3D Printing of an Oil/Water Mixture Separator with In Situ Demulsification and Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fe/PLA Composites Filament
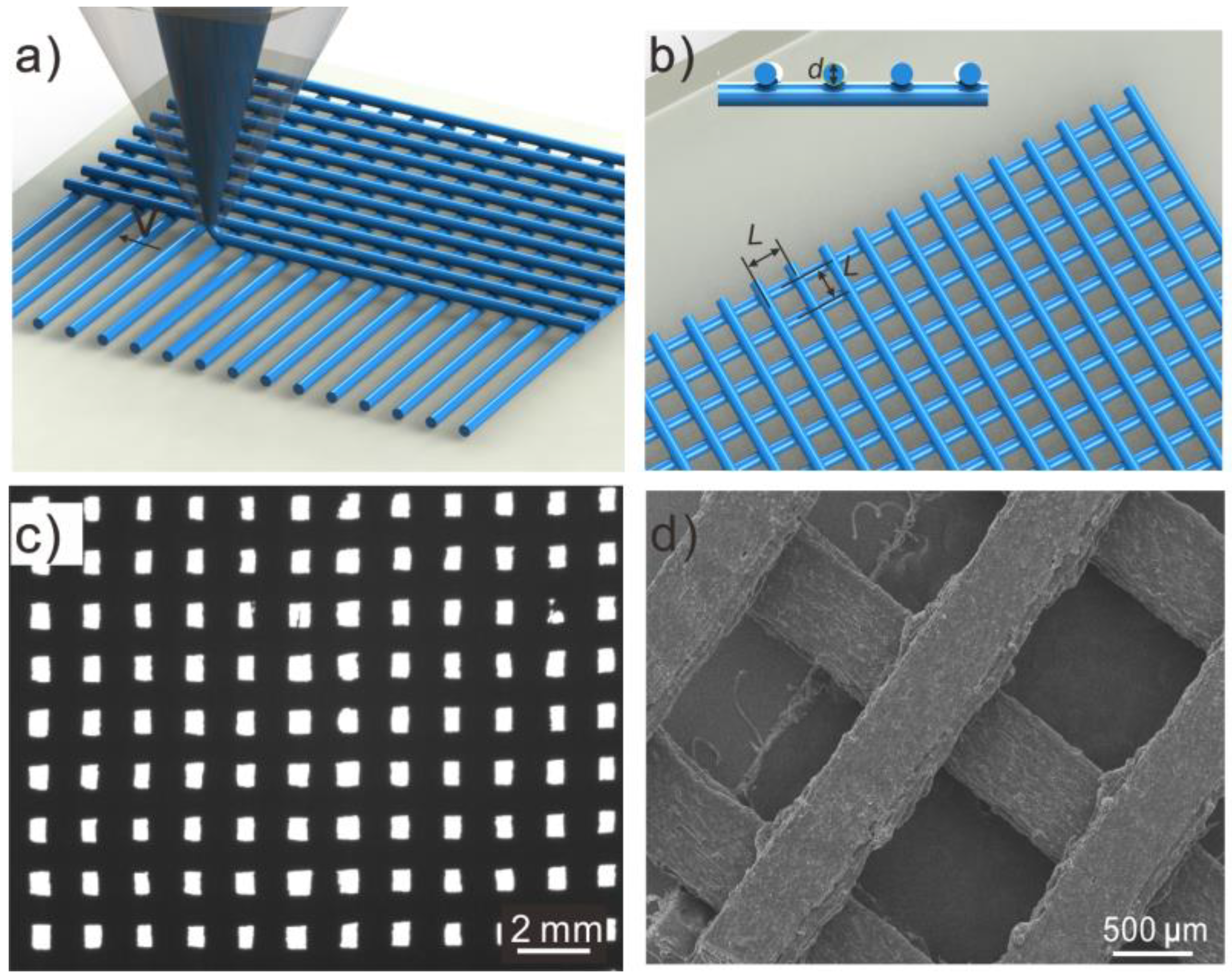
2.3. Printing Meshes
2.4. Fabrication of Salt-Containing S-USM
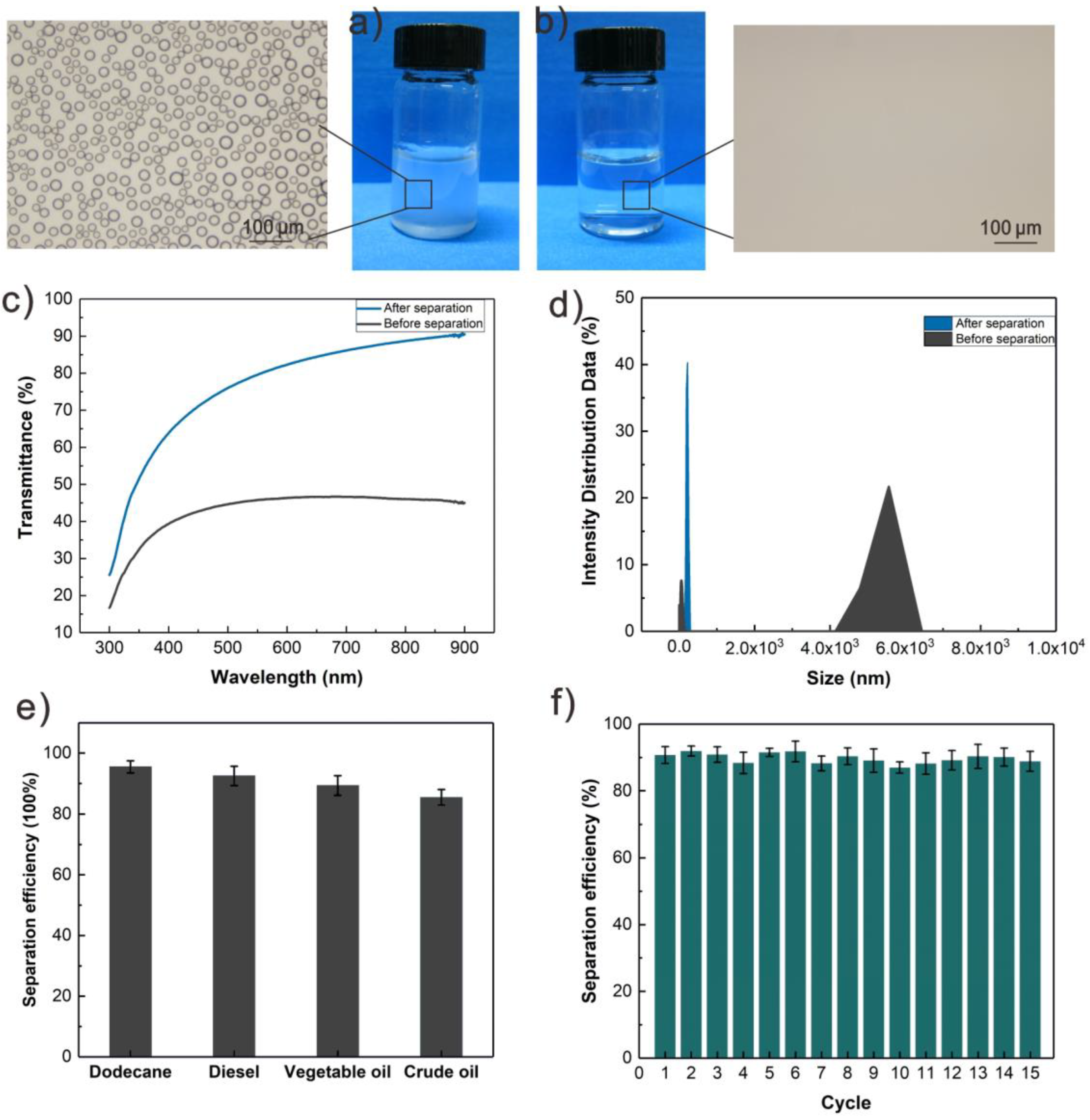
2.5. Separation Performance of Salt-Containing S-USM
2.6. Characterizations
3. Results and Discussion
3.1. Morphologies of 3D-Printed Mesh
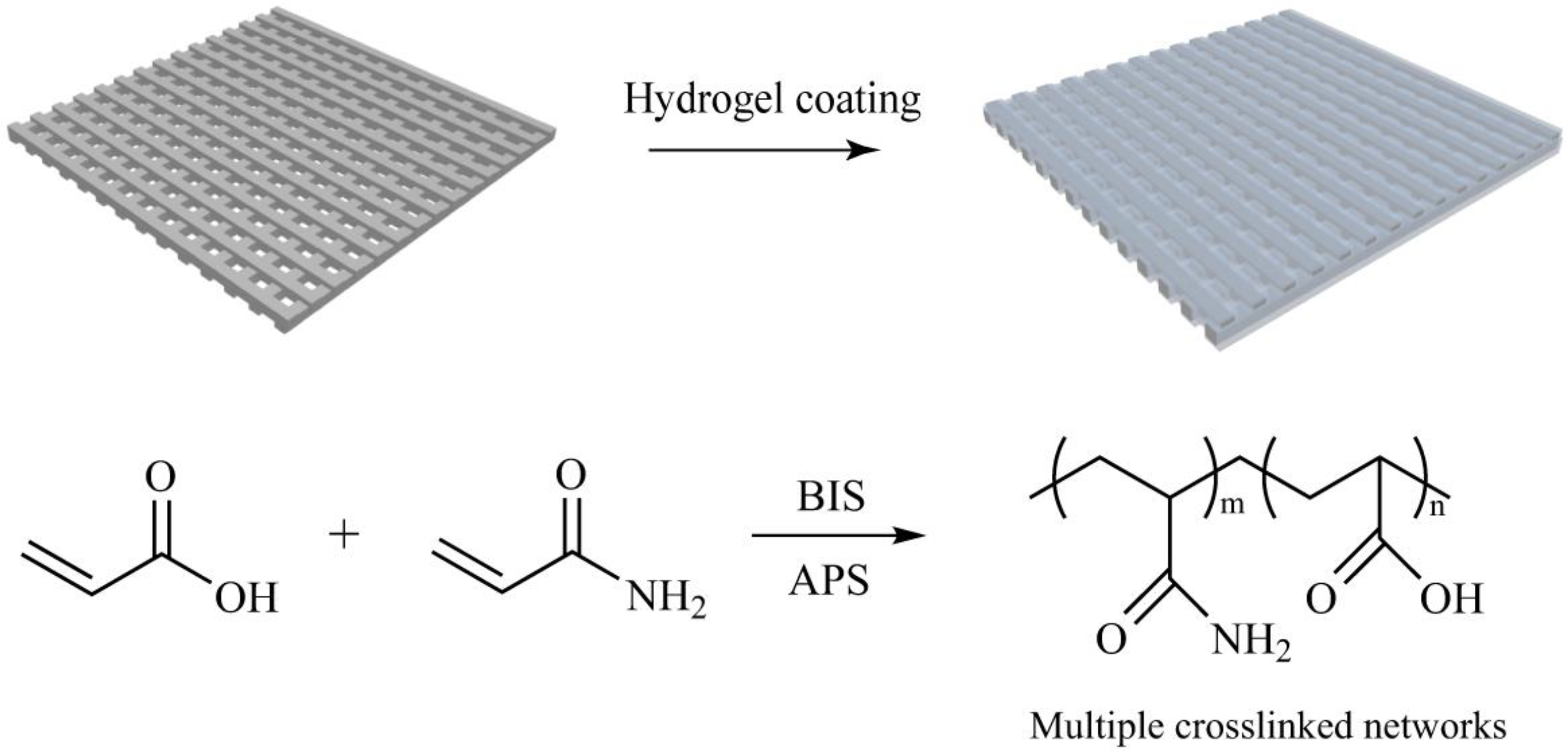
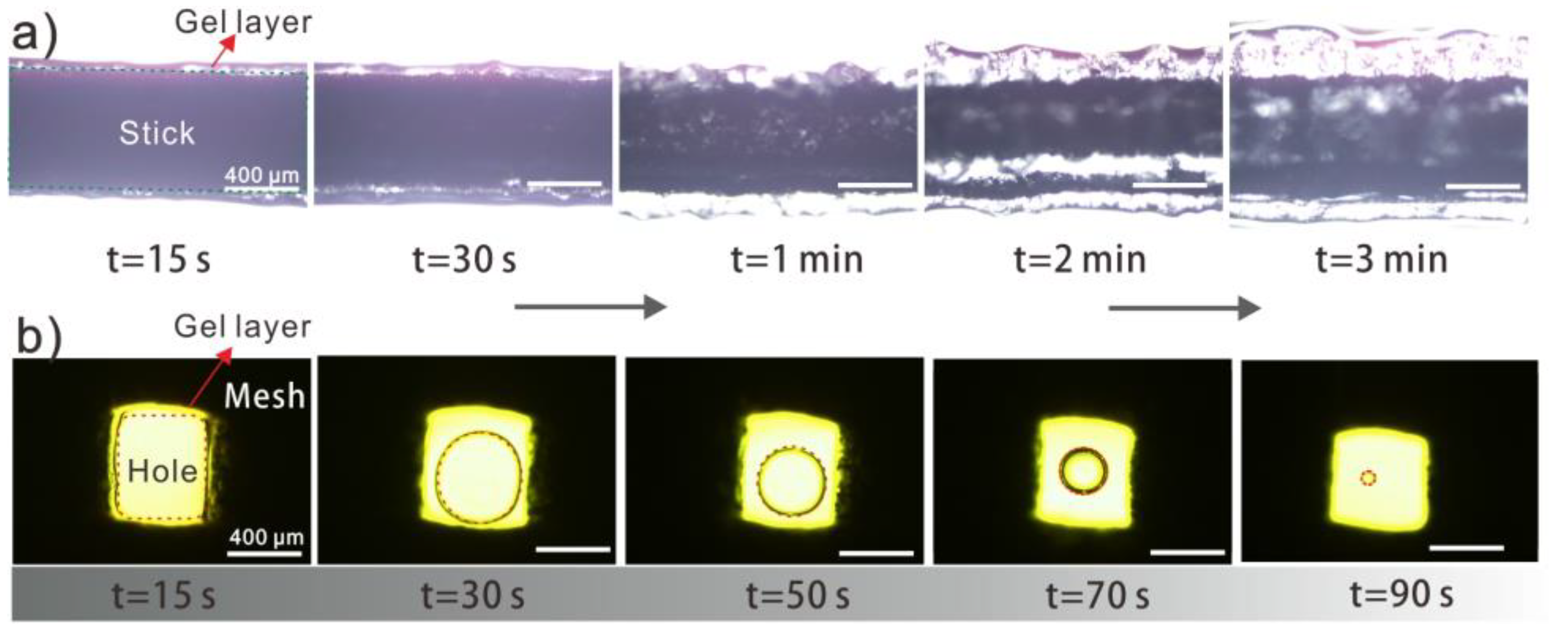
3.2. Fabrication of Hydrogel Coating
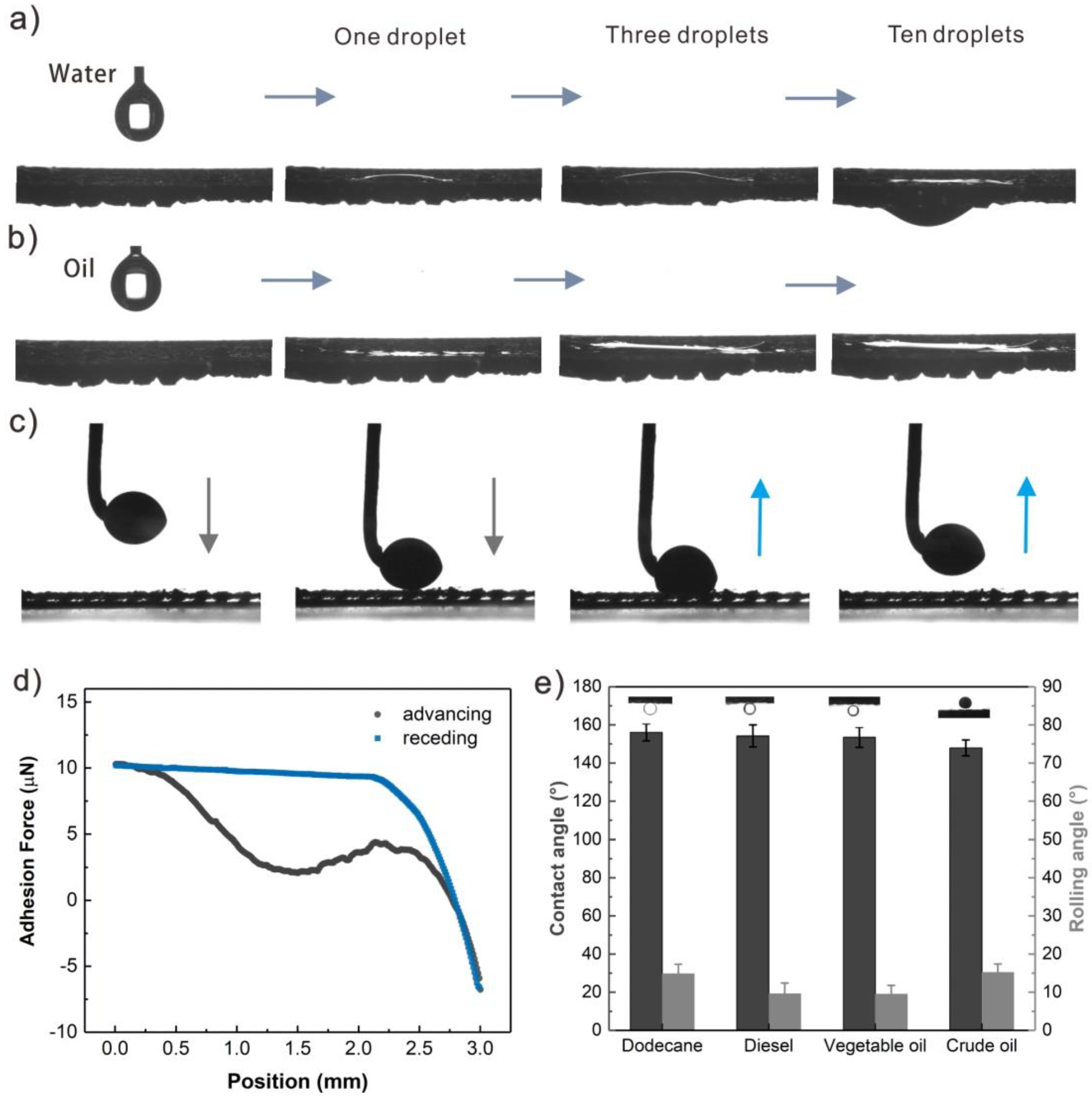
3.3. Wettability of S-USM
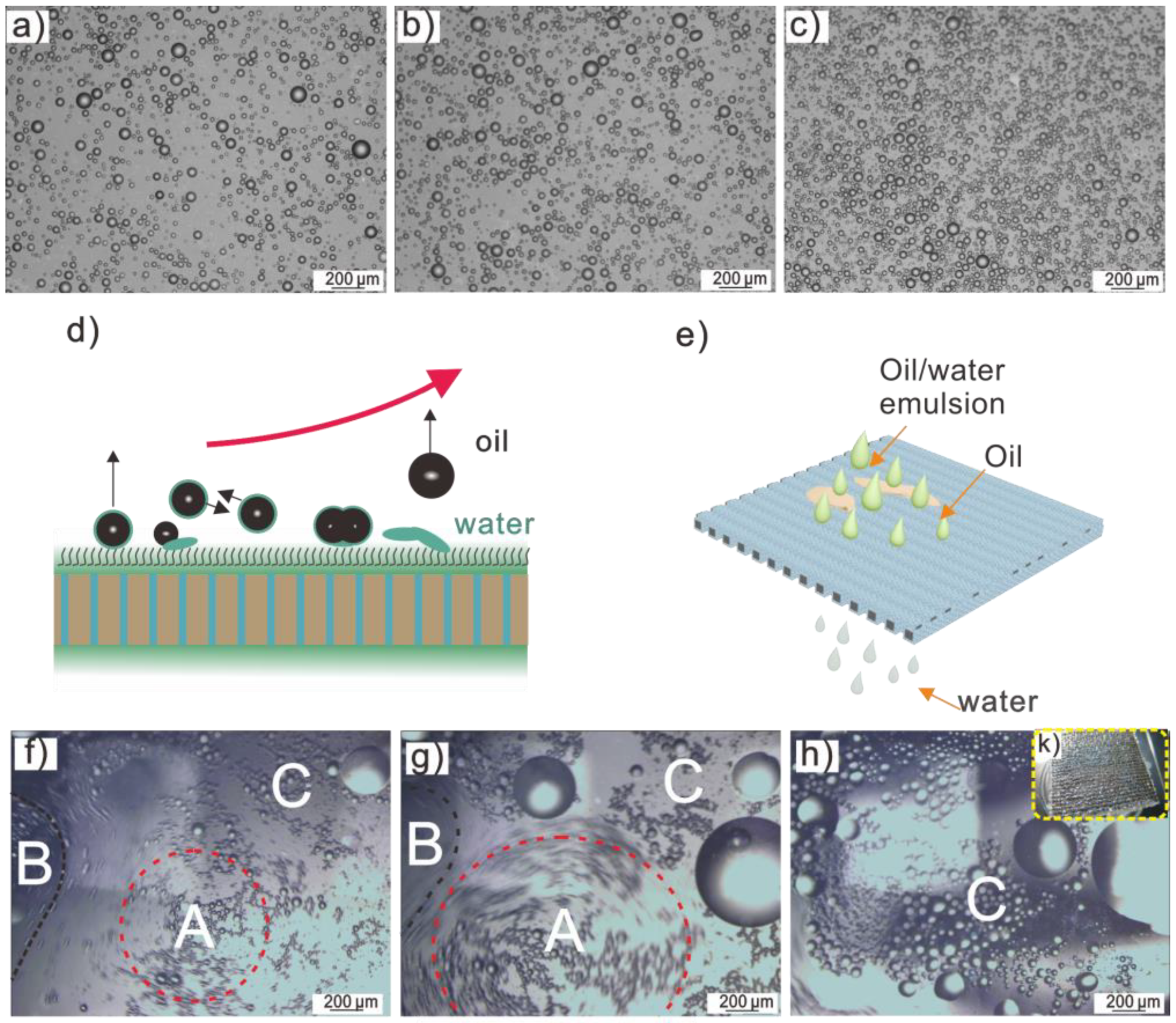
3.4. The Process of Oil/Water Mixture Separation Using Salt-Containing S-USM
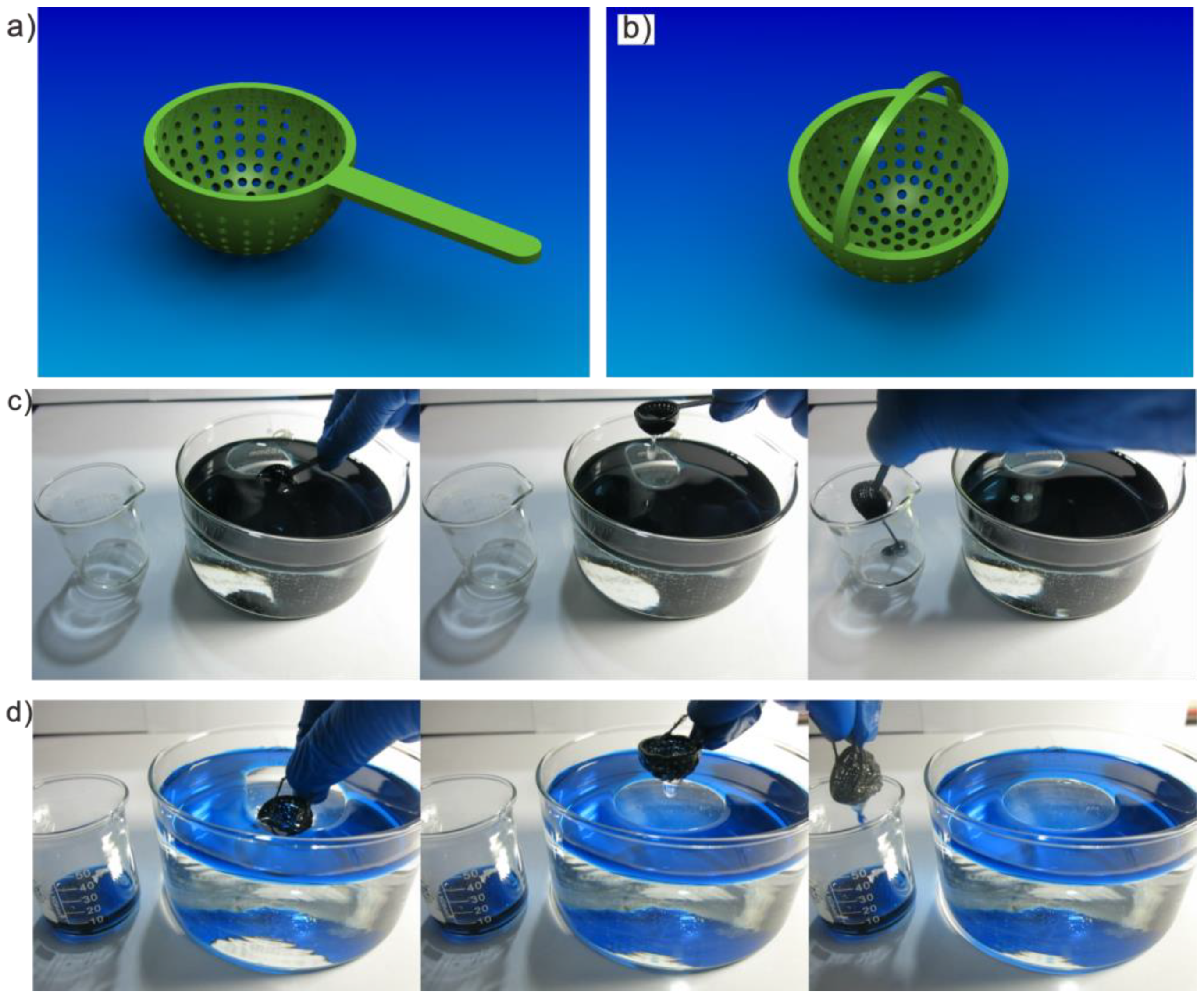
3.5. Special Oil Skimmers Made of S-USM
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Shi, Z.; Chen, X.; Wang, X.; Zhang, T.; Jin, J. Fabrication of Superstrong Ultrathin Free-Standing Single-Walled Carbon Nanotube Films via a Wet Process. Adv. Funct. Mater. 2011, 21, 4358–4363. [Google Scholar] [CrossRef]
- Chu, Z.; Feng, Y.; Seeger, S. Oil/water separation with selective superantiwetting/superwetting surface materials. Angew. Chem. 2015, 54, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, J.P.; Vadodariya, N.; Nataraj, S.K.; Meena, R. Chitosan-Based Aerogel Membrane for Robust Oil-in-Water Emulsion Separation. ACS Appl. Mater. Interfaces 2015, 7, 24957–24962. [Google Scholar] [CrossRef] [PubMed]
- Minas, C.; Carnelli, D.; Tervoort, E.; Studart, A.R. 3D Printing of Emulsions and Foams into Hierarchical Porous Ceramics. Adv. Mater. 2016, 28, 9993–9999. [Google Scholar] [CrossRef] [PubMed]
- Kwon, G.; Mabry, J.M.; Kota, A.K.; Choi, W.; Tuteja, A. Hygro-responsive membranes for effective oil–water separation. Nat. Commun. 2012, 3, 1025. [Google Scholar] [Green Version]
- Chen, P.-C.; Xu, Z.-K. Mineral-Coated Polymer Membranes with Superhydrophilicity and Underwater Superoleophobicity for Effective Oil/Water Separation. Sci. Rep. 2013, 3, 2776. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Li, X.; Zhang, W.; Fu, C.; Wang, Y.; Fu, S. Study on Demulsification-Flocculation Mechanism of Oil-Water Emulsion in Produced Water from Alkali/Surfactant/Polymer Flooding. Polymers 2019, 11, 395. [Google Scholar] [CrossRef]
- Wang, C.; Yao, T.; Wu, J.; Ma, C.; Fan, Z.; Wang, Z.; Cheng, Y.; Lin, Q.; Yang, B. Facile Approach in Fabricating Superhydrophobic and Superoleophilic Surface for Water and Oil Mixture Separation. ACS Appl. Mater. Interfaces 2009, 1, 2613–2617. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghoshal, A.; Purkait, M. Ultrafiltration of stable oil-in-water emulsion by polysulfone membrane. J. Membr. Sci. 2008, 325, 427–437. [Google Scholar] [CrossRef]
- Zhang, J.; Seeger, S. Polyester Materials with Superwetting Silicone Nanofilaments for Oil/Water Separation and Selective Oil Absorption. Adv. Funct. Mater. 2011, 21, 4699–4704. [Google Scholar] [CrossRef]
- Jin, M.; Wang, J.; Yao, X.; Liao, M.; Zhao, Y.; Jiang, L. Underwater Oil Capture by a Three-Dimensional Network Architectured Organosilane Surface. Adv. Mater. 2011, 23, 2861–2864. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Q.; Yang, W.; Xu, Y.; Zhang, M.; Deng, Q.; Liu, G. Facile Fabrication of Magnetic, Durable and Superhydrophobic Cotton for Efficient Oil/Water Separation. Polymers 2019, 11, 442. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, J.; Zhu, Y.; Wang, S.; Wang, X.; Lv, K. Novel Polymer Material for Efficiently Removing Methylene Blue, Cu(II) and Emulsified Oil Droplets from Water Simultaneously. Polymers 2018, 10, 1393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-P.; Yang, J.-H.; Li, L.-L.; Cui, C.-X.; Li, Y.; Liu, S.-Q.; Zhou, X.-M.; Qu, L.-B. Facile Fabrication of Superhydrophobic Copper-Foam and Electrospinning Polystyrene Fiber for Combinational Oil–Water Separation. Polymers 2019, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Bai, Q.; Li, C.; Lei, H.; Liu, C.; Shen, Y.; Uyama, H. Cost-Effective, Highly Selective and Environmentally Friendly Superhydrophobic Absorbent from Cigarette Filters for Oil Spillage Clean up. Polymers 2018, 10, 1101. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, W.B.; Shi, Z.; Wang, D.; Jin, J.; Jiang, L. Nanowire-Haired Inorganic Membranes with Superhydrophilicity and Underwater Ultralow Adhesive Superoleophobicity for High-Efficiency Oil/Water Separation. Adv. Mater. 2013, 25, 4192–4198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shi, Z.; Zhang, F.; Liu, X.; Jin, J.; Jiang, L. Superhydrophobic and Superoleophilic PVDF Membranes for Effective Separation of Water-in-Oil Emulsions with High Flux. Adv. Mater. 2013, 25, 2071–2076. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, X.; Peng, Y.; Min, X.; Yin, D.; Shao, L. MgAl-Layered-Double-Hydroxide/Sepiolite Composite Membrane for High-Performance Water Treatment Based on Layer-by-Layer Hierarchical Architectures. Polymers 2019, 11, 525. [Google Scholar] [CrossRef]
- Chen, W.; He, H.; Zhu, H.; Cheng, M.; Li, Y.; Wang, S. Thermo-Responsive Cellulose-Based Material with Switchable Wettability for Controllable Oil/Water Separation. Polymers 2018, 10, 592. [Google Scholar] [CrossRef] [PubMed]
- Striemer, C.C.; Gaborski, T.R.; McGrath, J.L.; Fauchet, P.M. Charge- and size-based separation of macromolecules using ultrathin silicon membranes. Nature 2007, 445, 749–753. [Google Scholar] [CrossRef]
- Wang, C.-F.; Tzeng, F.-S.; Chen, H.-G.; Chang, C.-J. Ultraviolet-Durable Superhydrophobic Zinc Oxide-Coated Mesh Films for Surface and Underwater–Oil Capture and Transportation. Langmuir 2012, 28, 10015–10019. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Huang, J.; Cui, Z.; Ge, M.; Zhang, K.Q.; Chen, Z.; Chi, L. Recent advances in TiO2-based nanostructured surfaces with controllable wettability and adhesion. Small 2016, 12, 2203–2224. [Google Scholar] [CrossRef]
- Zhu, M.; Xiong, R.; Huang, C. Bio-based and photocrosslinked electrospun antibacterial nanofibrous membranes for air filtration. Carbohydr. Polym. 2019, 205, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Zhang, M.; Liu, Z.; Kang, M.; Huang, C.; Fu, G. Fabrication of highly durable and robust superhydrophobic-superoleophilic nanofibrous membranes based on a fluorine-free system for efficient oil/water separation. J. Membr. Sci. 2019, 570–571, 303–313. [Google Scholar] [CrossRef]
- Ma, W.; Zhao, J.; Oderinde, O.; Han, J.; Liu, Z.; Gao, B.; Xiong, R.; Zhang, Q.; Jiang, S.; Huang, C. Durable superhydrophobic and superoleophilic electrospun nanofibrous membrane for oil-water emulsion separation. J. Colloid Interface Sci. 2018, 532, 12–23. [Google Scholar] [CrossRef]
- Ma, W.; Samal, S.K.; Liu, Z.; Xiong, R.; De Smedt, S.C.; Bhushan, B.; Zhang, Q.; Huang, C. Dual pH- and ammonia-vapor-responsive electrospun nanofibrous membranes for oil-water separations. J. Membr. Sci. 2017, 537, 128–139. [Google Scholar] [CrossRef]
- Xue, D.; Wang, Y.; Zhang, J.; Mei, D.; Wang, Y.; Chen, S. Projection-Based 3D Printing of Cell Patterning Scaffolds with Multiscale Channels. ACS Appl. Mater. Interfaces 2018, 10, 19428–19435. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, M.; Fu, Z.; Niu, K.; Yi, C.; Hua, S.; Teng, S.; Zhao, Q. Fabrication and characterization of electrospinning/3D printing bone tissue engineering scaffold. RSC Adv. 2016, 6, 110557–110565. [Google Scholar] [CrossRef]
- Scholze, M.; Singh, A.; Lozano, P.F.; Ondruschka, B.; Ramezani, M.; Werner, M.; Hammer, N. Utilization of 3D printing technology to facilitate and standardize soft tissue testing. Sci. Rep. 2018, 8, 11340. [Google Scholar] [CrossRef] [PubMed]
- Hardin, J.O.; Ober, T.J.; Valentine, A.D.; Lewis, J.A. Microfluidic Printheads for Multimaterial 3D Printing of Viscoelastic Inks. Adv. Mater. 2015, 27, 3279–3284. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Zhang, J.M.; Liu, Y.; Li, X.; Lv, P.; Jin, D.; Duan, H. A Modular Microfluidic Device via Multimaterial 3D Printing for Emulsion Generation. Sci. Rep. 2018, 8, 4791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, C.; Ji, Z.; Ma, S.; Wang, X.; Zhou, F. 3D Printing as Feasible Platform for On-Site Building Oil-Skimmer for Oil Collection from Spills. Adv. Mater. Interfaces 2016, 3, 1600015. [Google Scholar] [CrossRef]
- Wang, X.; Cai, X.; Guo, Q.; Zhang, T.; Kobe, B.; Yang, J. i3DP, a robust 3D printing approach enabling genetic post-printing surface modification. Chem. Commun. 2013, 49, 10064. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Zheng, X.; Chen, Z.; Zhou, Q.; Chen, Y. 3d-printed biomimetic super-hydrophobic structure for microdroplet manipulation and oil/water separation. Adv. Mater. 2018, 30, 1704912. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Günter, C.; Taubert, A. Co-Deposition of a Hydrogel/Calcium Phosphate Hybrid Layer on 3D Printed Poly(Lactic Acid) Scaffolds via Dip Coating: Towards Automated Biomaterials Fabrication. Polymers 2018, 10, 275. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Irvine, S.A.; Agrawal, A.; Cao, Y.; Lim, P.Q.; Tan, S.Y.; Venkatraman, S.S.; Ying, T.S.; Venkatraman, S. 3D patterned substrates for bioartificial blood vessels—The effect of hydrogels on aligned cells on a biomaterial surface. Acta Biomater. 2015, 26, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Raman, R.; Clay, N.E.; Sen, S.; Melhem, M.; Qin, E.; Kong, H.; Bashir, R. 3D printing enables separation of orthogonal functions within a hydrogel particle. Biomed. Microdevices 2016, 18, 49. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Wang, S.; Lin, L.; Chen, L.; Liu, M.; Feng, L.; Jiang, L. A Novel Superhydrophilic and Underwater Superoleophobic Hydrogel-Coated Mesh for Oil/Water Separation. Adv. Mater. 2011, 23, 4270–4273. [Google Scholar] [CrossRef]
- Fan, J.-B.; Song, Y.; Wang, S.; Meng, J.; Yang, G.; Guo, X.; Feng, L.; Jiang, L. Directly Coating Hydrogel on Filter Paper for Effective Oil-Water Separation in Highly Acidic, Alkaline, and Salty Environment. Adv. Funct. Mater. 2015, 25, 5368–5375. [Google Scholar] [CrossRef]
- Wong, S.; Teng, T.-T.; Ahmad, A.L.; Zuhairi, A.; Najafpour, G. Treatment of pulp and paper mill wastewater by polyacrylamide (PAM) in polymer induced flocculation. J. Hazard. Mater. 2006, 135, 378–388. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, C.; Zhang, J.; Pu, W. Feasibility investigation of oily wastewater treatment by combination of zinc and PAM in coagulation/flocculation. J. Hazard. Mater. 2007, 147, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Yakovchenko, M.; Pinchuk, L.G.; Kolosova, M.M.; Filipovich, L.A.; Masaev, V.Y. Intensification of Wastewater Treatment Processes during Coal Enrichment Using Modified Polyacrylamide Flocculants. IOP Conf. Ser. Earth Environ. Sci. 2019, 224, 012051. [Google Scholar] [CrossRef]
- Ma, S.; Yan, C.; Cai, M.; Yang, J.; Wang, X.; Zhou, F.; Liu, W. Continuous Surface Polymerization via Fe(II)-Mediated Redox Reaction for Thick Hydrogel Coatings on Versatile Substrates. Adv. Mater. 2018, 30, 1803371. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Rong, M.; Lin, P.; Bao, M.; Xie, J.; Wang, X.; Huck, W.T.S.; Zhou, F.; Liu, W. Fabrication of 3D Tubular Hydrogel Materials through On-Site Surface Free Radical Polymerization. Chem. Mater. 2018, 30, 6756–6768. [Google Scholar] [CrossRef]
- Yuan, S.; Tong, M.; Wu, G. Destabilization of emulsions by natural minerals. J. Hazard. Mater. 2011, 192, 1882–1885. [Google Scholar] [CrossRef]
- Tong, K.; Zhang, Y.; Chu, P.K. Evaluation of calcium chloride for synergistic demulsification of super heavy oil wastewater. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 419, 46–52. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, C.; Ma, S.; Ji, Z.; Guo, Y.; Liu, Z.; Zhang, X.; Wang, X. 3D Printing of an Oil/Water Mixture Separator with In Situ Demulsification and Separation. Polymers 2019, 11, 774. https://doi.org/10.3390/polym11050774
Yan C, Ma S, Ji Z, Guo Y, Liu Z, Zhang X, Wang X. 3D Printing of an Oil/Water Mixture Separator with In Situ Demulsification and Separation. Polymers. 2019; 11(5):774. https://doi.org/10.3390/polym11050774
Chicago/Turabian StyleYan, Changyou, Shuanhong Ma, Zhongying Ji, Yuxiong Guo, Zhilu Liu, Xiaoqin Zhang, and Xiaolong Wang. 2019. "3D Printing of an Oil/Water Mixture Separator with In Situ Demulsification and Separation" Polymers 11, no. 5: 774. https://doi.org/10.3390/polym11050774




