Butylglyceryl Pectin Nanoparticles: Synthesis, Formulation and Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
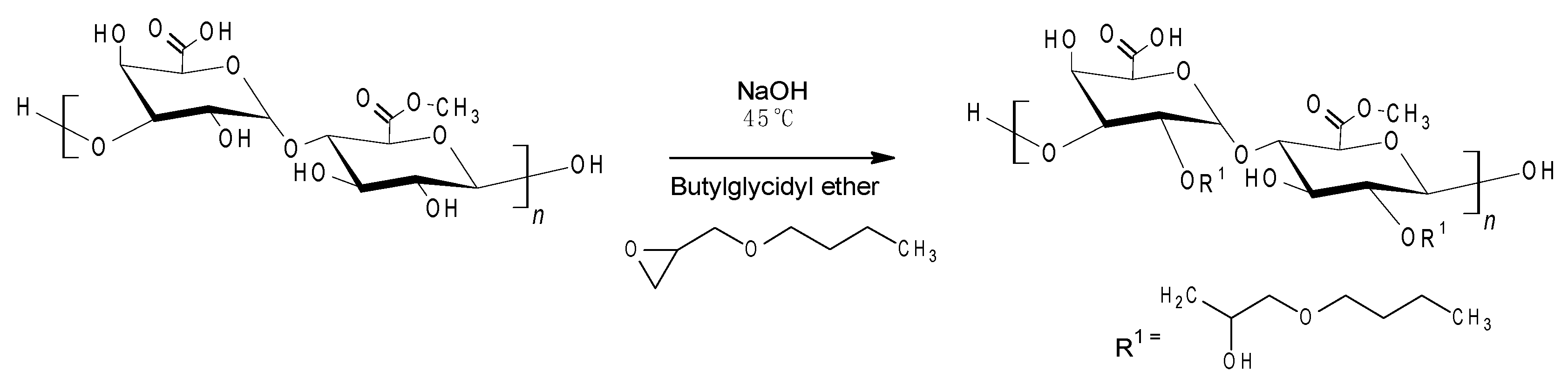
2.2. Synthesis of Butylglyceryl-Modified Pectin
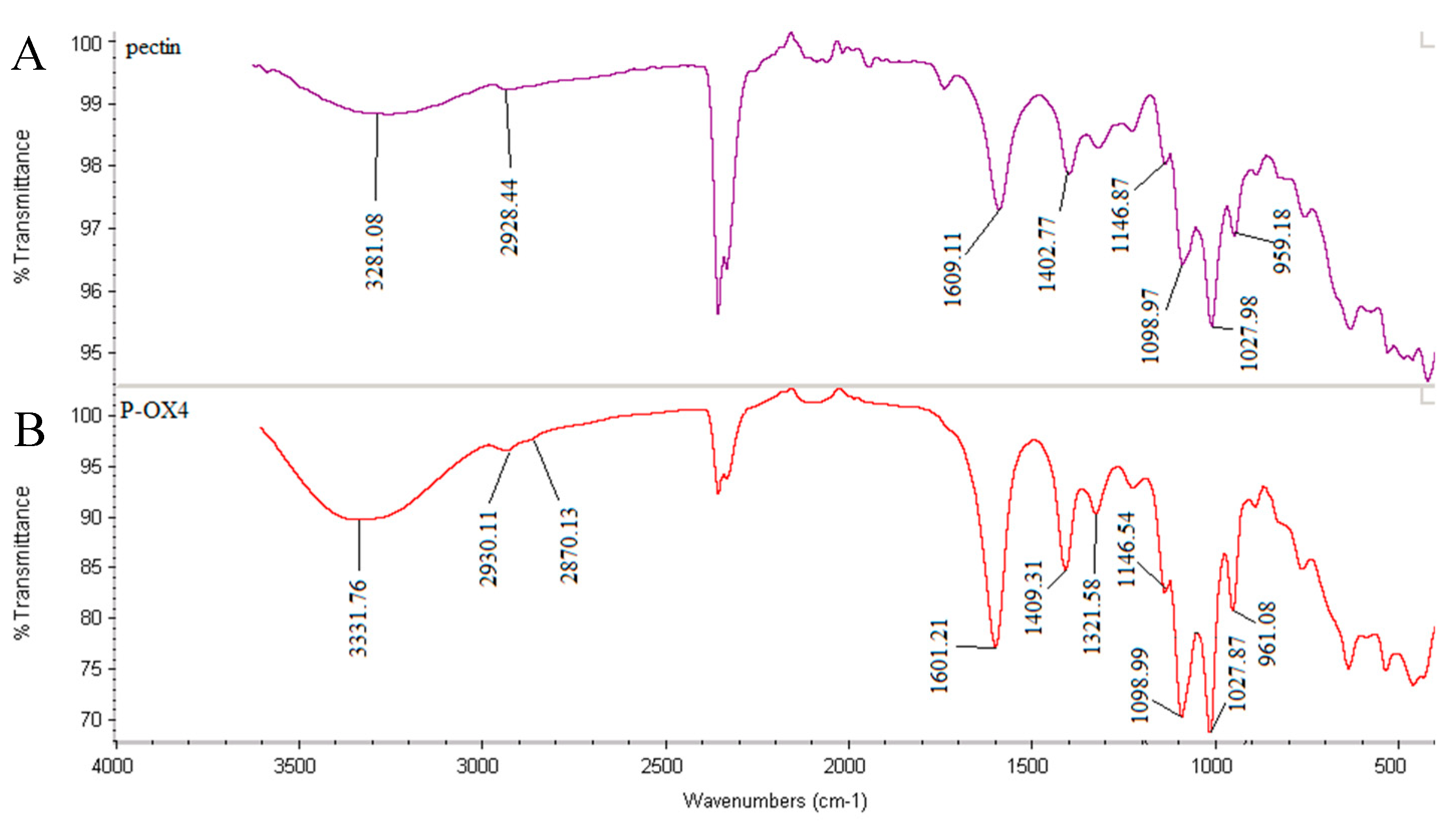
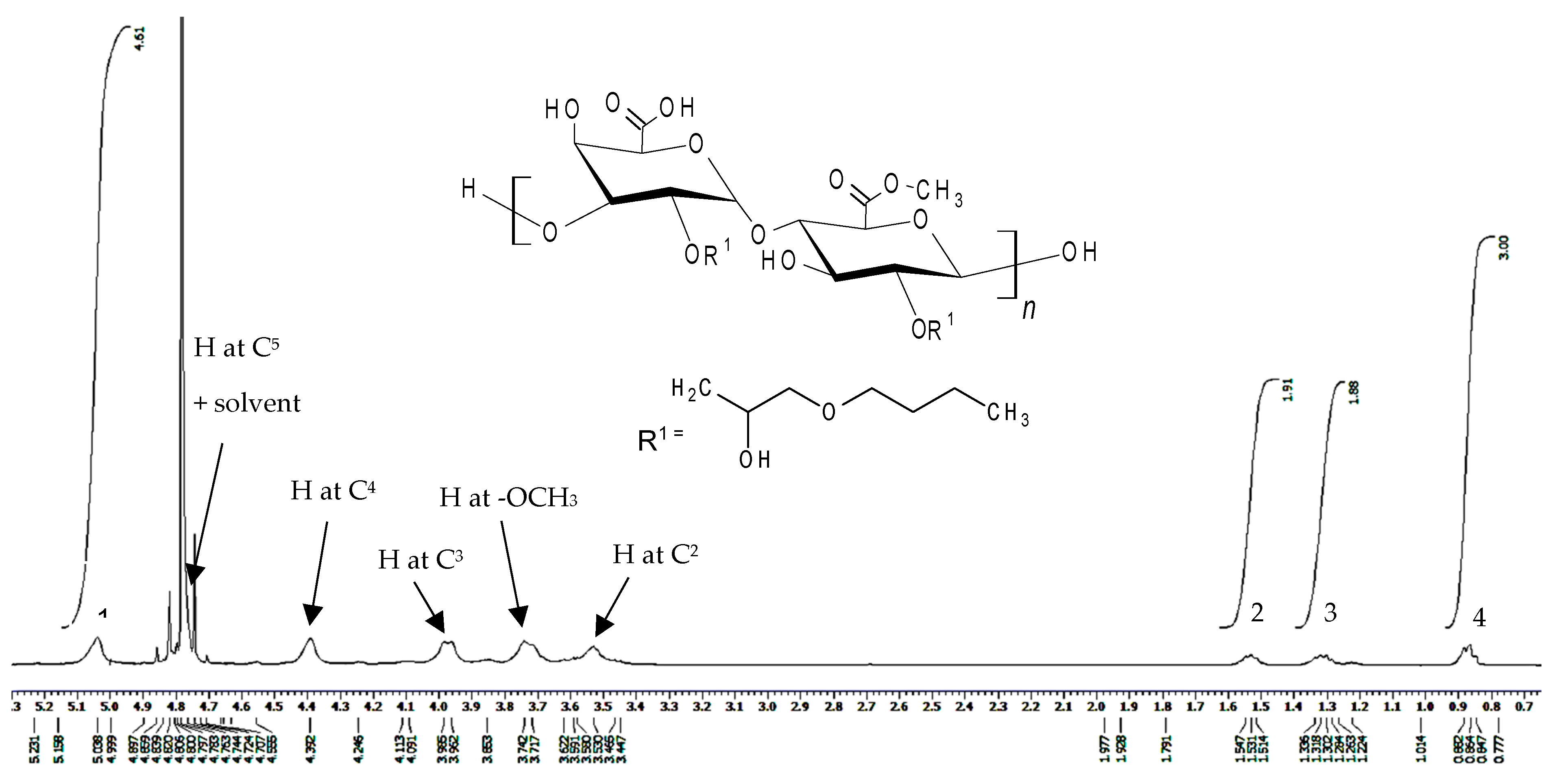
2.3. Characterization of Butylglyceryl-Modified Pectin
2.4. Formulation of Nanoparticles from Butylglyceryl-Modified Pectin
2.5. Physical Characterization of Butylglyceryl Pectin Nanoparticles
2.6. Loading Capacity of Butylglyceryl Pectin Nanoparticles
3. Results and Discussion
- DS (%) Degree of substitution (number of butylglyceryl chains attached to 100 sugar residue of pectin)
- A Integral value of the signal assigned to the alkyl chain-end CH3 group (δ 0.9 ppm)
- B Integral value of the signal assigned to the anomeric C1 proton of the glucopyranose ring (δ 5.0 ppm)
- η Viscosity (in centipoise, cp)
- ρt Density of ball (g/mL)
- ρ Density of liquid (g/mL)
- t Time of the ball descent in the liquid (mins)
- K Viscometer constant (depending on ball size)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Quiñones, J.P.; Peniche, H.; Peniche, C. Chitosan based self-assembled nanoparticles in drug delivery. Polymers 2018, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Tao, T.; Wen, Y.; Yi, J.; He, L.; Huang, Z.; Nie, Y.; Yao, X.; Wang, Y.; He, C.; et al. Novel delivery of mitoxantrone with hydrophobically modified pullulan nanoparticles to inhibit bladder cancer cell and the effect of nano-drug size on inhibition efficiency. Nanoscale Res. Lett. 2018, 13, 345. [Google Scholar] [CrossRef] [PubMed]
- Kamal, T.; Sarfraz, M.; Arafat, M.; Mikov, M.; Rahman, N. Cross-linked guar gum and sodium borate based microspheres as colon-targeted anticancer drug delivery systems for 5-fluorouracil. Pak. J. Pharm. Sci. 2017, 30, 2329–2336. [Google Scholar]
- Li, S.; Xiong, Q.; Lai, X.; Li, X.; Wan, M.; Zhang, J.; Yan, Y.; Cao, M.; Lu, L.; Guan, J. Molecular modification of polysaccharides and resulting bioactivities. Compr. Rev. Food Sci. Food Saf. 2016, 15, 237–250. [Google Scholar]
- Miao, X. Hydrophobically Modified Derivatives of Polysaccharides. Ph.D. Thesis, The University of Grenobl, Grenoble, France, 4 October 2011. [Google Scholar]
- Srivastava, P.; Malviya, R. Sources of pectin, extraction and its applications in pharmaceutical industry—An overview. Indian J. Nat. Prod. Resour. 2011, 2, 10–18. [Google Scholar]
- May, C.D. Industrial pectins: Sources, production and applications. Carbohydr. Polym. 1990, 12, 79–99. [Google Scholar] [CrossRef]
- BeMiller, J.N. An introduction to pectins: Structure and properties. ACS Symp. Ser. Am. Chem. Soc. 1986, 3, 2–12. [Google Scholar]
- Mishra, R.K.; Banthia, A.K.; Majeed, A.B.A. Pectin based formulations for biomedical applications: A review. Asian J. Pharm. Clin. Res. 2012, 5, 1–7. [Google Scholar]
- Sinha, V.R.; Kumria, R. Polysaccharides in colon-specific drug delivery. Int. J. Pharm. 2001, 224, 19–38. [Google Scholar] [CrossRef]
- Vandamme, T.F.; Lenourry, A.; Charrueau, C.; Chaumeil, J.C. The use of polysaccharides to target drugs to the colon. Carbohydr. Polym. 2002, 48, 219–231. [Google Scholar] [CrossRef]
- Munjeri, O.; Collett, J.H.; Fell, J.T. Amidated pectin hydrogel beads for colonic drug delivery—An in vitro study. Drug Deliv. 1997, 4, 207–211. [Google Scholar] [CrossRef]
- Munjeri, O.; Collett, J.H.; Fell, J.T. Hydrogel beads based on amidated pectins for colon-specific drug delivery: The role of chitosan in modifying drug release. J. Control. Release 1997, 46, 273–278. [Google Scholar] [CrossRef]
- Wakerly, Z.; Fell, J.; Attwood, D.; Parkins, D. Studies on amidated pectins as potential carriers in colonic drug delivery. J. Pharm. Pharmacol. 1997, 49, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Charlton, S.; Jones, N.S.; Davis, S.S.; Illum, L. Distribution and clearance of bioadhesive formulations from the olfactory region in man: Effect of polymer type and nasal delivery device. Eur. J. Pharm. Sci. 2007, 30, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Charlton, S.T.; Davis, S.S.; Illum, L. Evaluation of bioadhesive polymers as delivery systems for nose to brain delivery: In vitro characterisation studies. J. Control. Release 2007, 118, 225–234. [Google Scholar] [CrossRef]
- Zheng, X.F.; Lian, Q.; Yang, H.; Zhu, H. Alkyl pectin: Hydrophobic matrices for controlled drug release. J. Appl. Polym. Sci. 2015. [Google Scholar] [CrossRef]
- Erdlenbruch, B.; Jendrossek, V.; Eibl, H.; Lakomek, M. Transient and controllable opening of the blood-brain barrier to cytostatic and antibiotic agents by alkylglycerols in rats. Exp. Brain Res. 2000, 135, 417–422. [Google Scholar]
- Marigny, K.; Pedrono, F.; Martin-Chouly, C.A.; Youmine, H.; Saiag, B.; Legrand, A.B. Modulation of endothelial permeability by 1-O-alkylglycerols. Acta Physiol. Scand. 2002, 176, 263–268. [Google Scholar] [CrossRef]
- Erdlenbruch, B.; Alipour, M.; Fricker, G.; Miller, D.S.; Kugler, W.; Eibl, H.; Lakomek, M. Alkylglycerol opening of the blood–brain barrier to small and large fluorescence markers in normal and C6 glioma-bearing rats and isolated rat brain capillaries. Br. J. Pharmacol. 2003, 140, 1201–1210. [Google Scholar] [CrossRef] [Green Version]
- Molnár, E.; Barbu, E.; Lien, C.F.; Górecki, D.C.; Tsibouklis, J. Toward drug delivery into the brain: Synthesis, characterization, and preliminary in vitro assessment of alkylglyceryl-functionalized chitosan nanoparticles. Biomacromolecules 2010, 11, 2880–2889. [Google Scholar] [CrossRef]
- Toman, P.; Lien, C.F.; Ahmad, Z.; Dietrich, S.; Smith, J.R.; An, Q.; Molnár, E.; Pilkington, G.J.; Górecki, D.C.; Tsibouklis, J.; et al. Nanoparticles of alkylglyceryl-dextran-graft-poly(lactic acid) for drug delivery to the brain: Preparation and in vitro investigation. Acta Biomater. 2015, 22, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Arafat, M.; Kirchhoefer, C.; Mikov, M.; Sarfraz, M.; Löbenberg, R. Nanosized liposomes containing bile salt: A vesicular nanocarrier for enhancing oral bioavailability of BCS class III drug. J. Pharm. Pharm. Sci. 2017, 20, 305–318. [Google Scholar] [CrossRef]
- Arafat, M.; Kirchhoefer, C.; Mikov, M. Mixed micelles loaded with bile salt: An approach to enhance intestinal transport of the BCS class III drug cefotaxime in rats. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 635–645. [Google Scholar] [CrossRef]
- Mauleón, D.; Pujol, M.D.; Rosell, G. B-Adrenergic antagonists: N-alkyl and N-amidoethyl(arylalkoxy) propanolamines related to propranolol. Eur. J. Med. Chem. 1988, 23, 421–426. [Google Scholar] [CrossRef]
- Liu, T.; Li, B.; Zheng, X.; Liang, S.; Song, X.; Zhu, B.; Kennedy, J.F.; Xia, J. Effects of freezing on the condensed state structure of chitin in alkaline solution. Carbohydr. Polym. 2010, 82, 753–760. [Google Scholar] [CrossRef]
- Feng, F.; Liu, Y.; Hu, K. Influence of alkali-freezing treatment on the solid state structure of chitin. Carbohydr. Res. 2004, 393, 2321–2324. [Google Scholar] [CrossRef]
- Ström, A.; Schuster, E.; Goh, S.M. Rheological characterization of acid pectin samples in the absence and presence of monovalent ions. Carbohydr. Polym. 2014, 113, 336–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walkinshaw, M.D.; Arnott, S. Conformations and interactions of pectins: I. X-ray diffraction analyses of sodium pectate in neutral and acidified forms. J. Mol. Biol 1981, 153, 1055–1077. [Google Scholar] [CrossRef]
- Gnanasambandam, R.; Proctor, A. Determination of pectin degree of esterification by diffuse reflectance Fourier transform infrared spectroscopy. Food Chem. 2000, 68, 327–332. [Google Scholar] [CrossRef]
- Wang, Y.T.; Lien, L.L.; Chang, Y.C.; Wu, J.S.B. Pectin methyl esterase treatment on high-methoxy pectin for making fruit jam with reduced sugar content. J. Sci. Food Agric. 2013, 93, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Winning, H.; Viereck, N.; Nørgaard, L.; Larsen, J.; Engelsen, S.B. Quantification of the degree of blockiness in pectins using 1H NMR spectroscopy and chemometrics. Food Hydrocoll. 2007, 21, 256–266. [Google Scholar] [CrossRef]
- Pretsch, E.; Buehlmann, P.; Affolter, C.; Pretsch, E.; Bhuhlmann, P.; Affolter, C. Structure Determination of Organic Compounds; Springer: Berlin, Germany, 2000; p. 312. [Google Scholar]
- Morris, G.A.; Hromádková, Z.; Ebringerová, A.; Malovĺková, A.; Alföldi, J.; Harding, S.E. Modification of pectin with UV-absorbing substitutents and its effect on the structural and hydrodynamic properties of the water-soluble derivatives. Carbohydr. Polym. 2002, 48, 351–359. [Google Scholar] [CrossRef]
- Carey, F.A. Organic Chemistry: Conformations of Balkans and Cyclamates, 4th ed.; McGraw-Hill Education: New York, NY, USA, 2000; pp. 89–113. [Google Scholar]
- Almeida, E.A.; Facchi, S.P.; Martins, A.F.; Nocchi, S.; Schuquel, I.T.; Nakamura, C.V.; Rubira, A.F.; Muniz, E.C. Synthesis and characterization of pectin derivative with antitumor property against Caco-2 colon cancer cells. Carbohydr. Polym. 2015, 115, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.C.; Bulgakov, V.P.; Jinn, T.L. Pectin methylesterases: Cell wall remodeling proteins are required for plant response to heat stress. Front. Plant Sci. 2018, 9, 1612. [Google Scholar] [CrossRef]
- Le Devedec, F.; Won, A.; Oake, J.; Houdaihed, L.; Bohne, C.; Yip, C.M.; Allen, C. Postalkylation of a common mPEG-b-PAGE Precursor to produce tunable morphologies of spheres, filomicelles, disks, and polymersomes. ACS Macro Lett. 2016, 5, 128–133. [Google Scholar] [CrossRef]
- Thakur, V.K. Nanocellulose Polymer Nanocomposites: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Basu, S.; Shivhare, U.S.; Muley, S. Moisture adsorption isotherms and glass transition temperature of pectin. J. Food Sci. Technol. 2013, 50, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Sablani, S.S.; Kasapis, S.; Rahman, M.S. Evaluating water activity and glass transition concepts for food stability. J. Food Eng. 2007, 78, 266–271. [Google Scholar] [CrossRef]
- Roos, Y.; Karel, M. Differential scanning calorimetry study of phase transitions affecting the quality of dehydrated materials. Biotechnol. Prog. 1990, 6, 159–163. [Google Scholar] [CrossRef]
- Mishra, R.K.; Sutar, P.B.; Singhal, J.P.; Banthia, A.K. Graft copolymerization of pectin with polyacrylamide. Polym. Plast. Technol. Eng. 2007, 46, 1079–1085. [Google Scholar] [CrossRef]
- Roos, Y.H.; Drusch, S. Phase Transitions in Foods; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Arafat, M.; Ahmed, Z.; Arafat, O. Comparison between generic drugs and brand name drugs from bioequivalence and thermoequivalence prospective. Int. J. Pharm. Pharm. Sci. 2017, 9, 1–4. [Google Scholar] [CrossRef]
- Chen, N.X.; Zhang, J.H. The role of hydrogen-bonding interaction in poly(vinyl alcohol)/poly(acrylic acid) blending solutions and their films. Chin. J. Polym. Sci. 2010, 286, 903–911. [Google Scholar] [CrossRef]
- Aumelas, A.; Serrero, A.; Durand, A.; Dellacherie, E.; Leonard, M. Nanoparticles of hydrophobically modified dextrans as potential drug carrier systems. Colloids Surf. B 2007, 59, 74–80. [Google Scholar] [CrossRef]
- Hornig, S.; Heinze, T. Nanoscale structures of dextran esters. Carbohydr. Polym. 2007, 68, 280–286. [Google Scholar] [CrossRef]
- Khalid, N.; Sarfraz, M.; Arafat, M.; Akhtar, M.; Löbenberg, R.; Rehman, R. Nano-sized droplets of self-emulsifying system for enhancing oral bioavailability of chemotherapeutic agent VP-16 in rats: A nano lipid carrier for BCS class IV drugs. J. Pharm. Pharm. Sci. 2018, 21, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Zhou, G.; Ye, Q.; Kuang, J.; Ou, J.; Xu, Z.; Wen, Z.; Sha, L. In vitro anticancer activity of doxorubicin-loading pectin nanoparticles. J. Pharm. Biomed. Sci. 2016, 6, 338–342. [Google Scholar]


| Compound | Number Average Molecular Weight (Mn) | Weight Average Molecular Weight (Mw) | Polydispersity Index (PDI) |
|---|---|---|---|
| Pectin | 20,569 ± 276 | 25,037 ± 128 | 1.22 ± 0.10 |
| P-OX4 | 27,044 ± 298 | 29,111 ± 252 | 1.13 ± 0.06 |
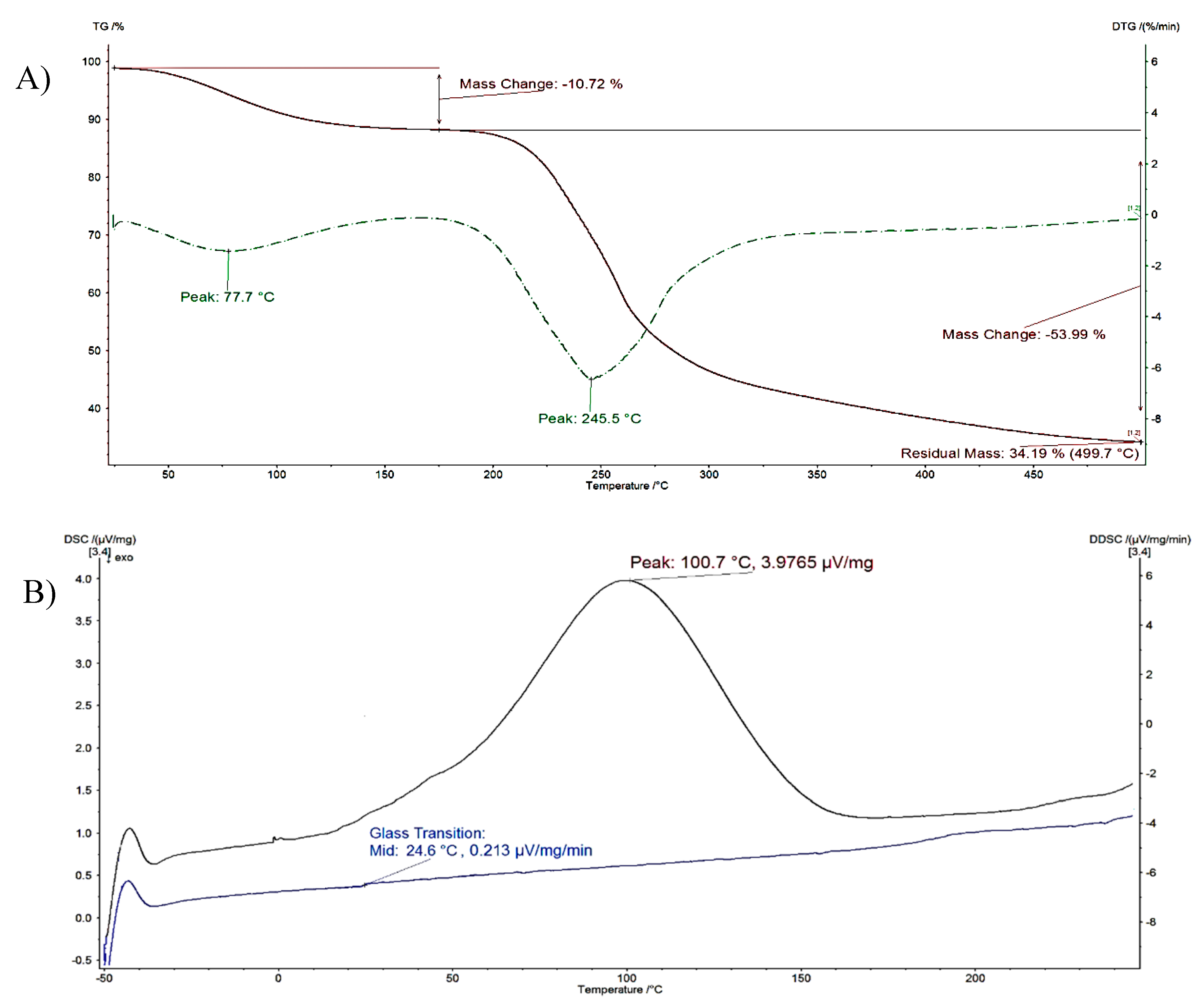
| Material | Water Evaporation | Decomposition | Glass Transition, Tg (°C) | ||
|---|---|---|---|---|---|
| DTG Peak (°C) | Mass Loss (%) | DTG Peak (°C) | Mass Loss (%) | ||
| Pectin | 56.2 ± 6.9 | 16.32 ± 8.1 | 215.4 ± 6.1 | 17.62 ± 7.4 | 22.4 ± 1.5 |
| P-OX4 | 77.7 ± 7.4 | 10.72 ± 8.2 | 245.5 ± 8.9 | 53.99 ± 7.1 | 24.7 ± 1.6 |
| Samples | Time of Descent t (min) | Viscosity η (cp) |
|---|---|---|
| Pectin | 0.80 ± 0.1 | 0.37 ± 0.1 |
| P-OX4 | 0.61 ± 0.1 | 0.28 ± 0.1 |
| Polymer | Concentration (mg/mL) | Diameter (nm) | Polydispersity Index | Zeta Potential (mV) |
|---|---|---|---|---|
| P-OX4 | 2 | 253.9 ± 11.1 | 0.18 ± 0.09 | −20.4 ± 2.1 |
| 5 | 251.1 ± 13.9 | 0.10 ± 0.08 | −22.7 ± 3.0 | |
| 10 | 252.9 ± 12.4 | 0.20 ± 0.10 | −24.2 ± 2.8 | |
| 15 | 279.9 ± 18.9 | 0.21 ± 0.12 | −26.2 ± 3.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bostanudin, M.F.; Arafat, M.; Sarfraz, M.; Górecki, D.C.; Barbu, E. Butylglyceryl Pectin Nanoparticles: Synthesis, Formulation and Characterization. Polymers 2019, 11, 789. https://doi.org/10.3390/polym11050789
Bostanudin MF, Arafat M, Sarfraz M, Górecki DC, Barbu E. Butylglyceryl Pectin Nanoparticles: Synthesis, Formulation and Characterization. Polymers. 2019; 11(5):789. https://doi.org/10.3390/polym11050789
Chicago/Turabian StyleBostanudin, Mohammad F., Mosab Arafat, Muhammad Sarfraz, Dariusz C. Górecki, and Eugen Barbu. 2019. "Butylglyceryl Pectin Nanoparticles: Synthesis, Formulation and Characterization" Polymers 11, no. 5: 789. https://doi.org/10.3390/polym11050789






