Dual Interactions of Amphiphilic Gelatin Copolymer and Nanocurcumin Improving the Delivery Efficiency of the Nanogels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
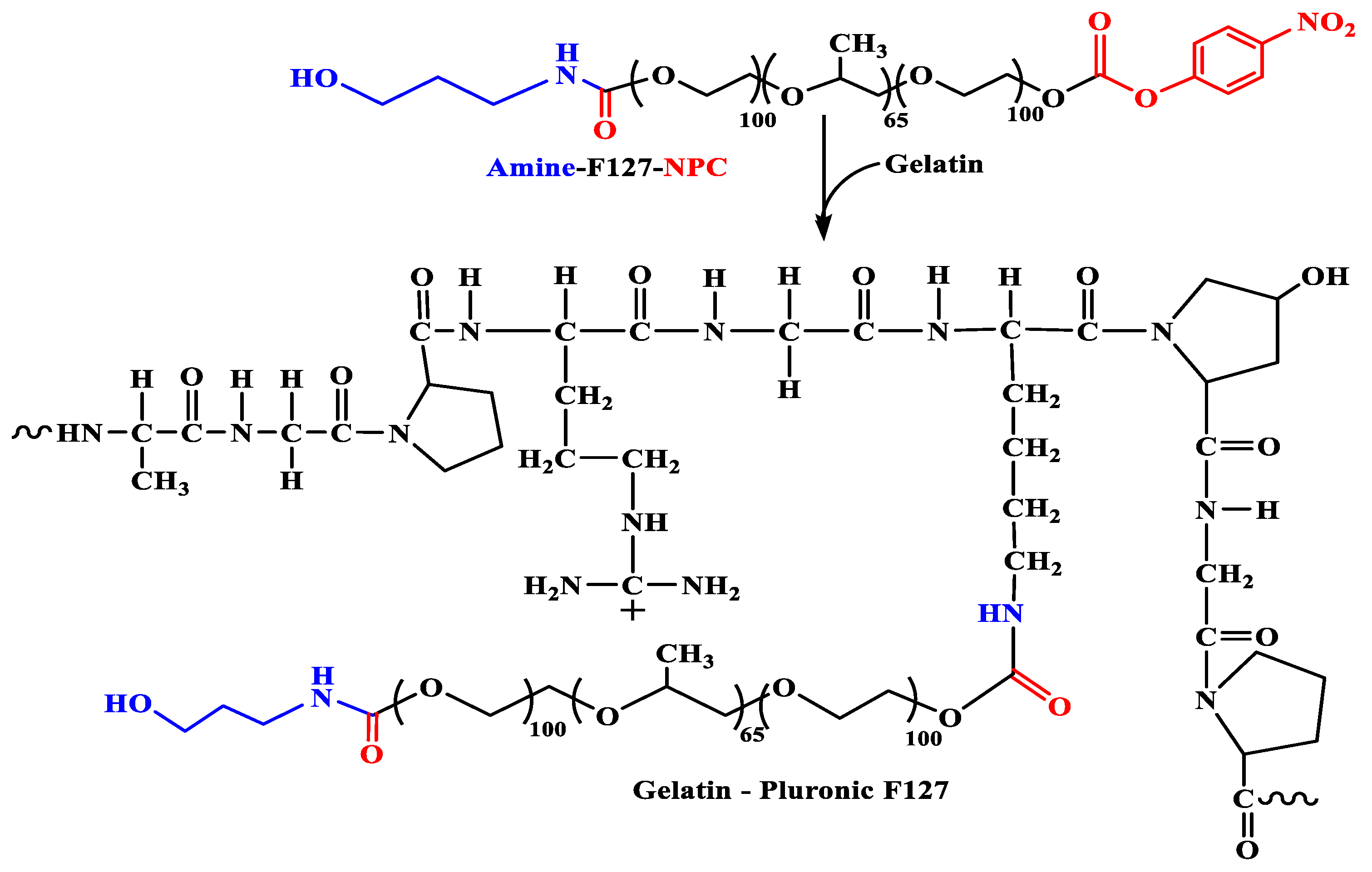
2.2. Synthesis of the Grafted GP Copolymers
2.3. Preparation of Cur-Loaded Pluronics and GP Nanogels
2.4. Release Behavior of Curcumin-Loaded Nanogels
2.5. In Vitro Cytotoxicity
3. Results and Discussion
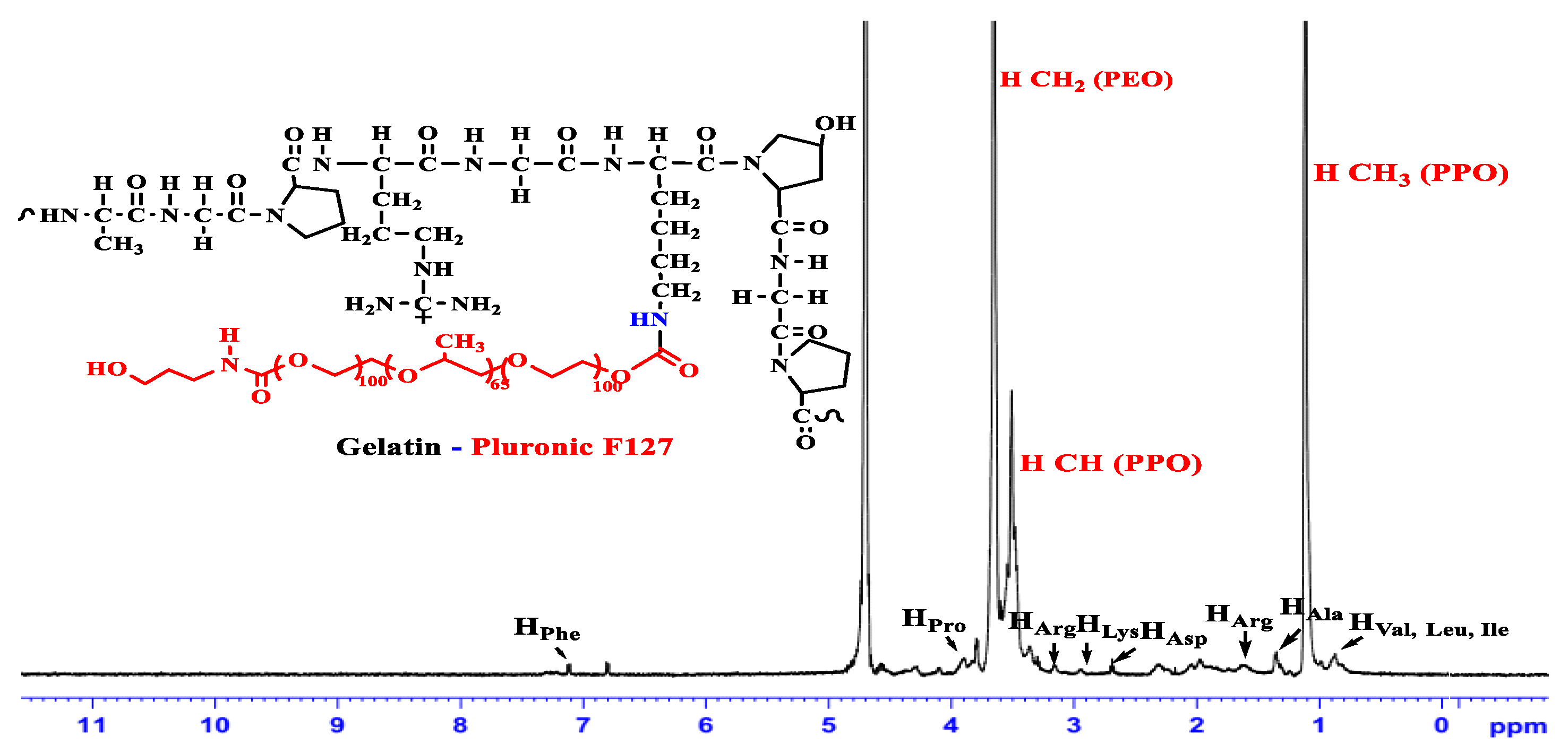
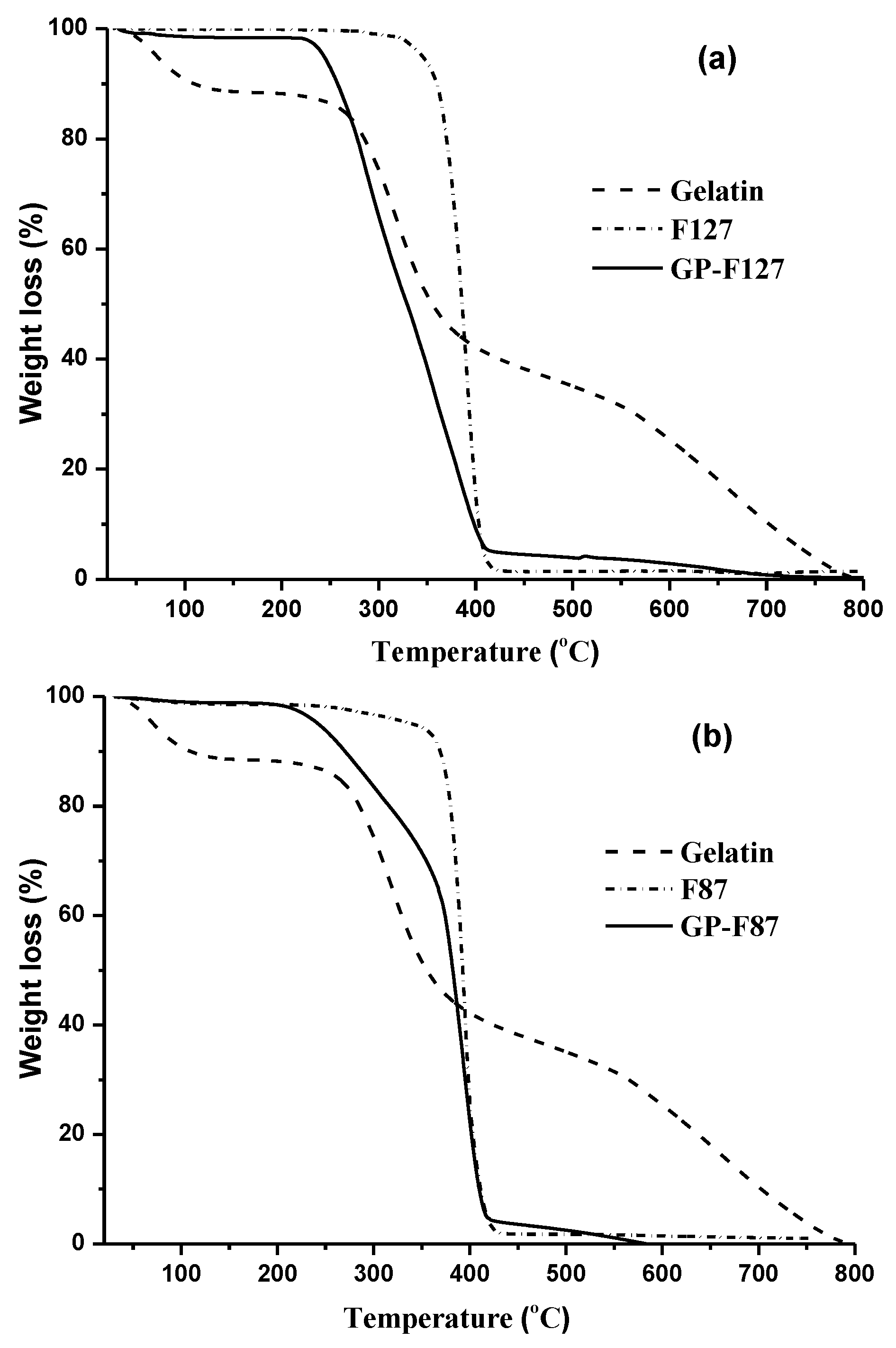
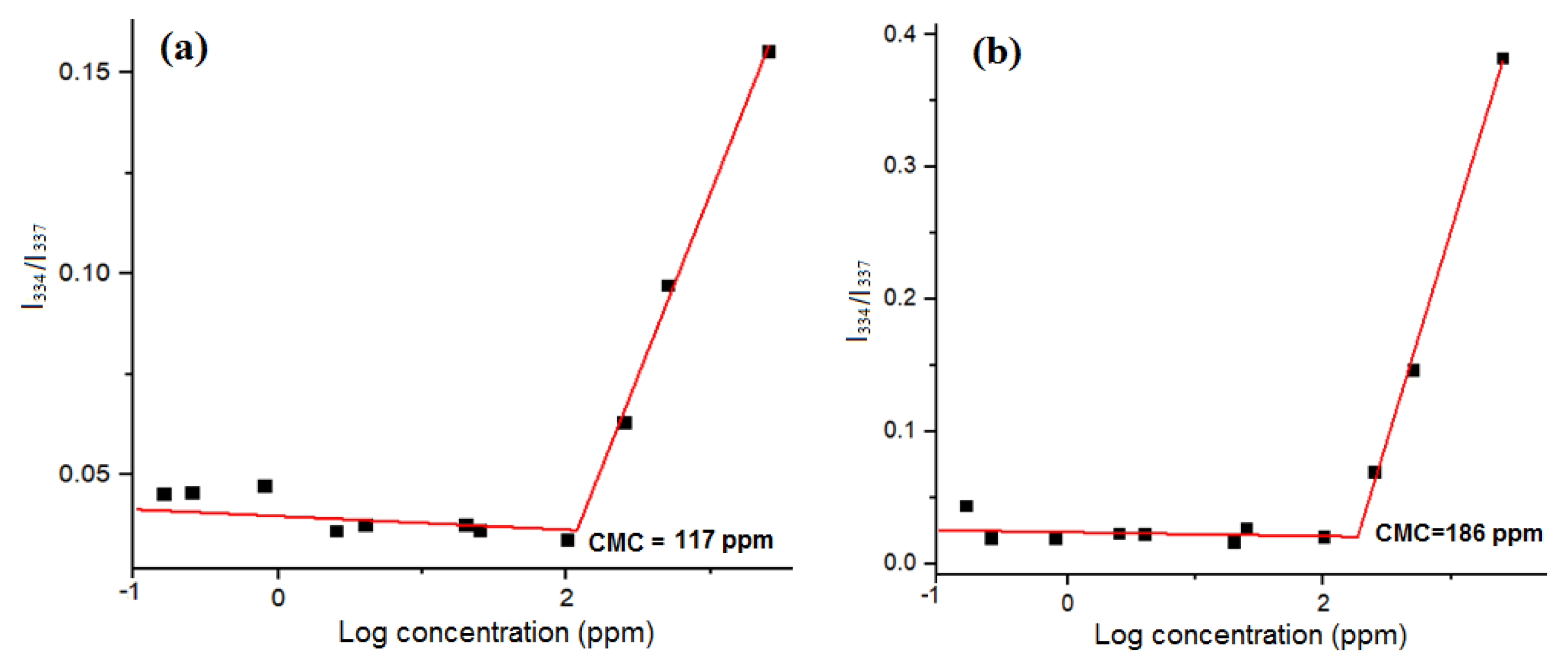
3.1. Characterization of the Amphiphilic GP Copolymers
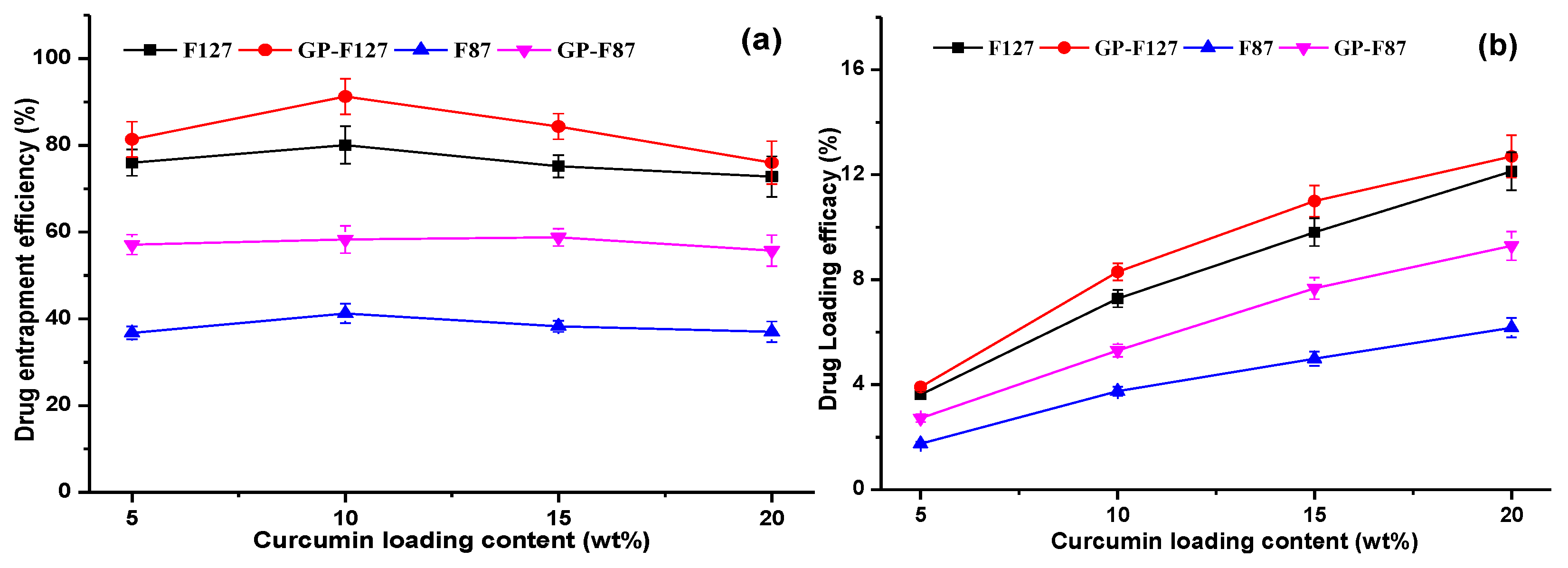
3.2. Optimization of Curcumin Loading Content
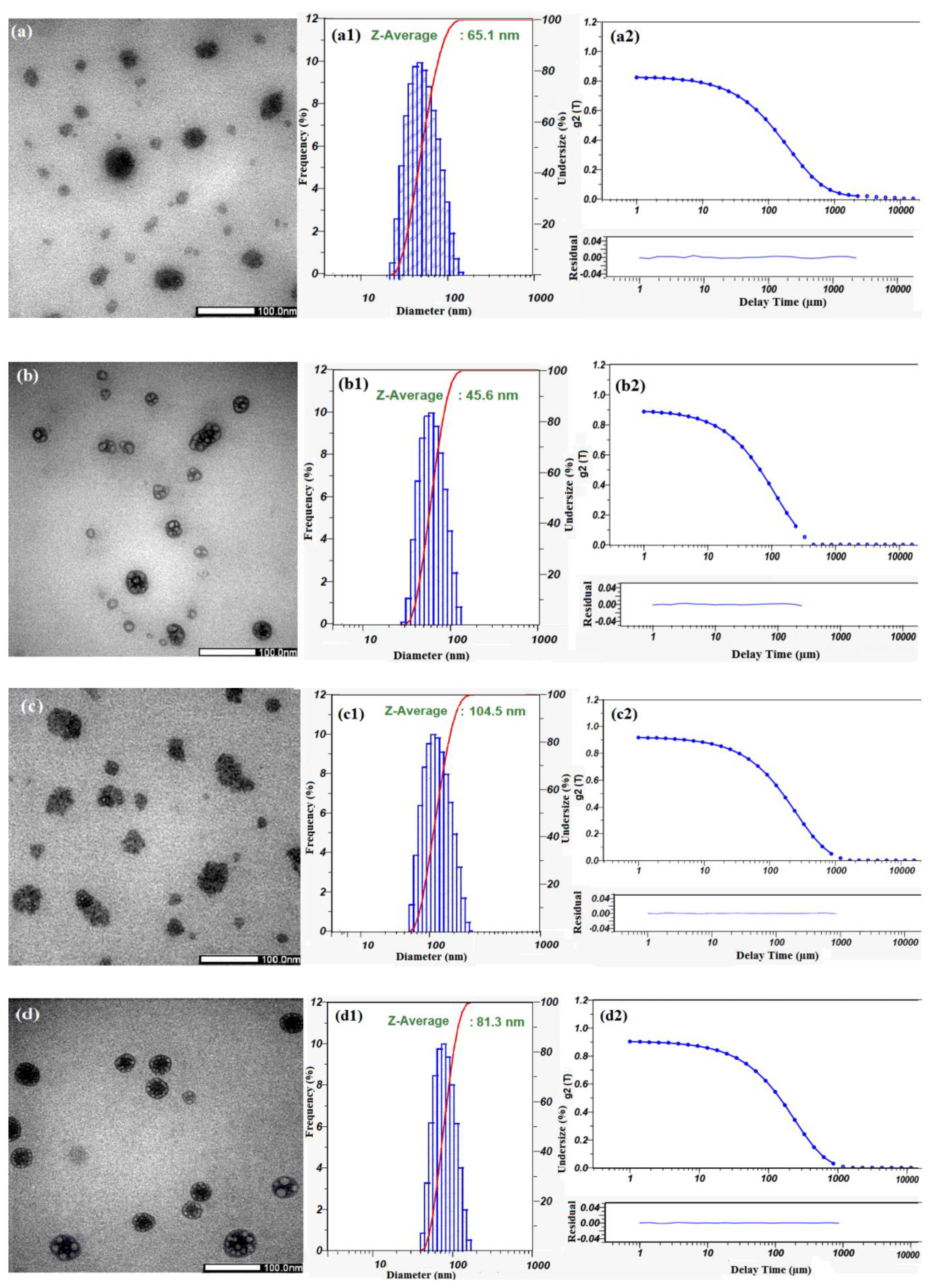
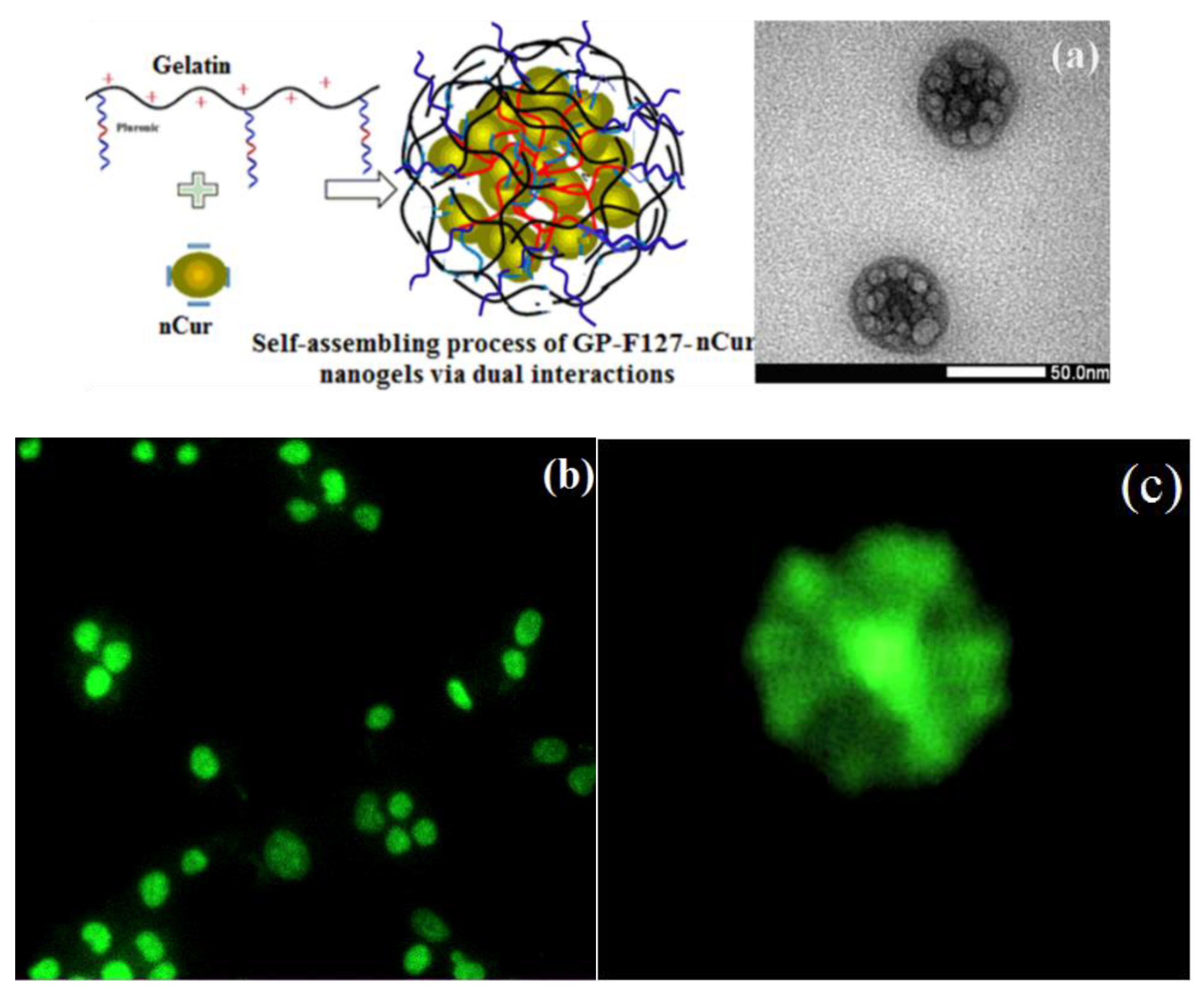
3.3. Characterization of Cur-Loaded Pluronics and GP Nanogels on Morphology and Stability
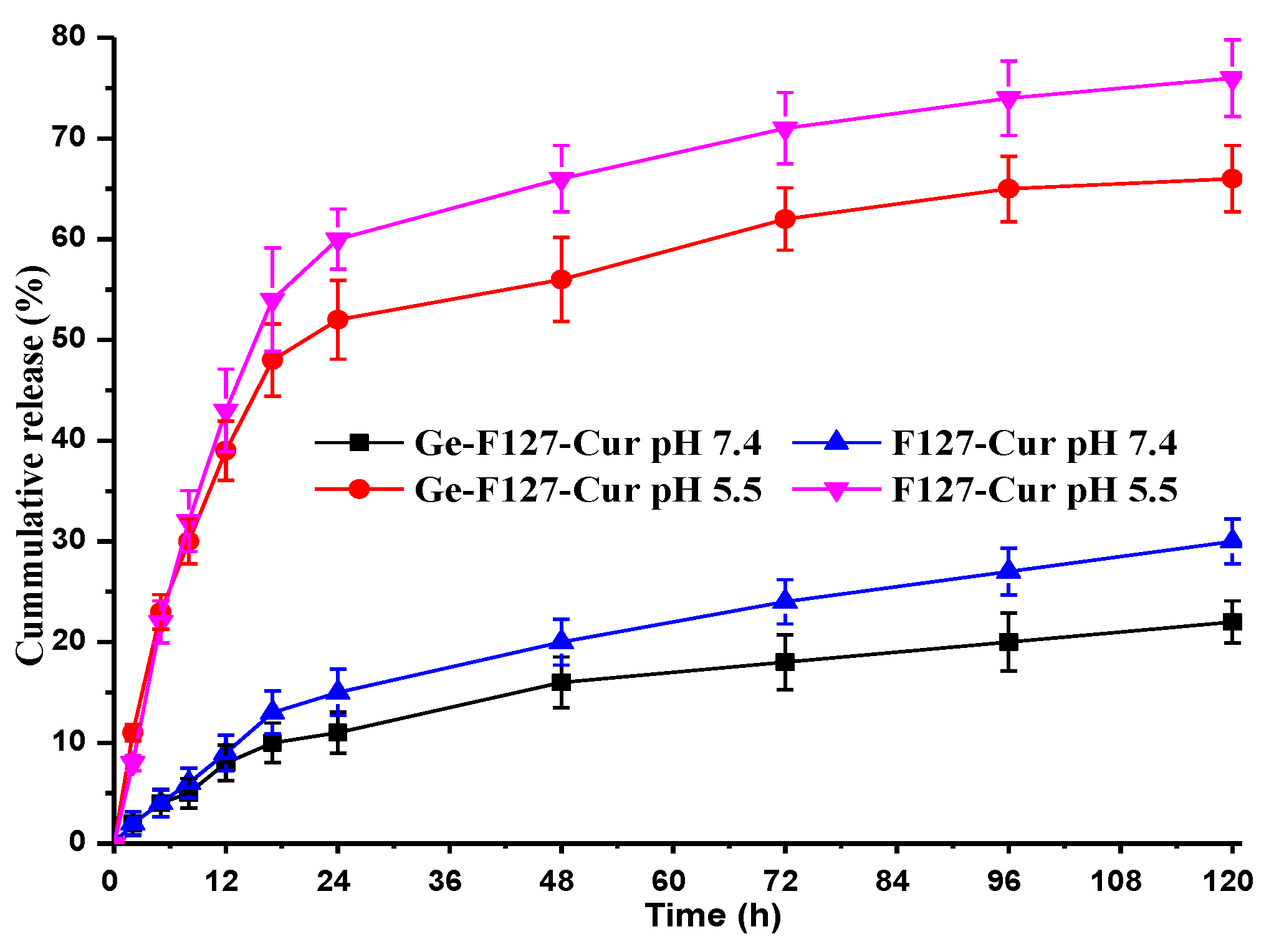
3.4. In Vitro Drug Release
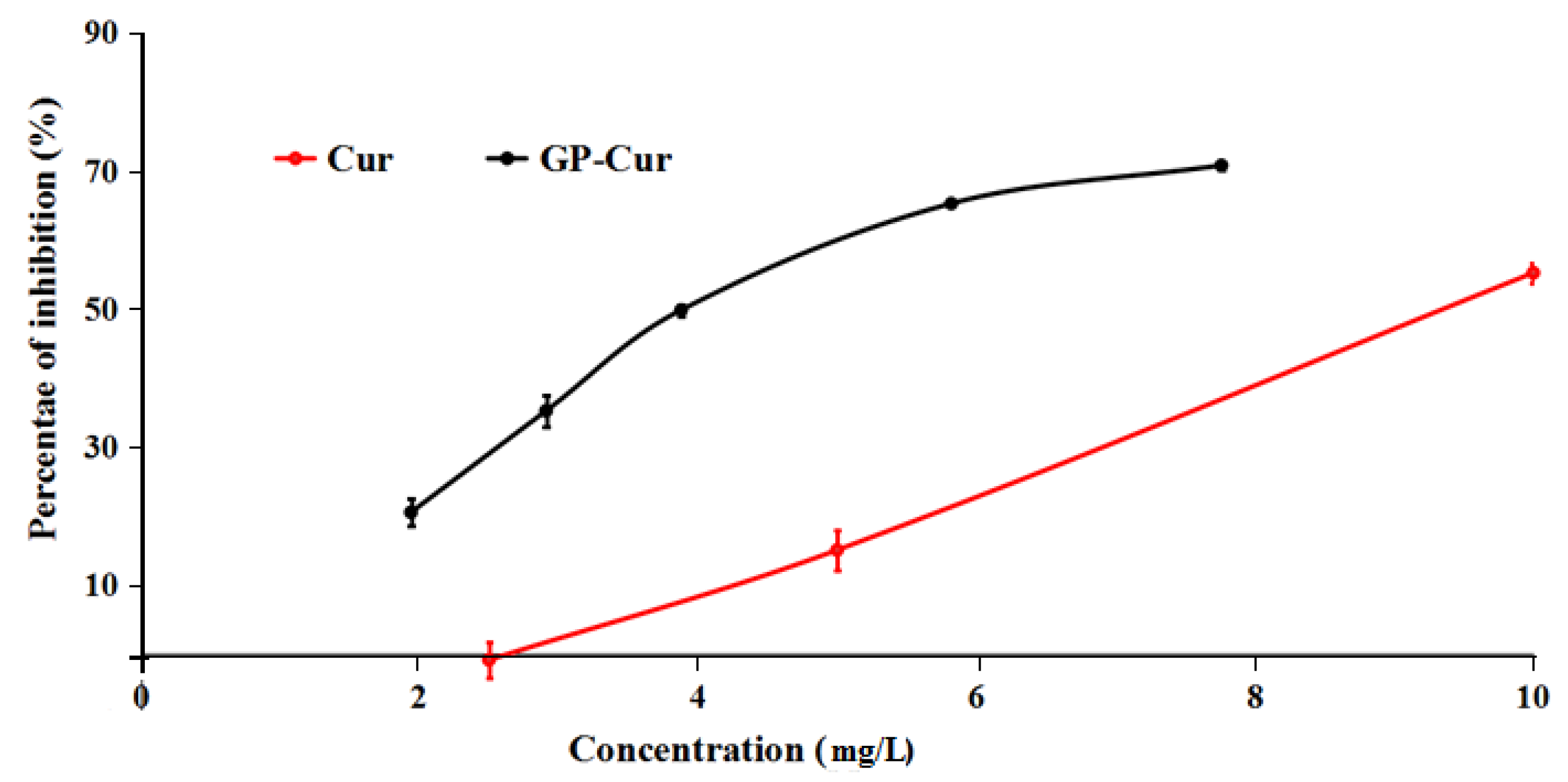
3.5. Cytotoxicity of nCur-Loaded GP Nanogels
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ree, B.J.; Satoh, Y.; Jin, K.S.; Isono, T.; Kim, W.J.; Kakuchi, T.; Satoh, T.; Ree, M. Well-defined and stable nanomicelles self-assembled from brush cyclic and tadpole copolymer amphiphiles: A versatile smart carrier platform. Npg Asia Mater. 2017, 9, e453. [Google Scholar] [CrossRef]
- Le, P.N.; Huynh, C.K.; Tran, N.Q. Advances in thermosensitive polymer-grafted platforms for biomedical applications. Mater. Sci. Eng. C 2018. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.C.; Lee, D.J.; Chen, J.K. Self-assembled supramolecular polymers with tailorable properties that enhance cell attachment and proliferation. Acta Biomater. 2017, 50, 476–483. [Google Scholar] [CrossRef]
- Le, P.N.; Nguyen, N.H.; Nguyen, C.K.; Tran, N.Q. Smart dendrimer-based nanogel for enhancing 5-fluorouracil loading efficiency against MCF7 cancer cell growth. Bull. Mater. Sci. 2016, 39, 1493–1500. [Google Scholar] [CrossRef]
- Ashraf, U.; Chat, O.A.; Maswal, M.; Jabeen, S.; Dar, A.A. An investigation of Pluronic P123–sodium cholate mixed system: Micellization, gelation and encapsulation behavior. RSC Adv. 2015, 5, 83608–83618. [Google Scholar] [CrossRef]
- Choi, J.H.; Joung, Y.K.; Bae, J.W.; Choi, J.W.; Quyen, T.N.; Park, K.D. Self-assembled nanogel of pluronic-conjugated heparin as a versatile drug nanocarrier. Macromol. Res. 2011, 19, 180–188. [Google Scholar] [CrossRef]
- Li, X.; Chen, G. Glycopolymer-based nanoparticles: Synthesis and application. Polym. Chem. 2015, 6, 1417–1430. [Google Scholar] [CrossRef]
- Tong, N.N.A.; Nguyen, T.P.; Nguyen, C.K.; Tran, N.Q. Aquated cisplatin and heparin-pluronic nanocomplexes exhibiting sustainable release of active platinum compound and NCI-H460 lung cancer cell antiproliferation. J. Biomater. Sci. Polym. Ed. 2016, 27, 709–720. [Google Scholar] [CrossRef]
- Dehvari, K.; Lin, K.-S.; Hammouda, B. Small-angle neutron scattering studies of microenvironmental and structural changes of Pluronic micelles upon encapsulation of paclitaxel. J. Taiwan Inst. Chem. Eng. 2017, 71, 405–413. [Google Scholar] [CrossRef]
- Talelli, M.; Barz, M.; Rijcken, C.J.; Kiessling, F.; Hennink, W.E.; Lammers, T. Core-crosslinked polymeric micelles: Principles, preparation, biomedical applications and clinical translation. Nano Today 2015, 10, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, J.; Yang, C.; Wang, W.; Chu, L.; Huang, F.; Liu, Q.; Deng, L.; Kong, D.; Liu, J. Folic acid-targeted disulfide-based cross-linking micelle for enhanced drug encapsulation stability and site-specific drug delivery against tumors. Int. J. Nanomed. 2016, 11, 1119. [Google Scholar] [CrossRef]
- Salim, N.V.; Hanley, T.L.; Waddington, L.; Hartley, P.G.; Guo, Q. A simple and effective approach to vesicles and large compound vesicles via complexation of amphiphilic block copolymer with polyelectrolyte in water. Macromol. Rapid Commun. 2012, 33, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Milani, R.; Houbenov, N.; Fernandez-Palacio, F.; Cavallo, G.; Luzio, A.; Haataja, J.; Giancane, G.; Saccone, M.; Priimagi, A.; Metrangolo, P. Hierarchical self-assembly of halogen-bonded block copolymer complexes into upright cylindrical domains. Chem 2017, 2, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Cu, T.S.; Nguyen, C.K.; Tran, N.Q. Preparation of silver core-chitosan shell nanoparticles using catechol-functionalized chitosan and antibacterial studies. Macromol. Res. 2014, 22, 418–423. [Google Scholar] [CrossRef]
- Hohl, L.; Röhl, S.; Stehl, D.; von Klitzing, R.; Kraume, M. Influence of Nanoparticles and Drop Size Distributions on the Rheology of w/o Pickering Emulsions. Chem. Ing. Tech. 2016, 88, 1815–1826. [Google Scholar] [CrossRef]
- Gröschel, A.H.; Müller, A.H. Self-assembly concepts for multicompartment nanostructures. Nanoscale 2015, 7, 11841–11876. [Google Scholar] [CrossRef]
- Grzelczak, M.; Vermant, J.; Furst, E.M.; Liz-Marzan, L.M. Directed self-assembly of nanoparticles. ACS Nano 2010, 4, 3591–3605. [Google Scholar] [CrossRef]
- Santos, A.C.; Pattekari, P.; Jesus, S.; Veiga, F.; Lvov, Y.; Ribeiro, A.N.J. Sonication-assisted layer-by-layer assembly for low solubility drug nanoformulation. ACS Appl. Mater. Interfaces 2015, 7, 11972–11983. [Google Scholar] [CrossRef]
- Guédra, M.; Valier-Brasier, T.; Conoir, J.-M.; Coulouvrat, F.; Astafyeva, K.; Thomas, J.-L. Influence of shell compressibility on the ultrasonic properties of polydispersed suspensions of nanometric encapsulated droplets. J. Acoust. Soc. Am. 2014, 135, 1044–1055. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Batrakova, E.V.; Alakhov, V.Y. Pluronic® block copolymers for overcoming drug resistance in cancer. Adv. Drug Deliv. Rev. 2002, 54, 759–779. [Google Scholar] [CrossRef]
- Sharma, P.K.; Bhatia, S.R. Effect of anti-inflammatories on Pluronic® F127: Micellar assembly, gelation and partitioning. Int. J. Pharm. 2004, 278, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Foster, B.; Cosgrove, T.; Espidel, Y. PFGSE-NMR Study of pH-Triggered Behavior in Pluronic−Ibuprofen Solutions. Langmuir 2009, 25, 6767–6771. [Google Scholar] [CrossRef]
- Alexander, S.; Cosgrove, T.; Prescott, S.W.; Castle, T.C. Flurbiprofen Encapsulation Using Pluronic Triblock Copolymers. Langmuir 2011, 27, 8054–8060. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.; De Vos, W.M.; Castle, T.C.; Cosgrove, T.; Prescott, S.W. Growth and shrinkage of pluronic micelles by uptake and release of flurbiprofen: Variation of pH. Langmuir 2012, 28, 6539–6545. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Song, C.; Han, Y.S.; Jang, J.D.; Choi, M.C. Spontaneous unilamellar polymer vesicles in aqueous solution. Soft Matter 2014, 10, 484–490. [Google Scholar] [CrossRef]
- Ghosh, S.; Kuchlyan, J.; Banik, D.; Kundu, N.; Roy, A.; Banerjee, C.; Sarkar, N. Organic additive, 5-methylsalicylic acid induces spontaneous structural transformation of aqueous pluronic triblock copolymer solution: A spectroscopic investigation of interaction of curcumin with pluronic micellar and vesicular aggregates. J. Phys. Chem. B 2014, 118, 11437–11448. [Google Scholar] [CrossRef]
- Dang, L.H.; Nguyen, T.H.; Tran, L.B.H.; Doan, N.V.; Tran, N.Q. Injectable Nanocurcumin-Formulated Chitosan-g-Pluronic Hydrogel Exhibiting a Great Potential for Burn Treatment. J. Healthc. Eng. 2018, 2018. [Google Scholar] [CrossRef]
- Nguyen, T.T.C.; Nguyen, C.K.; Nguyen, T.H.; Tran, N.Q. Highly lipophilic pluronics-conjugated polyamidoamine dendrimer nanocarriers as potential delivery system for hydrophobic drugs. Mater. Sci. Eng. C 2017, 70, 992–999. [Google Scholar] [CrossRef]
- Tong, N.N.A.; Tran, N.Q.; Nguyen, X.T.D.T.; Cao, V.D.; Nguyen, T.P.; Nguyen, C.K. Thermosensitive heparin-Pluronic® copolymer as effective dual anticancer drugs delivery system for combination cancer therapy. Int. J. Nanotechnol. 2018, 15, 174–187. [Google Scholar] [CrossRef]
- Dang, L.H.; Vu, M.T.; Chen, J.; Nguyen, C.K.; Bach, L.G.; Tran, N.Q.; Le, V.T. Effect of Ultrasonication on Self-Assembled Nanostructures Formed by Amphiphilic Positive-Charged Copolymers and Negative-Charged Drug. ACS Omega 2019, 4, 4540–4552. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, L.; Yang, L.; Zhu, F.; Ding, M.; Lin, F.; Wang, Z.; Yiwen, L. “Click” chemistry in polymeric scaffolds: Bioactive materials for tissue engineering. J. Control. Release 2018, 273, 160–179. [Google Scholar] [CrossRef]
- Gao, S.; Tang, G.; Hua, D.; Xiong, R.; Han, J.; Jiang, S.; Zhang, Q.; Huang, C. Stimuli-responsive bio-based polymeric systems and their applications. J. Mater. Chem. B 2019, 7, 709–729. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Nguyen, T.B.T.; Dang, L.H.; Nguyen, T.T.T.; Nguyen, D.H.; Nguyen, V.T.; Nguyen, C.K.; Nguyen, T.H.; Tran, N.Q. Green processing of thermosensitive nanocurcumin-encapsulated chitosan hydrogel towards biomedical application. Green Process. Synth. 2016, 5, 511–520. [Google Scholar] [CrossRef]
- Kim, H.; Park, J.; Tak, K.-H.; Bu, S.Y.; Kim, E. Chemopreventive effects of curcumin on chemically induced mouse skin carcinogenesis in BK5. insulin-like growth factor-1 transgenic mice. Vitr. Cell. Dev. Biol. Anim. 2014, 50, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Jain, R.; Das, M.; Agrawal, A.K.; Thanki, K.; Kushwah, V. Combinatorial bio-conjugation of gemcitabine and curcumin enables dual drug delivery with synergistic anticancer efficacy and reduced toxicity. RSC Adv. 2014, 4, 29193–29201. [Google Scholar] [CrossRef]
- Baek, J.-S.; Cho, C.-W. A multifunctional lipid nanoparticle for co-delivery of paclitaxel and curcumin for targeted delivery and enhanced cytotoxicity in multidrug resistant breast cancer cells. Oncotarget 2017, 8, 30369. [Google Scholar] [CrossRef]
- Jiang, H.; Geng, D.; Liu, H.; Li, Z.; Cao, J. Co-delivery of etoposide and curcumin by lipid nanoparticulate drug delivery system for the treatment of gastric tumors. Drug Deliv. 2016, 23, 3665–3673. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Si, X.; Han, M.K.; Viennois, E.; Zhang, M.; Merlin, D. Co-delivery of camptothecin and curcumin by cationic polymeric nanoparticles for synergistic colon cancer combination chemotherapy. J. Mater. Chem. B 2015, 3, 7724–7733. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Nguyen, N.N.T.; Tran, N.T.N.; Nguyen, N.H.; Le, P.N.; Nguyen, T.B.T.; Bach, L.G.; Doan, N.V.; Tran, L.B.H.; Le, V.T.; et al. Synergic Activity Against MCF-7 Breast Cancer Cell Growth of Nanocurcumin-Encapsulated and Cisplatin-Complexed Nanogels. Molecules 2018, 23, 3347. [Google Scholar] [CrossRef]
- Takahashi, M.; Uechi, S.; Takara, K.; Asikin, Y.; Wada, K. Evaluation of an oral carrier system in rats: Bioavailability and antioxidant properties of liposome-encapsulated curcumin. J. Agric. Food Chem. 2009, 57, 9141–9146. [Google Scholar] [CrossRef]
- Shaikh, J.; Ankola, D.; Beniwal, V.; Singh, D.; Kumar, M.R. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur. J. Pharm. Sci. 2009, 37, 223–230. [Google Scholar] [CrossRef]
- Nguyen, T.M.N.; Ho, H.T.D. Selective cytotoxicity of a Vietnamese traditional formula, Nam Dia long, against MCF-7 cells by synergistic effects. BMC Complementary Altern. Med. 2016, 16, 220. [Google Scholar] [CrossRef]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Farndale, R.W.; Hamaia, S.; Best, S.M.; Cameron, R.E. Evaluation of cell binding to collagen and gelatin: A study of the effect of 2D and 3D architecture and surface chemistry. J. Mater. Sci. Mater. Med. 2016, 27, 148. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Graulus, G.-J.; Samal, S.K.; Van Nieuwenhove, I.; Dubruel, P. Porous hydrogel biomedical foam scaffolds for tissue repair, in Biomedical foams for tissue engineering applications. Elsevier 2014, 335–390. [Google Scholar] [CrossRef]
- Dang, L.H.; Huynh, N.T.; Pham, N.O.; Nguyen, C.T.; Vu, M.T.; Tran, N.Q. Injectable nanocurcumin-dispersed gelatin–pluronic nanocomposite hydrogel platform for burn wound treatment. Bull. Mater. Sci. 2019, 42, 71. [Google Scholar] [CrossRef]
- Ding, W.; Sun, J.; Lian, H.; Xu, C.; Liu, X.; Zheng, S.; Zhang, D.; Han, X.; Liu, Y.; Chen, X.; et al. The Influence of Shuttle-Shape Emodin Nanoparticles on the Streptococcus suis Biofilm. Front. Pharmacol. 2018, 9, 227. [Google Scholar] [CrossRef]
- Bernert, D.B.; Isenbügel, K.; Ritter, H. Synthesis of a Novel Glycopeptide by Polymeranalogous Reaction of Gelatin with Mono-6-para-toluenesulfonyl-β-cyclodextrin and its Supramolecular Properties. Macromol. Rapid Commun. 2011, 32, 397–403. [Google Scholar] [CrossRef]
- Rahman, M.A.; Khan, M.A.; Tareq, S.M. Preparation and characterization of polyethylene oxide (PEO)/gelatin blend for biomedical application: Effect of gamma radiation. J. Appl. Polym. Sci. 2010, 117, 2075–2082. [Google Scholar] [CrossRef]
- Mirhosseini, M.M.; Haddadi-Asl, V.; Zargarian, S.S. Fabrication and characterization of polymer–ceramic nanocomposites containing pluronic F127 immobilized on hydroxyapatite nanoparticles. RSC Adv. 2016, 6, 80564–80575. [Google Scholar] [CrossRef]
- Liu, J.; Feng, N.; Chang, S.; Kang, H. Preparation and characterization of poly (glycidyl methacrylate) grafted from magnesium hydroxide particles via SI-ATRP. Appl. Surf. Sci. 2012, 258, 6127–6135. [Google Scholar] [CrossRef]
- Kozlov, M.Y.; Melik-Nubarov, N.S.; Batrakova, E.V.; Kabanov, A.V. Relationship between pluronic block copolymer structure, critical micellization concentration and partitioning coefficients of low molecular mass solutes. Macromolecules 2000, 33, 3305–3313. [Google Scholar] [CrossRef]
- Chen, L.C.; Chen, Y.C.; Su, C.Y.; Wong, W.P.; Sheu, M.T.; Ho, H.O. Corrigendum: Development and Characterization of Lecithin-based Self-assembling Mixed Polymeric Micellar (saMPMs) Drug Delivery Systems for Curcumin. Sci. Rep. 2017, 7, 44967. [Google Scholar] [CrossRef]
- Gyulai, G.; Magyar, A.; Rohonczy, J.; Orosz, J.; Yamasaki, M.; Bősze, S.; Kiss, É. Preparation and characterization of cationic Pluronic for surface modification and functionalization of polymeric drug delivery nanoparticles. Express Polym. Lett. 2016, 10, 216. [Google Scholar] [CrossRef]
- Mondal, S.; Ghosh, S.; Moulik, S.P. Stability of curcumin in different solvent and solution media: UV–visible and steady-state fluorescence spectral study. J. Photochem. Photobiol. B Biol. 2016, 158, 212–218. [Google Scholar] [CrossRef]
- Harigae, T.; Nakagawa, K.; Miyazawa, T.; Inoue, N.; Kimura, F.; Ikeda, I.; Miyazawa, T. Metabolic fate of poly-(lactic-co-glycolic acid)-based curcumin nanoparticles following oral administration. Int. J. Nanomed. 2016, 11, 3009. [Google Scholar] [CrossRef]
- Dende, C.; Meena, J.; Nagarajan, P.; Nagaraj, V.A.; Panda, A.K.; Padmanaban, G. Nanocurcumin is superior to native curcumin in preventing degenerative changes in Experimental Cerebral Malaria. Sci. Rep. 2017, 7, 10062. [Google Scholar] [CrossRef]
- Basak, R.; Bandyopadhyay, R. Encapsulation of hydrophobic drugs in Pluronic F127 micelles: Effects of drug hydrophobicity, solution temperature, and pH. Langmuir 2013, 29, 4350–4356. [Google Scholar] [CrossRef] [PubMed]
- Poma, P.; Notarbartolo, M.; Labbozzetta, M.; Maurici, A.; Carina, V.; Alaimo, A.; Rizzi, M.; Simoni, D.; D’Alessandro, N. The antitumor activities of curcumin and of its isoxazole analogue are not affected by multiple gene expression changes in an MDR model of the MCF-7 breast cancer cell line: Analysis of the possible molecular basis. Int. J. Mol. Med. 2007, 20, 329–335. [Google Scholar] [CrossRef] [PubMed]
| Sample | Gelatin (%) | Pluronic (%) | Grafting Yield (%) |
|---|---|---|---|
| GP-F127 | 10.10 | 89.90 | 49.45 |
| GP-F87 | 9.22 | 90.78 | 54.70 |
| Pluronic | HLB | Zeta (mV) | GP | Zeta (mV) | GP-Cur | Zeta (mV) |
|---|---|---|---|---|---|---|
| F127 | 22 | −22.67 ± 0.21 | GP-F127 | 7.67 ± 0.21 | GP-F127-nCur | −24.20 ± 0.53 |
| F87 | 24 | −29.77 ± 1.11 | GP-F87 | −7.9 ± 0.1 | GP-F87-nCur | −31.43 ± 0.74 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, D.T.; Dinh, V.T.; Dang, L.H.; Nguyen, D.N.; Giang, B.L.; Nguyen, C.T.; Nguyen, T.B.T.; Thu, L.V.; Tran, N.Q. Dual Interactions of Amphiphilic Gelatin Copolymer and Nanocurcumin Improving the Delivery Efficiency of the Nanogels. Polymers 2019, 11, 814. https://doi.org/10.3390/polym11050814
Nguyen DT, Dinh VT, Dang LH, Nguyen DN, Giang BL, Nguyen CT, Nguyen TBT, Thu LV, Tran NQ. Dual Interactions of Amphiphilic Gelatin Copolymer and Nanocurcumin Improving the Delivery Efficiency of the Nanogels. Polymers. 2019; 11(5):814. https://doi.org/10.3390/polym11050814
Chicago/Turabian StyleNguyen, Dinh Trung, Van Thoai Dinh, Le Hang Dang, Dang Nam Nguyen, Bach Long Giang, Cong Truc Nguyen, Thi Bich Tram Nguyen, Le Van Thu, and Ngoc Quyen Tran. 2019. "Dual Interactions of Amphiphilic Gelatin Copolymer and Nanocurcumin Improving the Delivery Efficiency of the Nanogels" Polymers 11, no. 5: 814. https://doi.org/10.3390/polym11050814
APA StyleNguyen, D. T., Dinh, V. T., Dang, L. H., Nguyen, D. N., Giang, B. L., Nguyen, C. T., Nguyen, T. B. T., Thu, L. V., & Tran, N. Q. (2019). Dual Interactions of Amphiphilic Gelatin Copolymer and Nanocurcumin Improving the Delivery Efficiency of the Nanogels. Polymers, 11(5), 814. https://doi.org/10.3390/polym11050814





