, M. Effects of Ball Milling Processes on the Microstructure and Rheological Properties of Microcrystalline Cellulose as a Sustainable Polymer Additive. Materials 2018, 11, 1057. [Google Scholar] [CrossRef]
- Yassin, S.; Goodwin, D.J.; Anderson, A.; Sibik, J.; Wilson, D.I.; Gladden, L.F.; Zeitler, J.A. The Disintegration Process in Microcrystalline Cellulose Based Tablets, Part 1: Influence of Temperature, Porosity and Superdisintegrants. J. Pharm. Sci. 2015, 104, 3440–3450. [Google Scholar] [CrossRef]
- Agrawal, A.; Dudhedia, M.; Deng, W.; Shepard, K.; Zhong, L.; Povilaitis, E.; Zimny, E. Development of Tablet Formulation of Amorphous Solid Dispersions Prepared by Hot Melt Extrusion Using Quality by Design Approach. AAPS PharmSciTech 2016, 17, 214–232. [Google Scholar] [CrossRef]
- Deve, A.S.; Kumaresan, K.; Rapheal, V.S. Extraction process optimization of polyphenols from Indian Citrus sinensis-as novel antiglycative agents in the management of diabetes mellitus. J. Diabetes Metab. Disord. 2014, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Park, H.J.; Kwon, S.H.; Jung, Y.J.; Lyu, H.N. Tellimoside, a Flavonol Glycoside from Brasenia schreberi, Inhibits the Growth of Cyanobacterium (Microcystis aeruginosa LB 2385). J. Korean Soc. Appl. Biol Chem. 2013, 56, 117–121. [Google Scholar] [CrossRef]
- Xie, J.H.; Tang, W.; Jin, M.L.; Li, J.E.; Xie, M.Y. Recent advances in bioactive polysaccharides from Lycium barbarum L., Zizyphus jujuba Mill, Plantago spp., and Morus spp.: Structures and functionalities. Food Hydrocoll. 2016, 60, 148–160. [Google Scholar] [CrossRef]
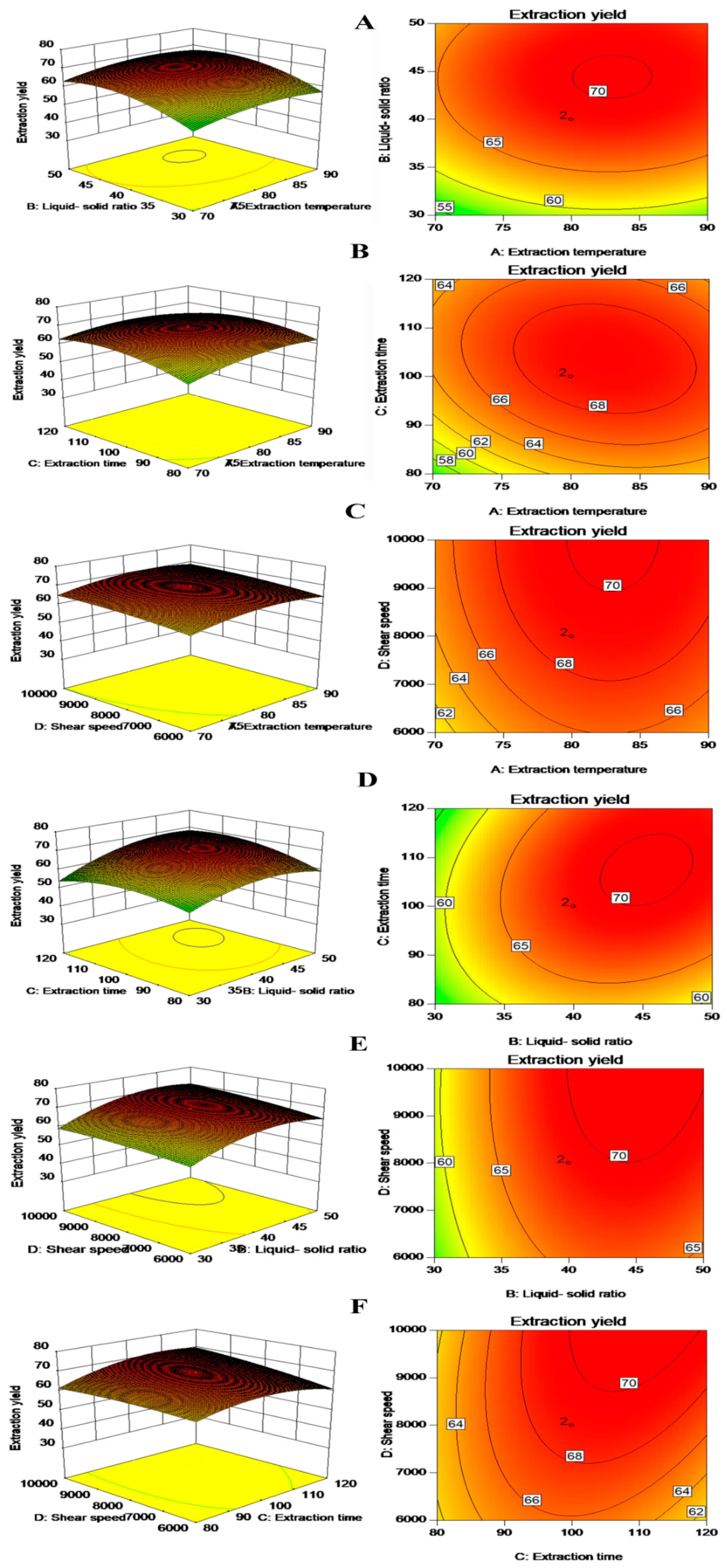

| Run | A (°C) | B (mL/g) | C (min) | D (rpm) | Extraction Temperature (°C) | Liquid-Solid Ratio (mL/g) | Extraction Time (min) | Shear Speed (rpm) | Extraction Yield (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 2 | 0 | 80 | 40 | 140 | 8000 | 54.755 |
| 2 | 1 | −1 | −1 | 1 | 90 | 30 | 80 | 10,000 | 54.450 |
| 3 | 1 | 1 | −1 | 1 | 90 | 50 | 80 | 10,000 | 56.776 |
| 4 | 0 | 0 | 0 | −2 | 80 | 40 | 100 | 4000 | 59.878 |
| 5 | 2 | 0 | 0 | 0 | 100 | 40 | 100 | 8000 | 59.549 |
| 6 | 0 | 0 | 0 | 0 | 80 | 40 | 100 | 8000 | 68.409 |
| 7 | 0 | 0 | −2 | 0 | 80 | 40 | 60 | 8000 | 47.141 |
| 8 | −1 | −1 | −1 | −1 | 70 | 30 | 80 | 6000 | 50.878 |
| 9 | 0 | 0 | 0 | 2 | 80 | 40 | 100 | 12,000 | 69.490 |
| 10 | −1 | −1 | 1 | 1 | 70 | 30 | 120 | 10,000 | 52.734 |
| 11 | −2 | 0 | 0 | 0 | 60 | 40 | 100 | 8000 | 52.123 |
| 12 | 1 | 1 | −1 | −1 | 90 | 50 | 80 | 6000 | 57.552 |
| 13 | 0 | 2 | 0 | 0 | 80 | 60 | 100 | 8000 | 61.735 |
| 14 | −1 | −1 | 1 | −1 | 70 | 30 | 120 | 6000 | 49.162 |
| 15 | −1 | 1 | −1 | 1 | 70 | 50 | 80 | 10,000 | 53.040 |
| 16 | 0 | 0 | 0 | 0 | 80 | 40 | 100 | 8000 | 69.490 |
| 17 | −1 | 1 | 1 | 1 | 70 | 50 | 120 | 10,000 | 67.469 |
| 18 | −1 | 1 | 1 | −1 | 70 | 50 | 120 | 6000 | 56.917 |
| 19 | 0 | −2 | 0 | 0 | 80 | 20 | 100 | 8000 | 32.736 |
| 20 | 1 | 1 | 1 | −1 | 90 | 50 | 120 | 6000 | 59.714 |
| 21 | −1 | −1 | −1 | 1 | 70 | 30 | 80 | 10,000 | 47.306 |
| 22 | 1 | −1 | 1 | −1 | 90 | 30 | 120 | 6000 | 45.755 |
| 23 | 1 | −1 | −1 | −1 | 90 | 30 | 80 | 6000 | 56.142 |
| 24 | −1 | 1 | −1 | −1 | 70 | 50 | 80 | 6000 | 48.387 |
| 25 | 1 | −1 | 1 | 1 | 90 | 30 | 120 | 10,000 | 56.612 |
| 26 | 1 | 1 | 1 | 1 | 90 | 50 | 120 | 10,000 | 68.080 |
| Source | Sum of Squares | df | Mean Square | F Value | P-Value (Prob > F) | Significance |
|---|---|---|---|---|---|---|
| Model | 1739.21 | 14 | 124.23 | 13.09 | <0.0001 | ** |
| A-Temperature | 80.81 | 1 | 80.81 | 8.52 | 0.0140 | * |
| B-Liquid-solid ratio | 531.04 | 1 | 531.04 | 55.97 | <0.0001 | ** |
| C-Time | 92.59 | 1 | 92.59 | 9.76 | 0.0097 | ** |
| D-Shear speed | 109.16 | 1 | 109.16 | 11.51 | 0.0060 | ** |
| AB | 0.74 | 1 | 0.74 | 0.077 | 0.7859 | |
| AC | 28.70 | 1 | 28.70 | 3.03 | 0.1098 | |
| AD | 0.15 | 1 | 0.15 | 0.016 | 0.9022 | |
| BC | 104.74 | 1 | 104.74 | 11.04 | 0.0068 | ** |
| BD | 11.61 | 1 | 11.61 | 1.22 | 0.2922 | |
| CD | 75.40 | 1 | 75.40 | 7.95 | 0.0167 | * |
| A2 | 180.33 | 1 | 180.33 | 19.01 | 0.0011 | ** |
| B2 | 502.27 | 1 | 502.27 | 52.94 | <0.0001 | ** |
| C2 | 343.50 | 1 | 343.50 | 36.20 | <0.0001 | ** |
| D2 | 17.53 | 1 | 17.53 | 1.85 | 0.2013 | |
| Residual | 104.37 | 11 | 9.49 | |||
| Lack of Fit | 103.78 | 10 | 10.38 | 17.76 | 0.1828 | Not significant |
| Pure Error | 0.58 | 1 | 0.58 | |||
| Cor Total | 1843.57 | 25 |
| Time (d) | Temperature (60 °C) | Humidity (95% ± 5%) | Illuminance (4500 lx ± 500 lx) |
|---|---|---|---|
| 0 (Powder weight) | 4.7494 g | 4.3586 g | 4.3205 g |
| 5 (Powder weight) | 4.8335 g | 5.6601 g | 4.7181 g |
| 10 (Powder weight) | 5.0036 g | 7.5229 g | 5.1489 g |
| 0 (Polysaccharide content) | 52.97% | 53.05% | 53.02% |
| 5 (Polysaccharide content) | 52.68% | 52.69% | 52.79% |
| 10 (Polysaccharide content) | 52.54% | 52.67% | 52.87% |
| Disintegrants | For-1 | For-2 | For-3 | For-4 | For-5 | For-6 | For-7 | For-8 |
|---|---|---|---|---|---|---|---|---|
| Else (mg) | 30.4 | 30.4 | 30.4 | 30.4 | 30.4 | 30.4 | 30.4 | 30.0 |
| CCS (mg) | 0.8 | — | 0.8 | — | — | 0.8 | — | — |
| CMS-Na (mg) | — | 0.8 | — | 0.8 | — | 0.8 | 1.2 | 1.5 |
| PVPP XL-10 (mg) | 0.8 | 0.8 | — | — | 0.8 | — | 0.4 | 0.5 |
| PVPP XL (mg) | — | — | 0.8 | 0.8 | 0.8 | — | — | — |
| Disintegration time (min) | >15 | 11 | >15 | 13 | >15 | 13 | 10 | 7 |
| Fillers | For-1 | For-2 | For-3 |
|---|---|---|---|
| MCC (Shanhe) (mg) | 160 | — | — |
| MCC (JRS) (mg) | — | 160 | — |
| Calcium hydrophosphate (mg) | — | — | 160 |
| Disintegration time (min) | 26 | 20 | 35 |
| Angle of repose (°) | 41.5 | 39.3 | 42.9 |
| Glidants | For-1 | For-2 | For-3 | For-4 | For-5 | For-6 |
|---|---|---|---|---|---|---|
| Colloidal silicon dioxide (mg) | — | 1.6 | 1.28 | 1.6 | 1.28 | — |
| Magnesium stearate (mg) | 3.2 | 1.6 | 1.92 | — | — | — |
| Sodium stearyl fumarate (mg) | — | — | — | 1.6 | 1.92 | 3.2 |
| Angle of repose (°) | 40.1 | 38.5 | 39.7 | 37.8 | 38.9 | 39.7 |
| Projects | Sample-1 | Sample-2 | Sample-3 | Mean Values |
|---|---|---|---|---|
| Water content (%) | 4.56 | 5.22 | 4.67 | 4.81 ± 0.402 |
| Weight (g) | 0.3276 | 0.3261 | 0.3282 | 0.32729 ± 0.007 |
| Polysaccharide content (mg/g) | 163.2 | 144.4 | 156.5 | 154.7 ± 0.95 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Q.; Wu, M.; Sun, Y.; Lv, J.; Zhang, Y.; Cao, H.; Wu, D.; Lin, D.; Zhang, Q.; Liu, Y.; et al. Optimizing the Extraction and Encapsulation of Mucilage from Brasenia Schreberi. Polymers 2019, 11, 822. https://doi.org/10.3390/polym11050822
Luo Q, Wu M, Sun Y, Lv J, Zhang Y, Cao H, Wu D, Lin D, Zhang Q, Liu Y, et al. Optimizing the Extraction and Encapsulation of Mucilage from Brasenia Schreberi. Polymers. 2019; 11(5):822. https://doi.org/10.3390/polym11050822
Chicago/Turabian StyleLuo, Qingying, Min Wu, Yanan Sun, Junxia Lv, Yu Zhang, Hongfu Cao, Dingtao Wu, Derong Lin, Qing Zhang, Yuntao Liu, and et al. 2019. "Optimizing the Extraction and Encapsulation of Mucilage from Brasenia Schreberi" Polymers 11, no. 5: 822. https://doi.org/10.3390/polym11050822
APA StyleLuo, Q., Wu, M., Sun, Y., Lv, J., Zhang, Y., Cao, H., Wu, D., Lin, D., Zhang, Q., Liu, Y., Qin, W., & Chen, H. (2019). Optimizing the Extraction and Encapsulation of Mucilage from Brasenia Schreberi. Polymers, 11(5), 822. https://doi.org/10.3390/polym11050822








