Time-Course Proteomic Analysis of Pseudomonas putida KT2440 during Mcl-Polyhydroxyalkanoate Synthesis under Nitrogen Deficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Media
2.2. Fermentation Conditions
2.3. Analytical Methods
2.4. Mcl-PHAs Extraction and Analysis
2.5. Protein Extraction and Labeling with Fluorescent Dyes
2.6. D-DIGE
2.7. Image Acquisition and Analysis
2.8. Protein Digestion and MALDI TOF/TOF Protein Identification
3. Results and Discussion
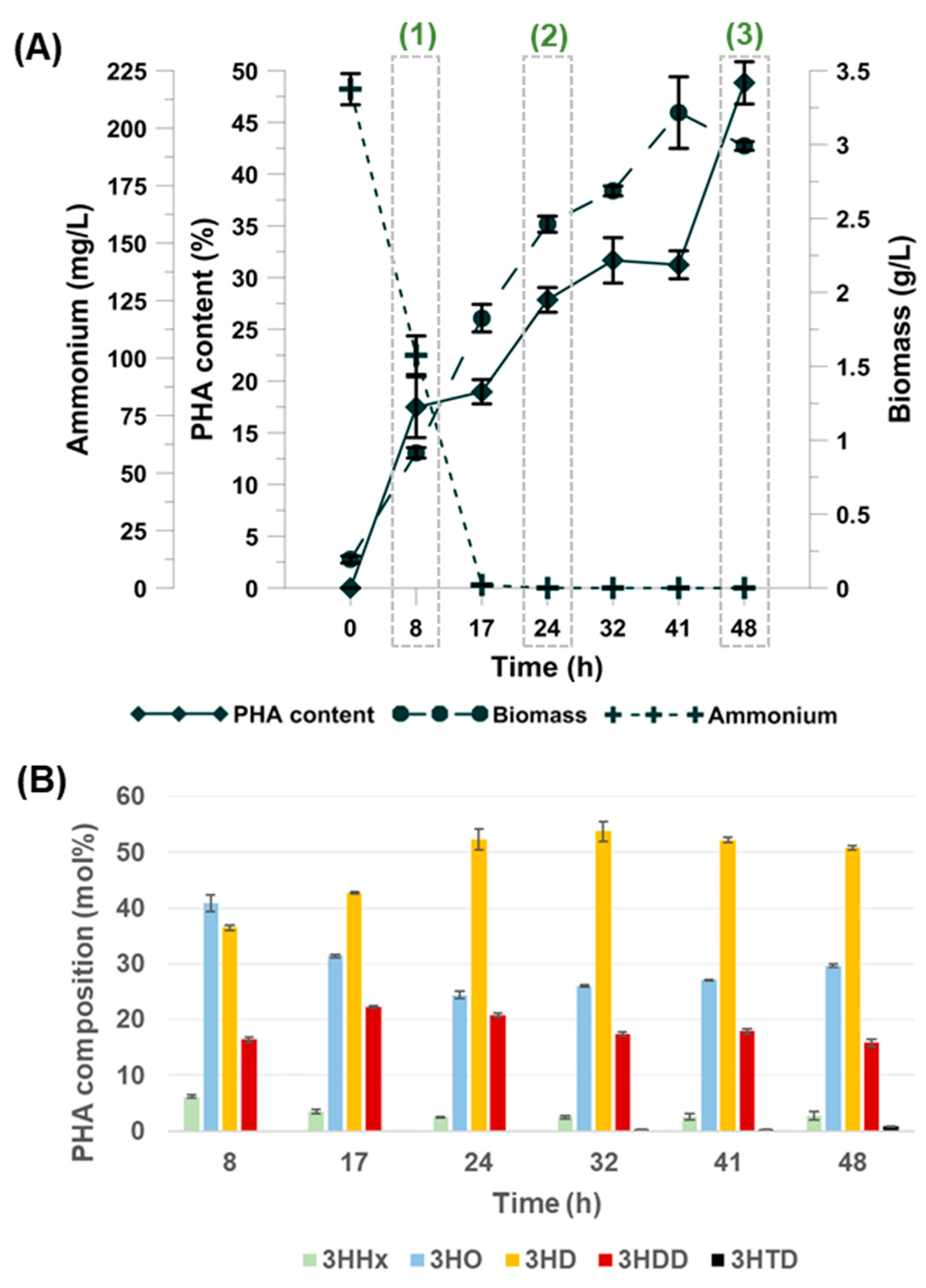
3.1. Fed-Batch Fermentation
3.2. PHAs Composition
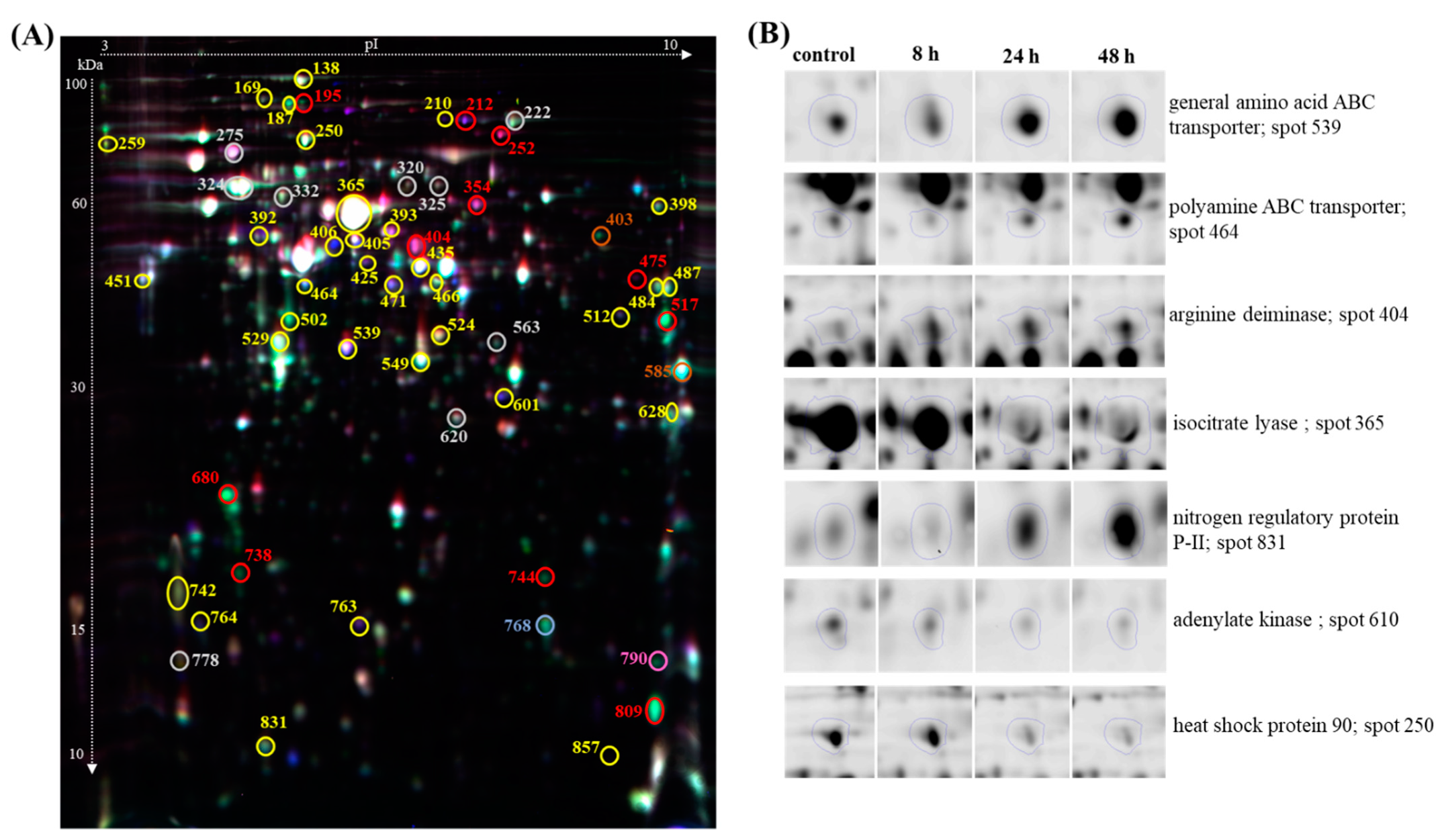
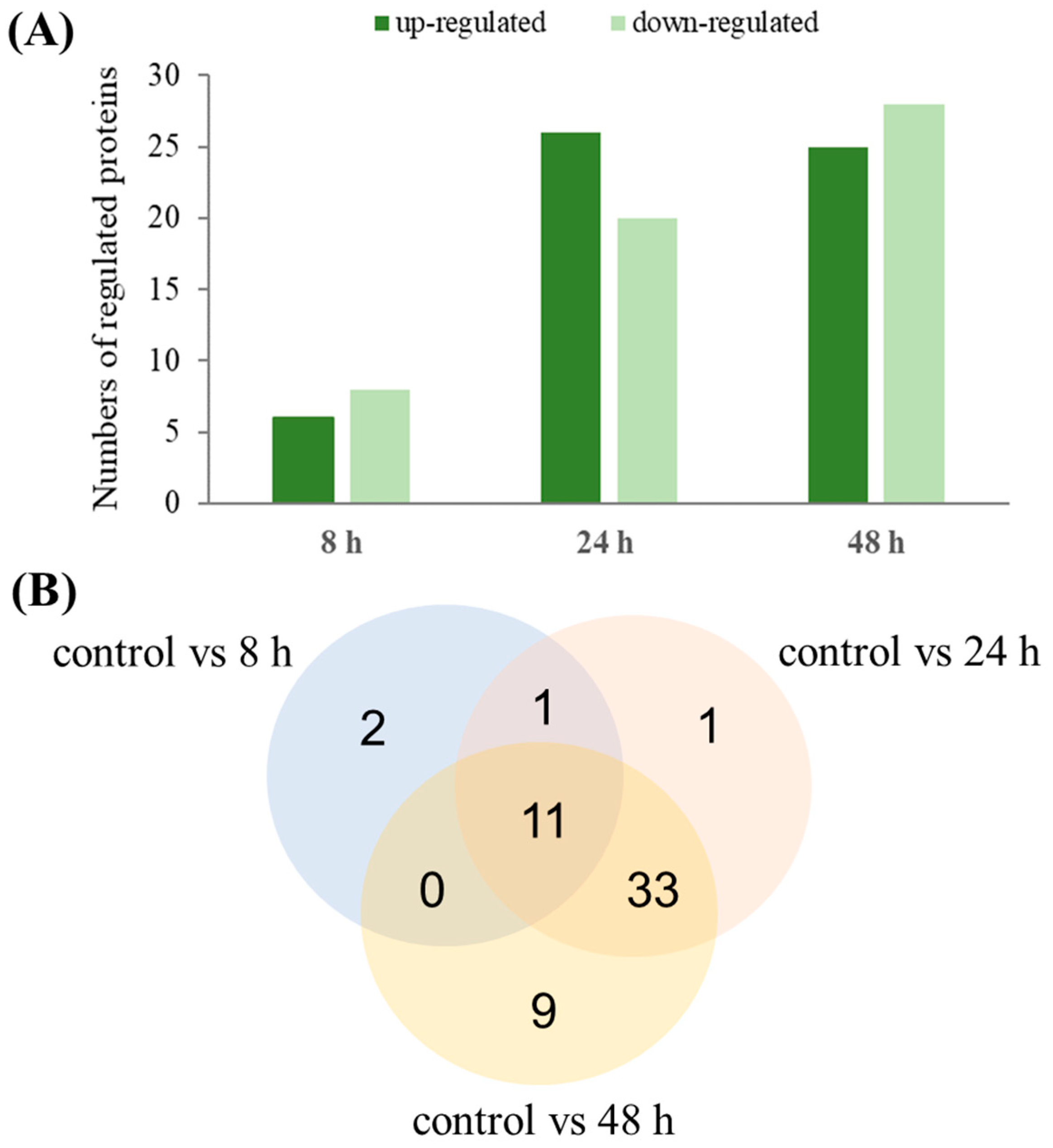
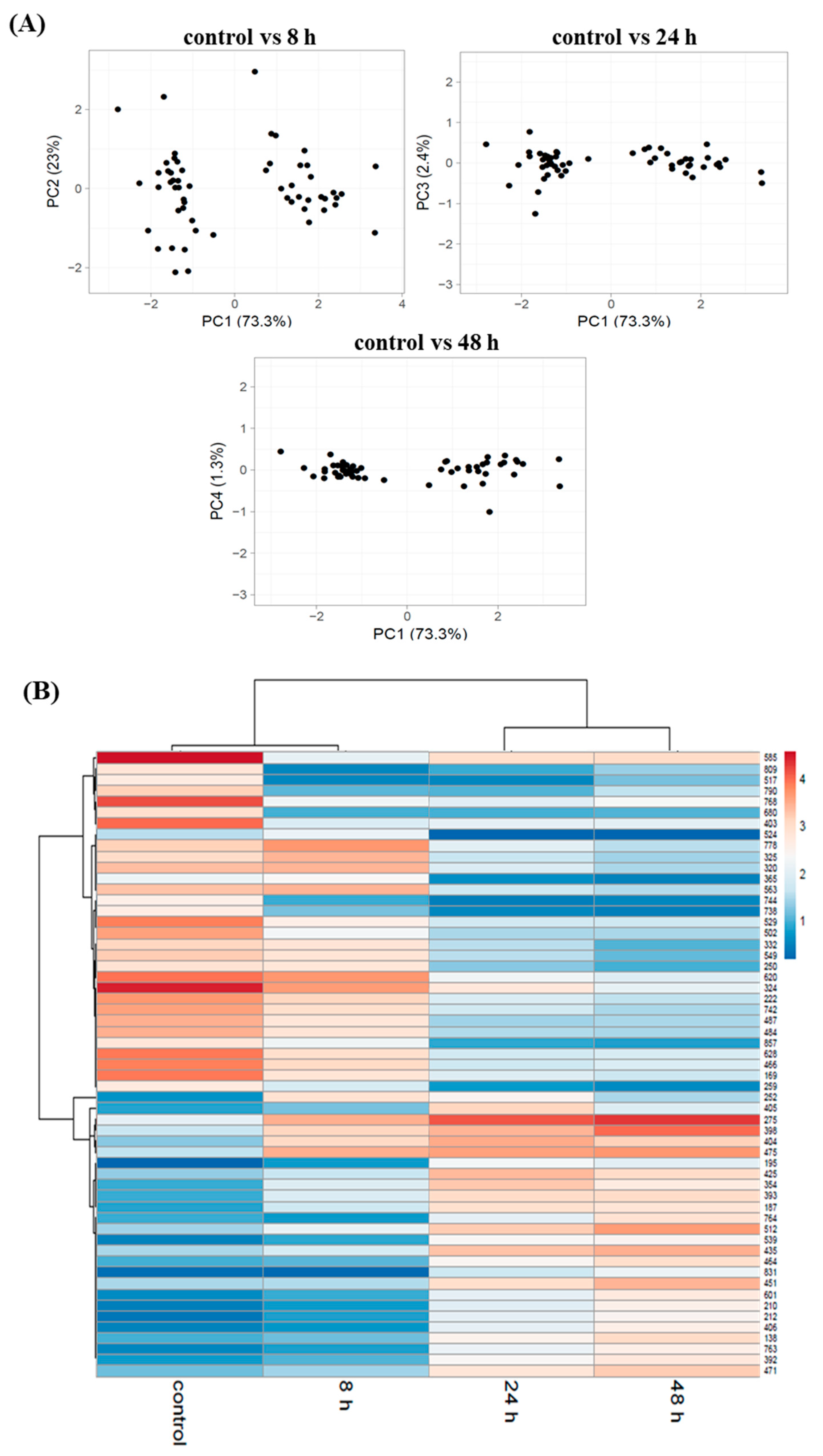
3.3. Proteome Changes during PHAs Synthesis under Nitrogen Starvation
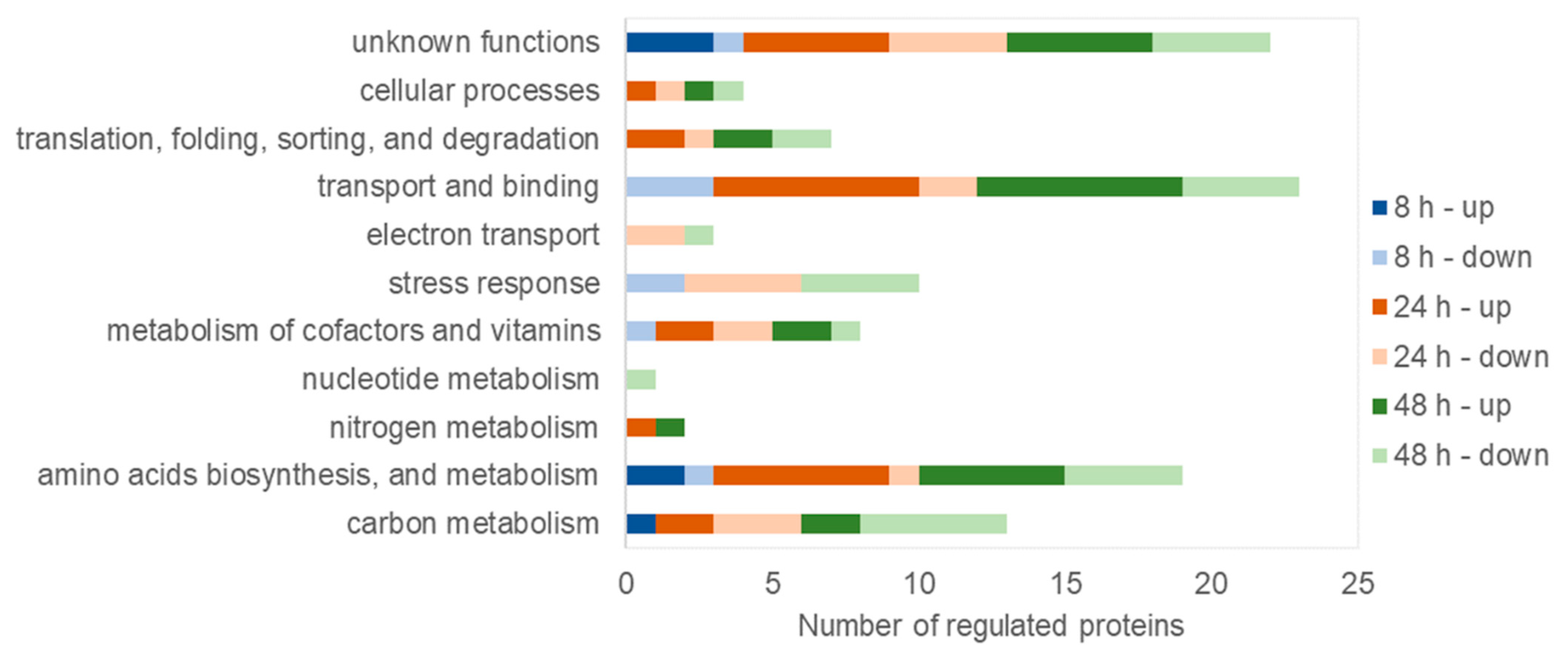
3.4. Activities of Proteins during Mcl-PHA Synthesis under Nitrogen Deficiency
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Panith, N.; Assavanig, A.; Lertsiri, S.; Bergkvist, M.; Surarit, R.; Niamsiri, N. Development of tunable biodegradable polyhydroxyalkanoates microspheres for controlled delivery of tetracycline for treating periodontal disease. J. Appl. Polym. Sci. 2016, 133, 44128–44140. [Google Scholar] [CrossRef]
- Lukasiewicz, B.; Basnett, P.; Nigmatullin, R.; Matharu, R.; Knowles, J.C.; Roy, I. Binary polyhydroxyalkanoate systems for soft tissue engineering. Acta Biomater. 2018, 71, 225–234. [Google Scholar] [CrossRef]
- Laycock, B.; Arcos-Hernandez, M.; Langford, A.; Pratt, S.; Werker, A.; Halley, P.; Lant, P. Crystallisation and fractionation of selected polyhydroxyalkanoates produced from mixed cultures. New Biotechnol. 2014, 31, 345. [Google Scholar] [CrossRef] [PubMed]
- Możejko, J.; Ciesielski, S. Pulsed feeding strategy is more favorable to medium-chain-length polyhydroxyalkanoates production from waste rapeseed oil. Biotechnol. Prog. 2014, 30, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Poblete-Castro, I.; Becker, J.; Dohnt, K.; dos Santos, V.M.; Wittmann, C. Industrial biotechnology of Pseudomonas putida and related species. Appl. Microbiol. Biotechnol. 2012, 93, 2279–2290. [Google Scholar] [CrossRef] [PubMed]
- Belda, E.; van Heck, R.G.; José Lopez-Sanchez, M.; Cruveiller, S.; Barbe, V.; Fraser, C.; Klenk, H.P.; Petersen, J.; Morgat, A.; Nikel, P.I.; et al. The revisited genome of Pseudomonas putida KT2440 enlightens its value as a robust metabolic chassis. Environ. Microbiol. 2016, 18, 3403–3424. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Munir, R.I.; de Kievit, T.; Levin, D.B. Synthesis of polyhydroxyalkanoates (PHAs) from vegetable oils and free fatty acids by wild-type and mutant strains of Pseudomonas chlororaphis. Can. J. Microbiol. 2017, 63, 1009–1024. [Google Scholar] [CrossRef] [PubMed]
- Escapa, I.F.; Morales, V.; Martino, V.P.; Pollet, E.; Avérous, L.; García, J.L.; Prieto, M.A. Disruption of β-oxidation pathway in Pseudomonas putida KT2442 to produce new functionalized PHAs with thioester groups. Appl. Microbiol. Biotechnol. 2011, 89, 1583–2598. [Google Scholar] [CrossRef]
- Borrero-de Acuña, J.M.; Bielecka, A.; Häussler, S.; Schobert, M.; Jahn, M.; Wittmann, C.; Jahn, D.; Poblete-Castro, I. Production of medium chain length polyhydroxyalkanoate in metabolic flux optimized Pseudomonas putida. Microb. Cell Fact. 2014, 13, 88. [Google Scholar] [CrossRef]
- Mozejko-Ciesielska, J.; Dabrowska, D.; Szalewska-Palasz, A.; Ciesielski, S. Medium-chain-length polyhydroxyalkanoates synthesis by Pseudomonas putida KT2440 relA/spoT mutant: Bioprocess characterization and transcriptome analysis. AMB Express 2017, 7, 92. [Google Scholar] [CrossRef]
- Mozejko-Ciesielska, J.; Pokoj, T.; Ciesielski, S. Transcriptome remodeling of Pseudomonas putida KT2440 during mcl-PHAs synthesis: Effect of different carbon sources and response to nitrogen stress. J. Ind. Microbiol. Biotechnol. 2018, 45, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Poblete-Castro, I.; Escapa, I.F.; Jäger, C.; Puchalka, J.; Lam, C.M.C.; Schomburg, D.; Prieto, M.A.; dos Santos, V.M. The metabolic response of P. putida KT2442 producing high levels of polyhydroxyalkanoate under single- and multiple-nutrient-limited growth: Highlights from a multi-level omics approach. Microb. Cell Fact. 2012, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Sharma, P.; Spicer, V.; Krokhin, O.V.; Zhang, X.; Fristensky, B.; Cicek, N.; Sparling, R.; Levin, D.B. Quantitative ’Omics Analyses of Medium Chain Length Polyhydroxyalkanaote Metabolism in Pseudomonas putida LS46 Cultured with Waste Glycerol and Waste Fatty Acids. PLoS ONE 2015, 10, e0142322. [Google Scholar] [CrossRef] [PubMed]
- Możejko, J.; Przybyłek, G.; Ciesielski, S. Waste rapeseed oil as a substrate for medium-chain-length polyhydroxyalkanoates production. Eur. J. Lipid Sci. Tech. 2011, 113, 1550–1557. [Google Scholar] [CrossRef]
- Chen, B.Y.; Hung, J.Y.; Shiau, T.J.; Wei, Y.H. Exploring two-stage fermentation strategy of polyhydroxyalkanoate production using Aeromonas hydrophila. Biochem. Eng. J. 2013, 78, 80–84. [Google Scholar] [CrossRef]
- Beckers, V.; Poblete-Castro, I.; Tomasch, J.; Wittmann, C. Integrated analysis of gene expression and metabolic fluxes in PHA-producing Pseudomonas putida grown on glycerol. Microb. Cell Fact. 2016, 15, 73. [Google Scholar] [CrossRef]
- Follonier, S.; Panke, S.; Zinn, M. A reduction in growth rate of Pseudomonas putida KT2442 counteracts productivity advances in medium-chain-length. polyhydroxyalkanoate production from gluconate. Microb. Cell Fact. 2011, 10, 25. [Google Scholar] [CrossRef]
- Furrer, P.; Panke, S.; Zinn, M.J. Efficient recovery of low endotoxin medium-chain-length poly([R]-3-hydroxyalkanoate) from bacterial biomass. Microbiol. Methods 2007, 69, 206–213. [Google Scholar] [CrossRef]
- Madison, L.L.; Huisman, G.W. Metabolic Engineering of Poly(3-Hydroxyalkanoates): From DNA to Plastic. Microbiol. Mol. Biol. Rev. 1999, 63, 21–53. [Google Scholar]
- Liu, G.; Cai, S.; Hou, J.; Zhao, D.; Han, J.; Zhou, J.; Xiang, H. Enoyl-CoA hydratase mediates polyhydroxyalkanoate mobilization in Haloferax mediterranei. Sci. Rep. 2016, 6, 24015–24026. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, S.Y. New FadB homologous enzymes and their use in enhanced biosynthesis of medium-chain-length Polyhydroxyalkanoates in fadB mutant Escherichia coli. Biotechnol. Bioeng. 2004, 86, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Luo, G.; Zhou, X.R.; Chen, G.Q. Biosynthesis of poly(3-hydroxydecanoate) and 3-hydroxydodecanoate dominating polyhydroxyalkanoates by β-oxidation pathway inhibited Pseudomonas putida. Metab. Eng. 2011, 13, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.P.; Luo, R.C.; Chen, S.S.; Liu, Q.; Chung, A.; Wu, Q.; Chen, G.Q. Production of polyhydroxyalkanoates with high 3-hydroxydodecanoate monomer content by fadB and fadA knockout mutant of Pseudomonas putida KT2442. Biomacromolecules 2007, 8, 2504–2511. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; de Roo, G.; Witholt, B.; Zinn, M.; Thöny-Meyer, L. Influence of growth stage on activities of polyhydroxyalkanoate (PHA) polymerase and PHA depolymerase in Pseudomonas putida U. BMC Microbiol. 2010, 10, 254. [Google Scholar] [CrossRef]
- Lee, I.Y.; Kim, M.K.; Chang, H.N.; Park, Y.H. Regulation of poly-β-hydroxybutyrate biosynthesis by nicotinamide nucleotide in Alcaligenes eutrophus. FEMS Microbiol. Lett. 1995, 131, 35–39. [Google Scholar] [CrossRef]
- Simon, O.; Klaiber, I.; Huber, A.; Pfannstiel, J. Comprehensive proteome analysis of the response of Pseudomonas putida KT2440 to the flavor compound vanillin. J. Proteomics 2014, 109, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Commichau, F.M.; Forchhammer, K.; Stülke, J. Regulatory links between carbon and nitrogen metabolism. Curr. Opin. Microbiol. 2006, 9, 167–172. [Google Scholar] [CrossRef]
- Huergo, L.F.; Chandra, G.; Merrick, M. P(II) signal transduction proteins: Nitrogen regulation and beyond. FEMS Microbiol. Rev. 2013, 37, 251–283. [Google Scholar] [CrossRef]
- Hillar, A.; Peters, B.; Pauls, R.; Loboda, A.; Zhang, H.; Mauk, A.G.; Loewen, P.C. Modulation of the Activities of Catalase-Peroxidase HPI of Escherichia coli by Site-Directed Mutagenesis. Biochem. 2000, 39, 5868–5875. [Google Scholar] [CrossRef]
- Bai, X.; Song, H.; Lavoie, M.; Zhu, K.; Su, Y.; Ye, H.; Chen, S.; Fu, Z.; Qian, H. Proteomic analyses bring new insights into the effect of a dark stress on lipid biosynthesis in Phaeodactylum tricornutum. Sci. Rep. 2016, 6, 25494. [Google Scholar] [CrossRef]
- Follonier, S.; Escapa, I.F.; Fonseca, P.M.; Henes, B.; Panke, S.; Zinn, M.; Prieto, M.A. New insights on the reorganization of gene transcription in Pseudomonas putida KT2440 at elevated pressure. Microb. Cell Fact. 2013, 12, 30. [Google Scholar] [CrossRef]
- Czapski, T.R.; Trun, N. Expression of csp gene in E. coli K-12 in defined rich and defined minimal media during normal growth, and after cold-shock. Gene 2014, 547, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Brandi, A.; Spurio, R.; Gualerzi, C.O.; Pon, C.L. Massive presence of the Escherichia coli ‘major cold-shock protein’ CspA under non-stress conditions. EMBO J. 1999, 18, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Macé, K.; Giudice, E.; Chat, S.; Gillet, R. The structure of an elongation factor G-ribosome complex captured in the absence of inhibitors. Nucleic Acids Res. 2018, 46, 3211–3217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray-Soni, A.; Bellecourt, M.J.; Landick, R. Mechanisms of Bacterial Transcription Termination: All Good Things Must End. Annu. Rev. Biochem. 2016, 85, 319–347. [Google Scholar] [CrossRef]
- Gudkov, A.T. The L7/L12 ribosomal domain of the ribosome: Structural and functional studies. FEBS Lett. 1997, 407, 253–256. [Google Scholar] [CrossRef]
- Adams, H.; Zeder-Lutz, G.; Schalk, I.; Pattus, F.; Celia, H. Interaction of TonB with the Outer Membrane Receptor FpvA of Pseudomonas aeruginosa. J. Bacteriol. 2006, 188, 5752–5761. [Google Scholar] [CrossRef]
- He, Y.; Xu, T.; Fossheim, L.E.; Zhang, X.H. FliC, a flagellin protein, is essential for the growth and virulence of fish pathogen Edwardsiella tarda. PLoS ONE 2012, 7, e45070. [Google Scholar] [CrossRef]
- Margolin, W. FtsZ and the division of prokaryotic cells and organelles. Nat. Rev. Mol. Cell. Biol. 2005, 6, 862–871. [Google Scholar] [CrossRef]
- Lee, E.J.; Groisman, E.A. Control of a Salmonella virulence locus by an ATP-sensing leader messenger RNA. Nature 2012, 486, 271–275. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Możejko-Ciesielska, J.; Mostek, A. Time-Course Proteomic Analysis of Pseudomonas putida KT2440 during Mcl-Polyhydroxyalkanoate Synthesis under Nitrogen Deficiency. Polymers 2019, 11, 748. https://doi.org/10.3390/polym11050748
Możejko-Ciesielska J, Mostek A. Time-Course Proteomic Analysis of Pseudomonas putida KT2440 during Mcl-Polyhydroxyalkanoate Synthesis under Nitrogen Deficiency. Polymers. 2019; 11(5):748. https://doi.org/10.3390/polym11050748
Chicago/Turabian StyleMożejko-Ciesielska, Justyna, and Agnieszka Mostek. 2019. "Time-Course Proteomic Analysis of Pseudomonas putida KT2440 during Mcl-Polyhydroxyalkanoate Synthesis under Nitrogen Deficiency" Polymers 11, no. 5: 748. https://doi.org/10.3390/polym11050748