Degradable Polymer Stars Based on Tannic Acid Cores by ATRP
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Material Characterization
2.3. Instrumentation
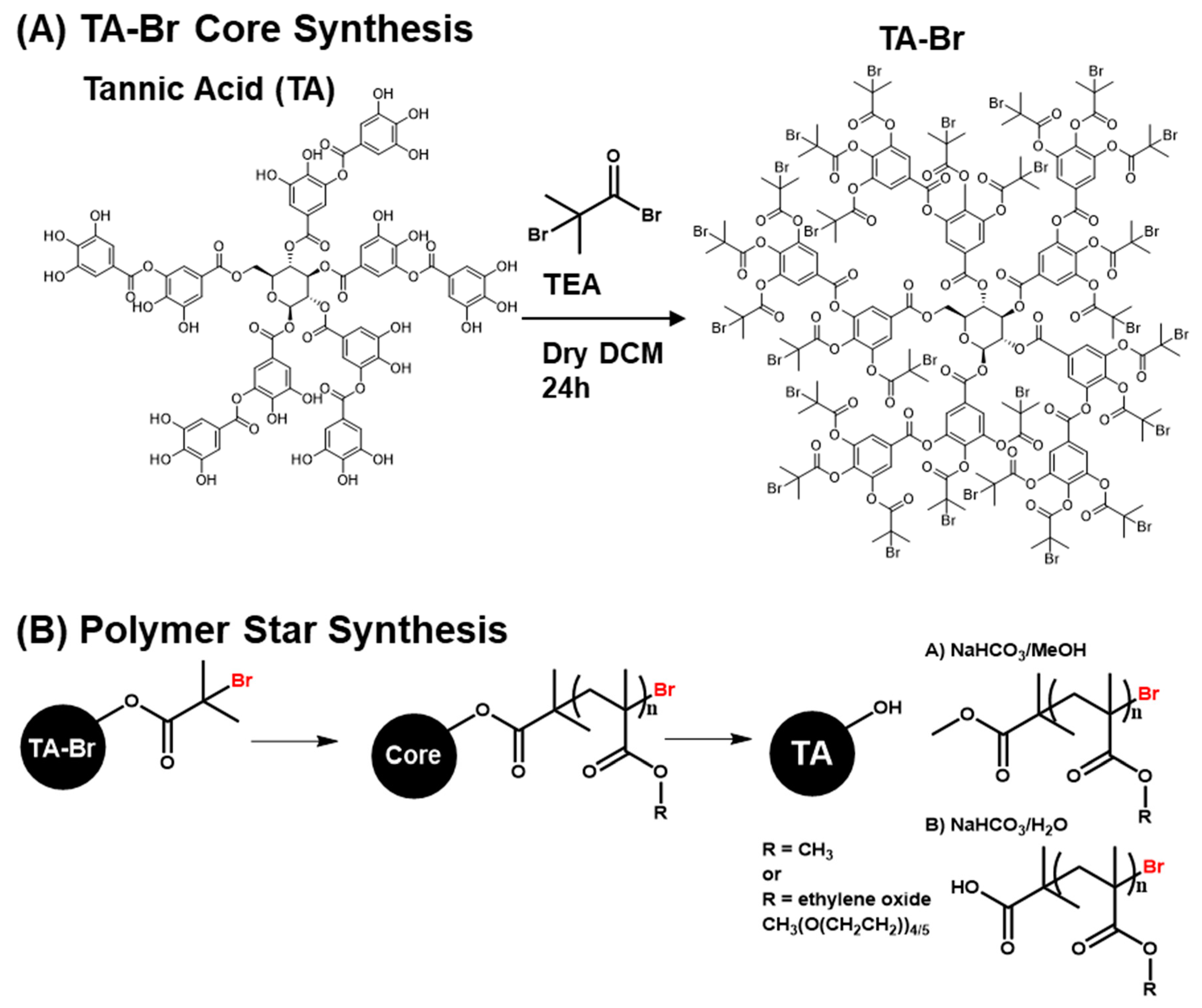
2.4. TA-Br Synthesis
2.5. General Polymer Star Synthesis
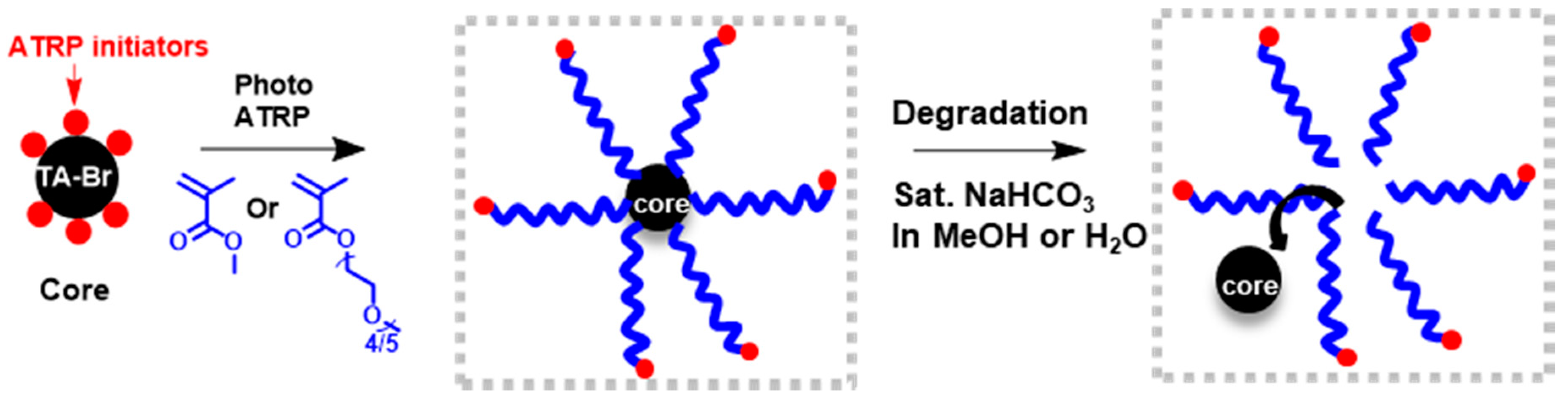
2.6. Degradation Procedure
2.7. Cell Culture
2.8. Cytotoxicity Assay
3. Results and Discussion
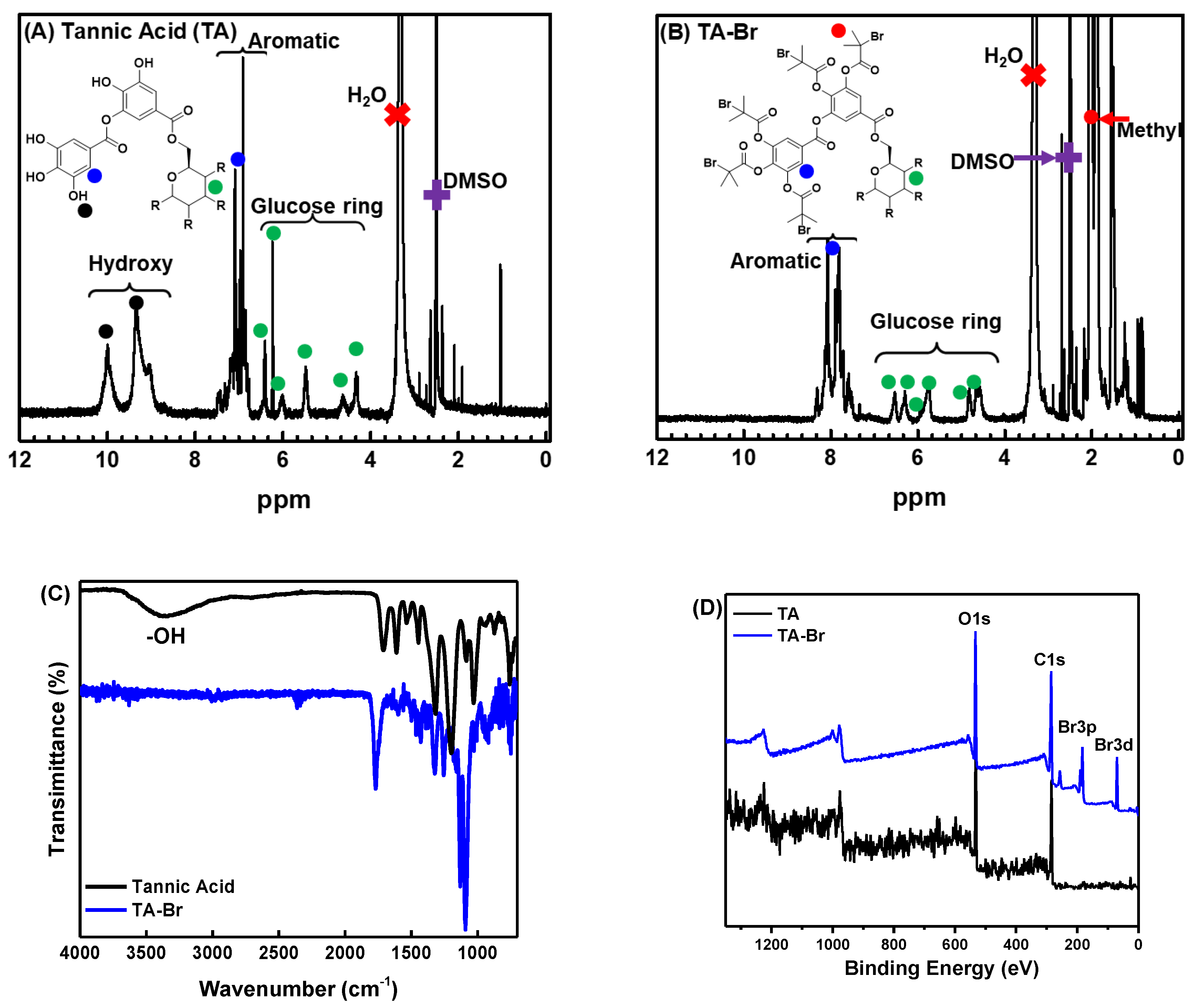
3.1. Synthesis and Characterization of TA-Br
3.2. Synthesis of Polymer Stars by Photo ATRP
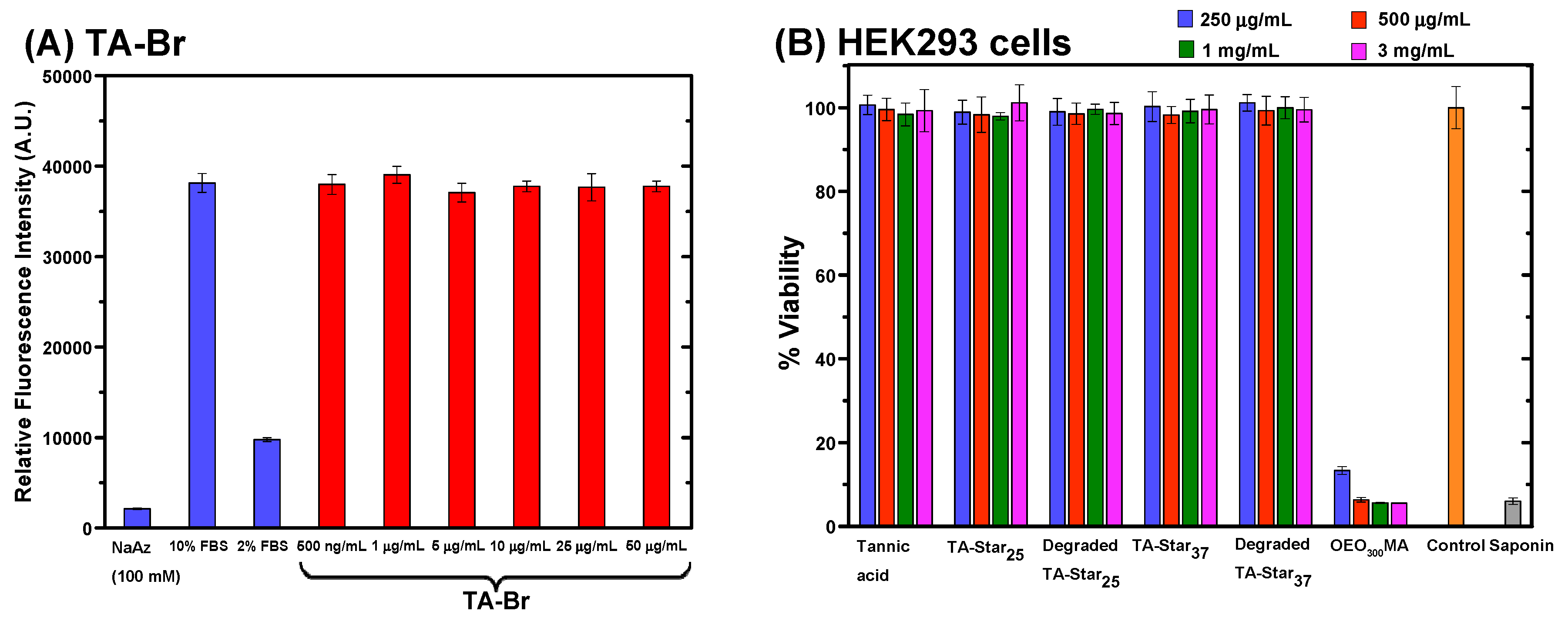
3.3. Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ren, X. Biodegradable plastics: A solution or a challenge? J. Clean. Prod. 2003, 11, 27–40. [Google Scholar] [CrossRef]
- Cheng, H.N.; Smith, P.B.; Gross, R.A. Green Polymer Chemistry: A Brief Review. ACS Symp. Ser. 2013, 1144, 1–12. [Google Scholar]
- Xu, L.Q.; Neoh, K.-G.; Kang, E.-T. Natural polyphenols as versatile platforms for material engineering and surface functionalization. Prog. Sci. 2018, 87, 165–196. [Google Scholar] [CrossRef]
- Shin, M.; Kim, K.; Shim, W.; Yang, J.W.; Lee, H. Tannic Acid as a Degradable Mucoadhesive Compound. ACS Biomater. Sci. Eng. 2016, 2, 687–696. [Google Scholar] [CrossRef]
- Singh, B.; Bhat, T.; Sharma, O. Biodegradation of tannic acid in an in vitro ruminal system. Livest. Prod. Sci. 2001, 68, 259–262. [Google Scholar] [CrossRef]
- Mahendran, B.; Raman, N.; Kim, D.-J. Purification and characterization of tannase from paecilomyces variotii: Hydrolysis of tannic acid using immobilized tannase. Appl. Microbiol. Biotechnol. 2006, 70, 444–450. [Google Scholar] [CrossRef]
- Rodriguez, H.; Rivas, B.D.L.; Gómez-Cordovés, C.; Muñoz, R. Degradation of tannic acid by cell-free extracts of Lactobacillus plantarum. Food Chem. 2008, 107, 664–670. [Google Scholar] [CrossRef]
- Scalbert, A. Antimicrobial properties of tannins. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Pranantyo, D.; Xu, L.Q.; Neoh, K.-G.; Ng, Y.X.; Teo, S.L.-M.; Kang, E.-T. Tea Stains-Inspired Initiator Primer for Surface Grafting of Antifouling and Antimicrobial Polymer Brush Coatings. Biomacromolecules 2015, 16, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Montanari, E.; Gennari, A.; Pelliccia, M.; Gourmel, C.; Lallana, E.; Matricardi, P.; McBain, A.J.; Tirelli, N. Hyaluronan/Tannic Acid Nanoparticles Via Catechol/Boronate Complexation as a Smart Antibacterial System. Macromol. Biosci. 2016, 16, 1815–1823. [Google Scholar] [CrossRef]
- Lee, J.; Cho, H.; Choi, J.; Kim, D.; Hong, D.; Park, J.H.; Yang, S.H.; Choi, I.S. Chemical sporulation and germination: Cytoprotective nanocoating of individual mammalian cells with a degradable tannic acid–feiii complex. Nanoscale 2015, 7, 18918–18922. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, N.; Sagbas, S.; Aktas, N. Single step natural poly(tannic acid) particle preparation as multitalented biomaterial. Mater. Sci. Eng. C 2015, 49, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Wang, J.; Zhang, Q.; Jin, Z. Tannic Acid-Based Multifunctional Hydrogels with Facile Adjustable Adhesion and Cohesion Contributed by Polyphenol Supramolecular Chemistry. ACS Omega 2017, 2, 6668–6676. [Google Scholar] [CrossRef]
- Zheng, L.-Y.; Shi, J.-M.; Chi, Y.-H. Tannic Acid Physically Cross-Linked Responsive Hydrogel. Macromol. Chem. Phys. 2018, 219, 1800234. [Google Scholar] [CrossRef]
- Shin, M.; Ryu, J.H.; Park, J.P.; Kim, K.; Yang, J.W.; Lee, H. DNA/Tannic Acid Hybrid Gel Exhibiting Biodegradability, Extensibility, Tissue Adhesiveness, and Hemostatic Ability. Adv. Funct. Mater. 2015, 25, 1270–1278. [Google Scholar] [CrossRef]
- Nam, H.G.; Nam, M.G.; Yoo, P.J.; Kim, J.-H. Hydrogen bonding-based strongly adhesive coacervate hydrogels synthesized using poly(N-vinylpyrrolidone) and tannic acid. Soft Matter. 2019, 15, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Hawker, C.J. Architectural Control in “Living” Free Radical Polymerizations: Preparation of Star and Graft Polymers†. Angew. Chem. Int. Ed. 1995, 34, 1456–1459. [Google Scholar] [CrossRef]
- Gao, H.; Matyjaszewski, K. Synthesis of functional polymers with controlled architecture by CRP of monomers in the presence of cross-linkers: From stars to gels. Prog. Sci. 2009, 34, 317–350. [Google Scholar] [CrossRef]
- Gao, H.; Matyjaszewski, K. Structural Control in ATRP Synthesis of Star Polymers Using the Arm-First Method. Macromolecules 2006, 39, 3154–3160. [Google Scholar] [CrossRef]
- Gao, H.; Matyjaszewski, K. Synthesis of Star Polymers by A New “Core-First” Method: Sequential Polymerization of Cross-Linker and Monomer. Macromolecules 2008, 41, 1118–1125. [Google Scholar] [CrossRef]
- Aali, N.; Massoumi, B.; Jaymand, M. Novel nanostructured star-shaped polyaniline derivatives and their electrospun nanofibers with gelatin. RSC Adv. 2015, 5, 107680–107693. [Google Scholar]
- Massoumi, B.; Jaymand, M. Nanostructured star-shaped polythiophene with tannic acid core: Synthesis, characterization, and its physicochemical properties. J. Appl. Sci. 2016, 133, 133. [Google Scholar] [CrossRef]
- Luo, S.; Yang, K.; Zhong, Z.; Wu, X.; Ren, T. Facile preparation of degradable multi-arm-star-branched waterborne polyurethane with bio-based tannic acid. RSC Adv. 2018, 8, 37765–37773. [Google Scholar] [CrossRef]
- Xu, G.; Liu, P.; Pranantyo, D.; Neoh, K.-G.; Kang, E.-T.; Lay-Ming Teo, S. One-step anchoring of tannic acid-scaffolded bifunctional coatings of antifouling and antimicrobial polymer brushes. ACS Sustain. Chem. Eng. 2019, 7, 1786–1795. [Google Scholar] [CrossRef]
- Wang, J.-S.; Matyjaszewski, K. Controlled/"living" radical polymerization. atom transfer radical polymerization in the presence of transition-metal complexes. J. Am. Chem. Soc. 1995, 117, 5614–5615. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Xia, J. Atom Transfer Radical Polymerization. Chem. Rev. 2001, 101, 2921–2990. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom Transfer Radical Polymerization (ATRP): Current Status and Future Perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Advanced Materials by Atom Transfer Radical Polymerization. Adv. Mater. 2018, 30, 1706441. [Google Scholar] [CrossRef] [PubMed]
- Si, S.; Gao, T.; Wang, J.; Liu, Q.; Zhou, G. Mussel inspired polymerized P(TA-TETA) for facile functionalization of carbon nanotube. Appl. Surf. Sci. 2018, 433, 94–100. [Google Scholar] [CrossRef]
- Chmielarz, P.; Pacześniak, T.; Rydel-Ciszek, K.; Zaborniak, I.; Biedka, P.; Sobkowiak, A.; Waldvogel, S.R. Synthesis of naturally-derived macromolecules through simplified electrochemically mediated ATRP. Beilstein J. Org. Chem. 2017, 13, 2466–2472. [Google Scholar] [CrossRef] [PubMed]
- Bhat, T.K.; Singh, B.; Sharma, O.P. Microbial degradation of tannins—A current perspective. Biogeochemistry 1998, 9, 343–357. [Google Scholar] [CrossRef]
- Xia, Z.; Singh, A.; Kiratitanavit, W.; Mosurkal, R.; Kumar, J.; Nagarajan, R. Unraveling the mechanism of thermal and thermo-oxidative degradation of tannic acid. Thermochim. Acta 2015, 605, 77–85. [Google Scholar] [CrossRef]

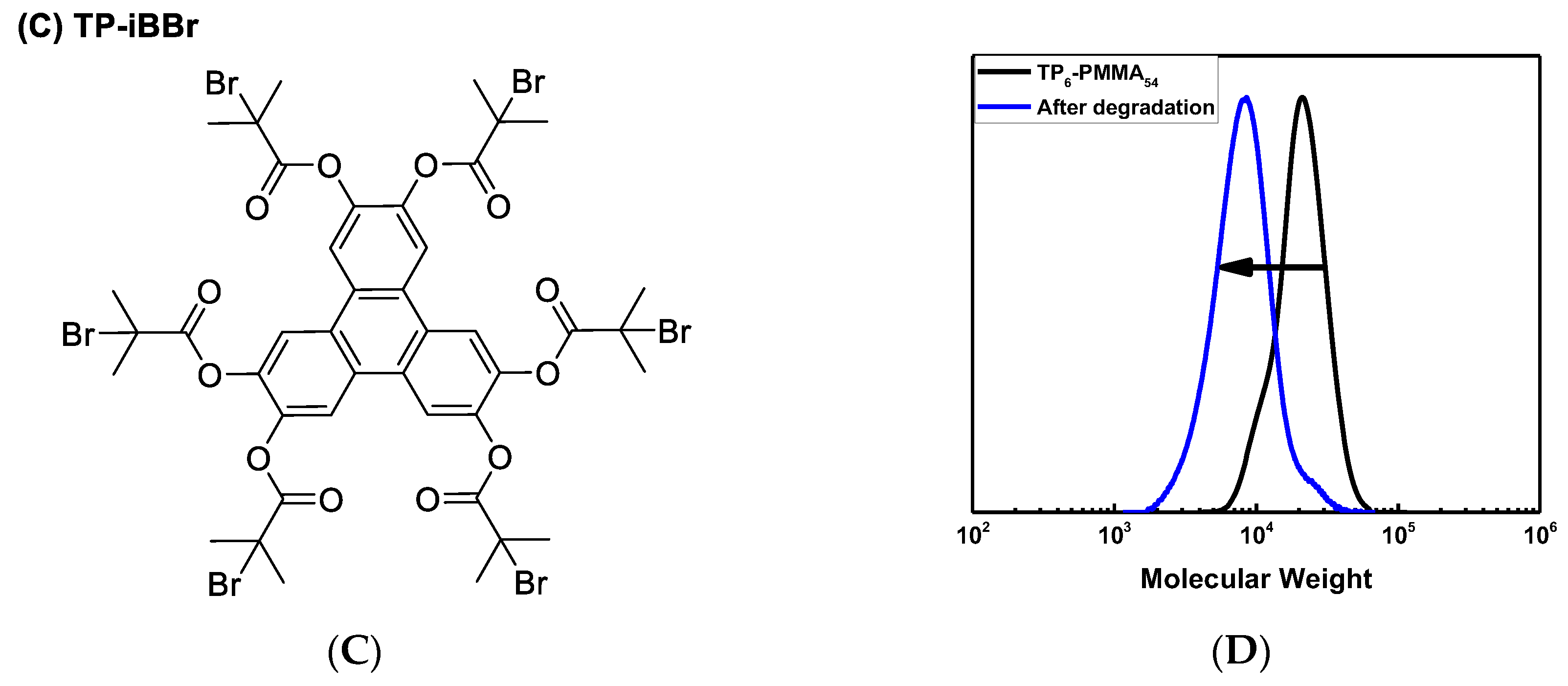
| Entry 1 | TA-Polymer Star | Arm DPtheo 2 | Mn, theo × 10−3 | Mn, GPC × 10−3 | Ð | f 3 |
|---|---|---|---|---|---|---|
| 1 | TA-PMMA7 | 7 | 23.6 | 11.1 | 1.23 | - |
| 2 | Polymer arms post degradation | 7 | 0.905 | 2.86 | 1.18 | 32% |
| 3 | TA-PMMA25 | 25 | 67.5 | 39.4 | 1.26 | - |
| 4 | Polymer arms post degradation | 25 | 2.66 | 5.03 | 1.26 | 53% |
| 5 | TA-P(OEO300MA)25 | 25 | 200 | 78.1 | 1.26 | - |
| 6 | Polymer arms post degradation | 25 | 7.98 | 17.6 | 1.56 | 45% |
| 7 | TA-P(OEO300MA)37 | 37 | 287 | 125 | 1.54 | - |
| 8 | Polymer arms post degradation | 37 | 11.4 | 32.7 | 1.84 | 35% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuthbert, J.; Yerneni, S.S.; Sun, M.; Fu, T.; Matyjaszewski, K. Degradable Polymer Stars Based on Tannic Acid Cores by ATRP. Polymers 2019, 11, 752. https://doi.org/10.3390/polym11050752
Cuthbert J, Yerneni SS, Sun M, Fu T, Matyjaszewski K. Degradable Polymer Stars Based on Tannic Acid Cores by ATRP. Polymers. 2019; 11(5):752. https://doi.org/10.3390/polym11050752
Chicago/Turabian StyleCuthbert, Julia, Saigopalakrishna S. Yerneni, Mingkang Sun, Travis Fu, and Krzysztof Matyjaszewski. 2019. "Degradable Polymer Stars Based on Tannic Acid Cores by ATRP" Polymers 11, no. 5: 752. https://doi.org/10.3390/polym11050752
APA StyleCuthbert, J., Yerneni, S. S., Sun, M., Fu, T., & Matyjaszewski, K. (2019). Degradable Polymer Stars Based on Tannic Acid Cores by ATRP. Polymers, 11(5), 752. https://doi.org/10.3390/polym11050752







