Facile Fabrication of Environmentally-Friendly Hydroxyl-Functionalized Multiwalled Carbon Nanotubes/Soy Oil-Based Polyurethane Nanocomposite Bioplastics with Enhanced Mechanical, Thermal, and Electrical Conductivity Properties
Abstract
1. Introduction
2. Experimental
2.1. Materials
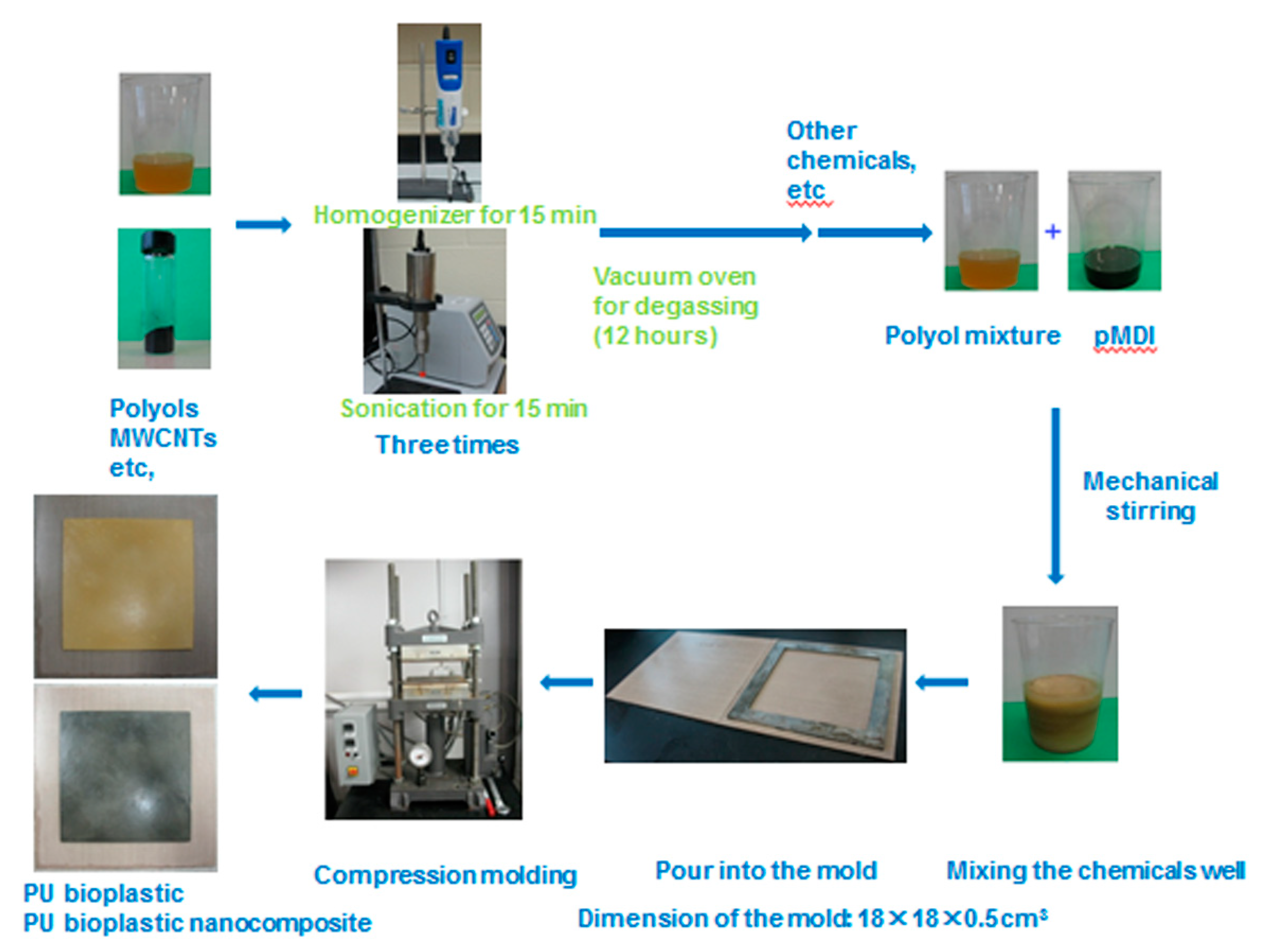
2.2. Procedure for Nanocomposites Fabrication
2.3. Characterization
3. Results and Discussion
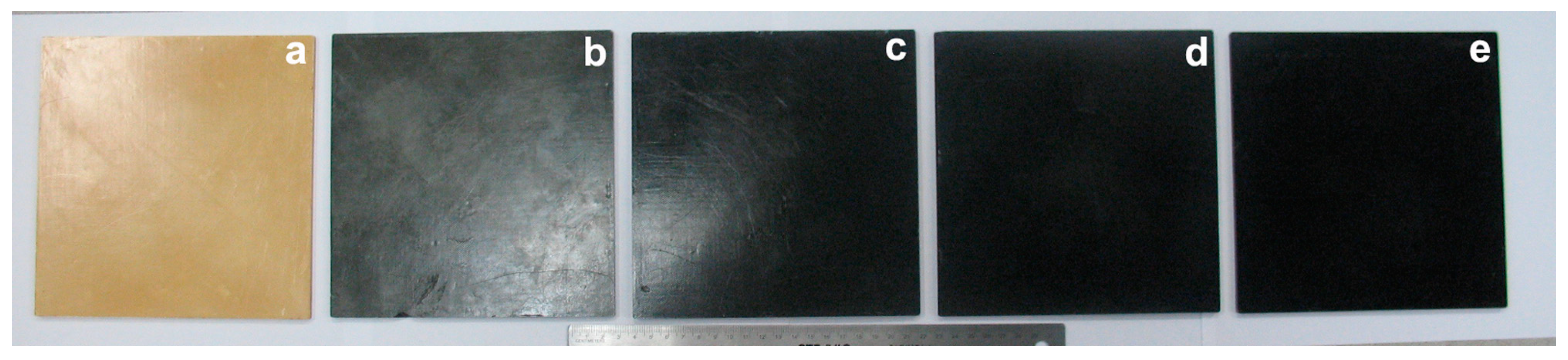
3.1. Photographs of the Nanocomposite Bioplastics
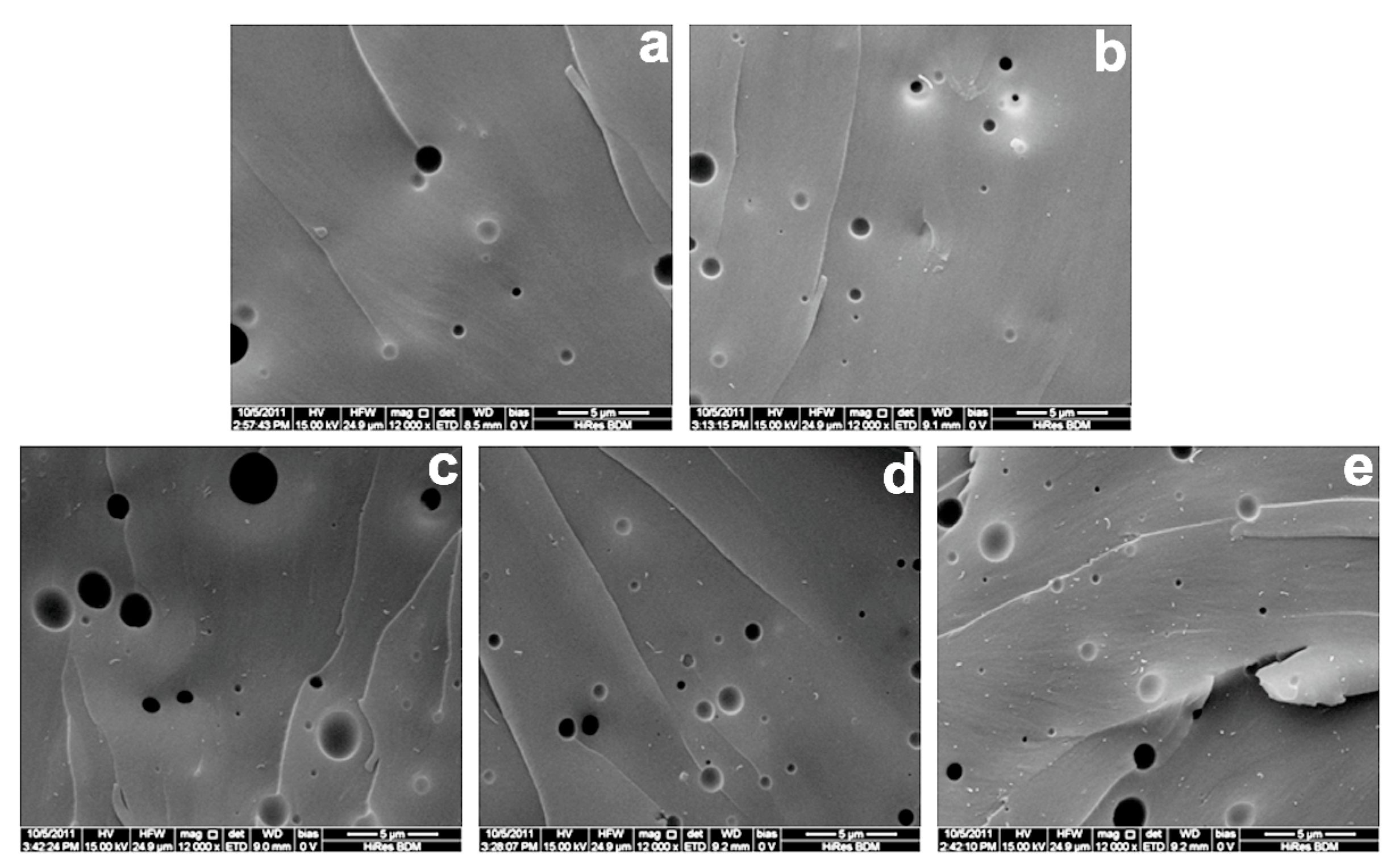
3.2. Dispersion of Hydroxyl-Functionalized Multiwalled Carbon Nanotubes in the Nanocomposite Bioplastics
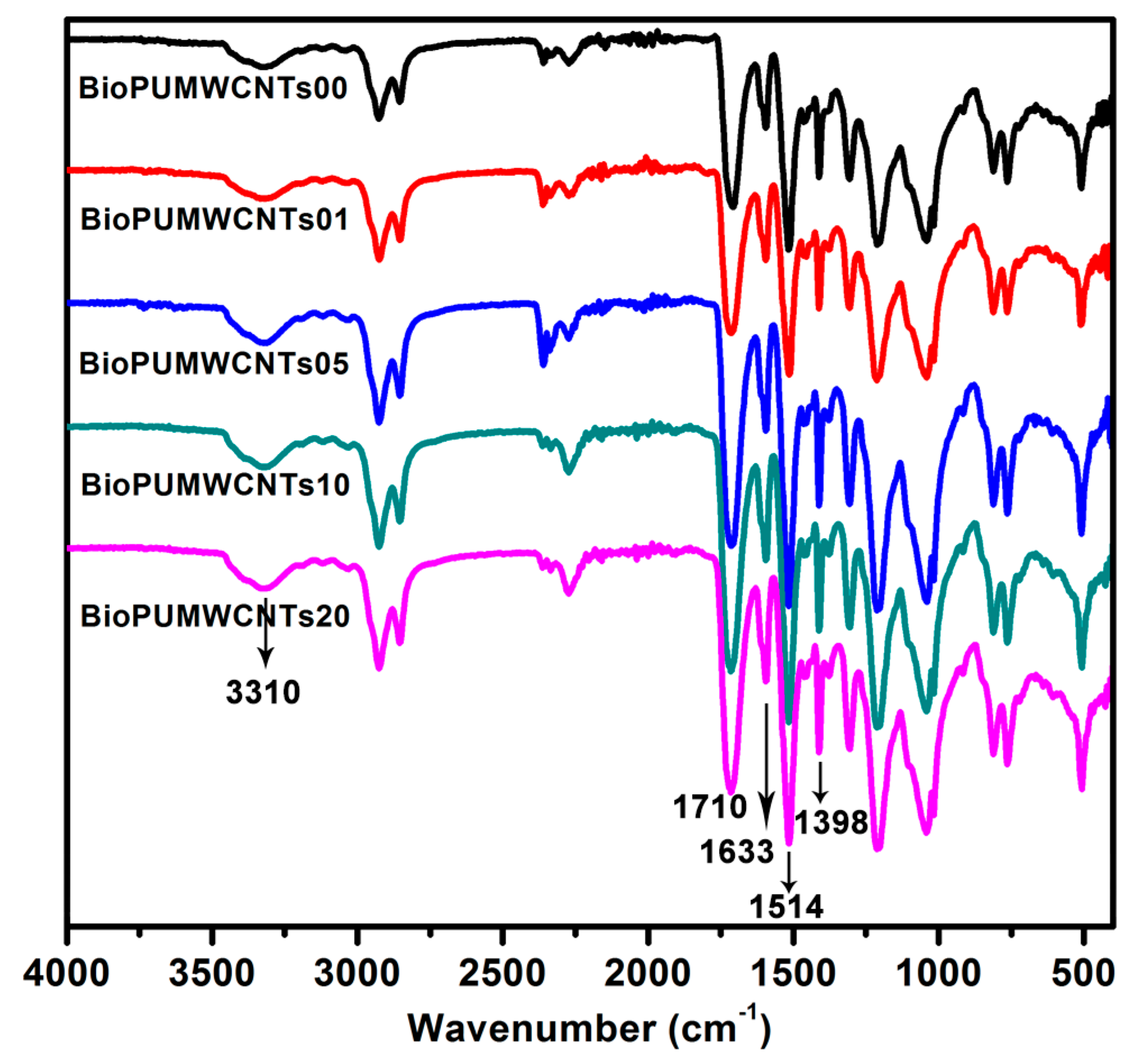
3.3. Fourier Transform Infrared of the Nanocomposite Bioplastics
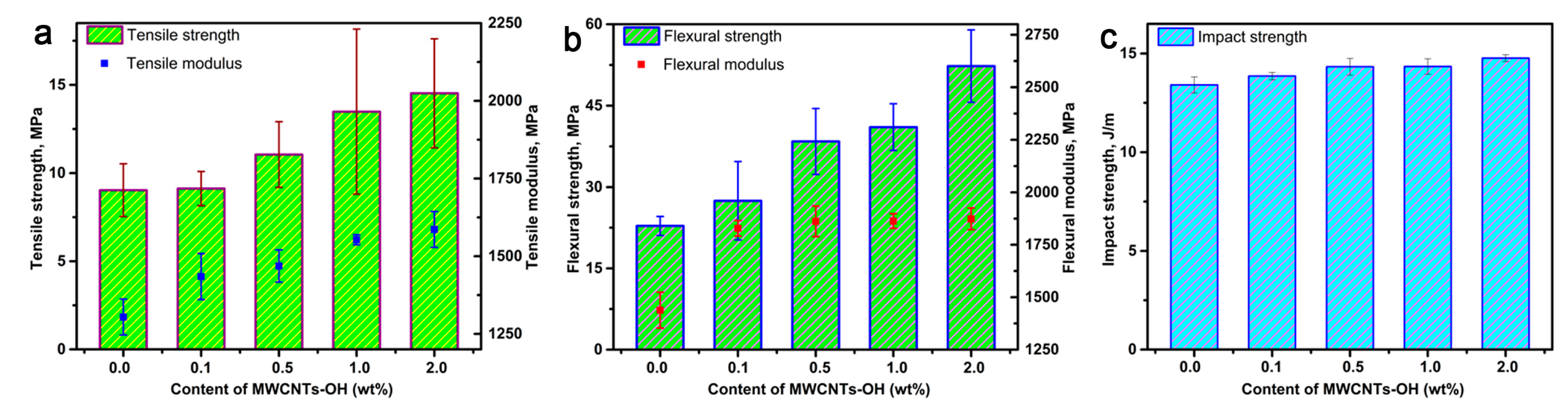
3.4. Mechanical Properties of Nanocomposite Bioplastics
3.5. Dynamic Mechanical Analysis of the Nanocomposite Bioplastics
3.6. Thermogravimetric Analysis of the Nanocomposite Bioplastics
3.7. DC Volume Resistivity of the Nanocomposite Bioplastics
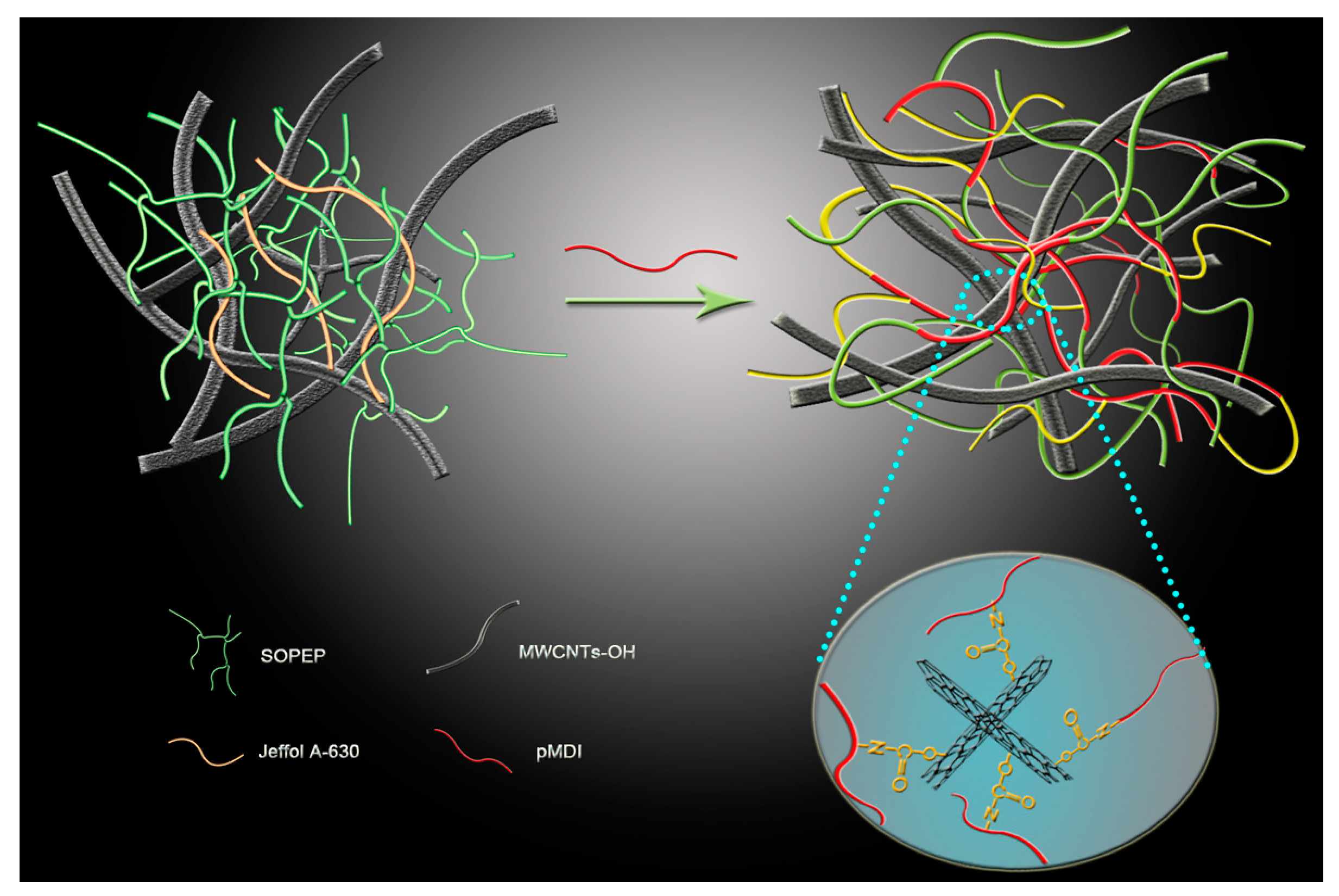
3.8. Schematic Diagram of the Three-Dimensioonal Macromolecular Network Structure Formation of the Nanocomposite Bioplastic
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, Y.; Luo, X.; Hu, S. Polyols and Polyurethanes from Vegetable Oils and Their Derivatives. In Bio-Based Polyols and Polyurethanes; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 15–43. [Google Scholar]
- Iwata, T. Biodegradable and Bio-Based Polymers: Future Prospects of Eco-Friendly Plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef] [PubMed]
- Desroches, M.; Escouvois, M.; Auvergne, R.; Caillol, S.; Boutevin, B. From vegetable oils to polyurethanes: Synthetic routes to polyols and main industrial products. Polym. Rev. 2012, 52, 38–79. [Google Scholar] [CrossRef]
- Raquez, J.M.; Deléglise, M.; Lacrampe, M.F.; Krawczak, P. Thermosetting (bio)materials derived from renewable resources: A critical review. Prog. Polym. Sci. 2010, 35, 487–509. [Google Scholar] [CrossRef]
- Dwan’Isa, J.P.L.; Mohanty, A.K.; Misra, M.; Drzal, L.T.; Kazemizadeh, M. Novel biobased polyurethanes synthesized from soybean phosphate ester polyols: Thermomechanical properties Evaluations. J. Polym. Environ. 2003, 11, 161–168. [Google Scholar] [CrossRef]
- Kairytė, A.; Vaitkus, S.; Vėjelis, S.; Girskas, G.; Balčiūnas, G. Rapeseed-based polyols and paper production waste sludge in polyurethane foam: Physical properties and their prediction models. Ind. Crop. Prod. 2018, 112, 119–129. [Google Scholar] [CrossRef]
- Sun, X.; Sun, H.; Li, H.; Peng, H. Developing Polymer Composite Materials: Carbon Nanotubes or Graphene? Adv. Mater. 2013, 25, 5153–5176. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Rubi, Y.; Ashrafi, B.; Jakubinek, M.B.; Zou, S.; Laqua, K.; Barnes, M.; Simard, B. Fabrication of High Content Carbon Nanotube–Polyurethane Sheets with Tailorable Properties. ACS Appl. Mater. Interfaces 2017, 9, 30840–30849. [Google Scholar] [CrossRef] [PubMed]
- Basirjafari, S. Effects of CNT loading on cellular structures and sound absorption of PU foams. Micro Nano Lett. 2018, 13, 1501–1505. [Google Scholar] [CrossRef]
- Caglayan, C.; Gurkan, I.; Gungor, S.; Cebeci, H. The effect of CNT-reinforced polyurethane foam cores to flexural properties of sandwich composites. Compos. Part A Appl. Sci. Manuf. 2018, 115, 187–195. [Google Scholar] [CrossRef]
- Tran, L.; Kim, J. A Comparative Study of the Thermoplastic Polyurethane/Carbon Nanotube and Natural Rubber/Carbon Nanotube Composites According to Their Mechanical and Electrical Properties. Fibers Polym. 2018, 19, 1948–1955. [Google Scholar] [CrossRef]
- Bauhofer, W.; Kovacs, J.Z. A review and analysis of electrical percolation in carbon nanotube polymer composites. Compos. Sci. Technol. 2009, 69, 1486–1498. [Google Scholar] [CrossRef]
- Luo, X.; Mohanty, A.; Misra, M. Lignin as a reactive reinforcing filler for water-blown rigid biofoam composites from soy oil-based polyurethane. Ind. Crop. Prod. 2013, 47, 13–19. [Google Scholar] [CrossRef]
- Luo, X.; Cai, Y.; Liu, L.; Zhang, F.; Wu, Q.; Zeng, J. Soy Oil-Based Rigid Polyurethane Biofoams Obtained by a Facile One-Pot Process and Reinforced with Hydroxyl-Functionalized Multiwalled Carbon Nanotube. J. Am. Oil Chem. Soc. 2019. [Google Scholar] [CrossRef]
- Luo, X.; Xiao, Y.; Wu, Q.; Zeng, J. Development of high-performance biodegradable rigid polyurethane foams using all bioresource-based polyols: Lignin and soy oil-derived polyols. Int. J. Biol. Macromol. 2018, 115, 786–791. [Google Scholar] [CrossRef]
- Liu, T.; Phang, I.Y.; Shen, L.; Chow, S.Y.; Zhang, W.-D. Morphology and Mechanical Properties of Multiwalled Carbon Nanotubes Reinforced Nylon-6 Composites. Macromolecules 2004, 37, 7214–7222. [Google Scholar] [CrossRef]
- Zhu, J.; Peng, H.; Rodriguez-Macias, F.; Margrave, J.L.; Khabashesku, V.N.; Imam, A.M.; Lozano, K.; Barrera, E.V. Reinforcing epoxy polymer composites through covalent integration of functionalized nanotubes. Adv. Funct. Mater. 2004, 14, 643–648. [Google Scholar] [CrossRef]
- Zhang, F.; Luo, X. Systematic study on substituting petroleum-based polyols with soy-based polyol for developing renewable hybrid biofoam by self-catalyzing/rising process. Ind. Crop. Prod. 2015, 77, 175–179. [Google Scholar] [CrossRef]
- Luo, X.; Mohanty, A.; Misra, M. Green Composites from Soy-Based Biopolyurethane with Microcrystalline Cellulose. Macromol. Mater. Eng. 2013, 298, 412–418. [Google Scholar] [CrossRef]
- Hayashida, K.; Matsuoka, Y. Electromagnetic interference shielding properties of polymer-grafted carbon nanotube composites with high electrical resistance. Carbon 2015, 85, 363–371. [Google Scholar] [CrossRef]
- Szycher, M. Handbook of Polyurethanes; CardioTech International Inc.: Woburn, MA, USA, 1999. [Google Scholar]
- Bian, X.-C.; Tang, J.-H.; Li, Z.-M.; Lu, Z.-Y.; Lu, A. Dependence of flame-retardant properties on density of expandable graphite filled rigid polyurethane foam. J. Appl. Polym. Sci. 2007, 104, 3347–3355. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef]
- Xie, X.-L.; Mai, Y.-W.; Zhou, X.-P. Dispersion and alignment of carbon nanotubes in polymer matrix: A review. Mater. Sci. Eng. R Rep. 2005, 49, 89–112. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Ghosh, A.; Sawai, P.; Pareek, K.; Banerjee, S.; Das, A.; Pötschke, P.; Heinrich, G.; Voit, B. Electromagnetic interference shielding effectiveness of MWCNT filled poly(ether sulfone) and poly(ether imide) nanocomposites. Polym. Eng. Sci. 2014, 54, 2560–2570. [Google Scholar] [CrossRef]
- Raja, M.; Ryu, S.H.; Shanmugharaj, A.M. Thermal, mechanical and electroactive shape memory properties of polyurethane (PU)/poly (lactic acid) (PLA)/CNT nanocomposites. Eur. Polym. J. 2013, 49, 3492–3500. [Google Scholar] [CrossRef]
- Rahimpour, A.; Jahanshahi, M.; Khalili, S.; Mollahosseini, A.; Zirepour, A.; Rajaeian, B. Novel functionalized carbon nanotubes for improving the surface properties and performance of polyethersulfone (PES) membrane. Desalination 2012, 286, 99–107. [Google Scholar] [CrossRef]
- Li, S.; Du, X.; Hou, C.; Hao, X.; Jia, J.; Guan, T.; Yi, T.; Ma, G. One-pot two-step perfluoroalkylsilane functionalization of multi-walled carbon nanotubes for polyurethane-based composites. Compos. Sci. Technol. 2017, 143, 46–55. [Google Scholar] [CrossRef]
- Wu, Q.; Henriksson, M.; Liu, X.; Berglund, L.A. A high strength nanocomposite based on microcrystalline cellulose and polyurethane. Biomacromolecules 2007, 8, 3687–3692. [Google Scholar] [CrossRef]
- Coleman, J.N.; Khan, U.; Gun’ko, Y.K. Mechanical reinforcement of polymers using carbon nanotubes. Adv. Mater. 2006, 18, 689–706. [Google Scholar] [CrossRef]
- Yeung, P.; Broutman, L.J. 32nd Annual Technical Conference, Rein forced Plastics Division; The Society of the Plastics Industry: New York, NY, USA, 1977. [Google Scholar]
- Latere Dwan’isa, J.P.; Mohanty, A.K.; Misra, M.; Drzal, L.T.; Kazemizadeh, M. Biobased polyurethane and its composite with glass fiber. J. Mater. Sci. 2004, 39, 2081–2087. [Google Scholar] [CrossRef]
- Wirpsza, Z.; Kemp, T.J.; Skup, A. Polyurethanes: Chemistry, Technology and Applications; Ellis Horwood: Hemel Hempstead, UK, 1993; Volume 575. [Google Scholar]
- Yang, W.P.; Macosko, C.W.; Wellinghoff, S.T. Thermal degradation of urethanes based on 4,4′-diphenylmethane diisocyanate and 1,4-butanediol (MDI/BDO). Polymer 1986, 27, 1235–1240. [Google Scholar] [CrossRef]
- Guo, A.; Javni, I.; Petrovic, Z. Rigid polyurethane foams based on soybean oil. J. Appl. Polym. Sci. 2000, 77, 467–473. [Google Scholar] [CrossRef]
- Gu, R.; Konar, S.; Sain, M. Preparation and Characterization of Sustainable Polyurethane Foams from Soybean Oils. J. Am. Oil Chem. Soc. 2012, 89, 2103–2111. [Google Scholar] [CrossRef]
- Xia, H.; Song, M. Preparation and characterization of polyurethane–carbon nanotube composites. Soft Matter 2005, 1, 386–394. [Google Scholar] [CrossRef]
- Nayak, S.; Sahoo, B.; Kumar Chaki, T.; Khastgir, D. Development of polyurethane-titania nanocomposites as dielectric and piezoelectric material. RSC Adv. 2013, 3, 2620–2631. [Google Scholar] [CrossRef]
- Yamamoto, N.; de Villoria, R.G.; Wardle, B.L. Electrical and thermal property enhancement of fiber-reinforced polymer laminate composites through controlled implementation of multi-walled carbon nanotubes. Compos. Sci. Technol. 2012, 72, 2009–2015. [Google Scholar] [CrossRef]
- Nayak, S.; Chaki, T.K.; Khastgir, D. Development of Flexible Piezoelectric Poly(dimethylsiloxane)–BaTiO3 Nanocomposites for Electrical Energy Harvesting. Ind. Eng. Chem. Res. 2014, 53, 14982–14992. [Google Scholar] [CrossRef]
- Hayashida, K.; Tanaka, H. Ultrahigh Electrical Resistance of Poly(cyclohexyl methacrylate)/Carbon Nanotube Composites Prepared Using Surface-Initiated Polymerization. Adv. Funct. Mater. 2012, 22, 2338–2344. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Yu, Z.; Cai, Y.; Wu, Q.; Zeng, J. Facile Fabrication of Environmentally-Friendly Hydroxyl-Functionalized Multiwalled Carbon Nanotubes/Soy Oil-Based Polyurethane Nanocomposite Bioplastics with Enhanced Mechanical, Thermal, and Electrical Conductivity Properties. Polymers 2019, 11, 763. https://doi.org/10.3390/polym11050763
Luo X, Yu Z, Cai Y, Wu Q, Zeng J. Facile Fabrication of Environmentally-Friendly Hydroxyl-Functionalized Multiwalled Carbon Nanotubes/Soy Oil-Based Polyurethane Nanocomposite Bioplastics with Enhanced Mechanical, Thermal, and Electrical Conductivity Properties. Polymers. 2019; 11(5):763. https://doi.org/10.3390/polym11050763
Chicago/Turabian StyleLuo, Xiaogang, Zengcheng Yu, Yixin Cai, Qiangxian Wu, and Jian Zeng. 2019. "Facile Fabrication of Environmentally-Friendly Hydroxyl-Functionalized Multiwalled Carbon Nanotubes/Soy Oil-Based Polyurethane Nanocomposite Bioplastics with Enhanced Mechanical, Thermal, and Electrical Conductivity Properties" Polymers 11, no. 5: 763. https://doi.org/10.3390/polym11050763
APA StyleLuo, X., Yu, Z., Cai, Y., Wu, Q., & Zeng, J. (2019). Facile Fabrication of Environmentally-Friendly Hydroxyl-Functionalized Multiwalled Carbon Nanotubes/Soy Oil-Based Polyurethane Nanocomposite Bioplastics with Enhanced Mechanical, Thermal, and Electrical Conductivity Properties. Polymers, 11(5), 763. https://doi.org/10.3390/polym11050763




