Synthesis of Cyano-Substituted Conjugated Polymers for Photovoltaic Applications
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Instruments
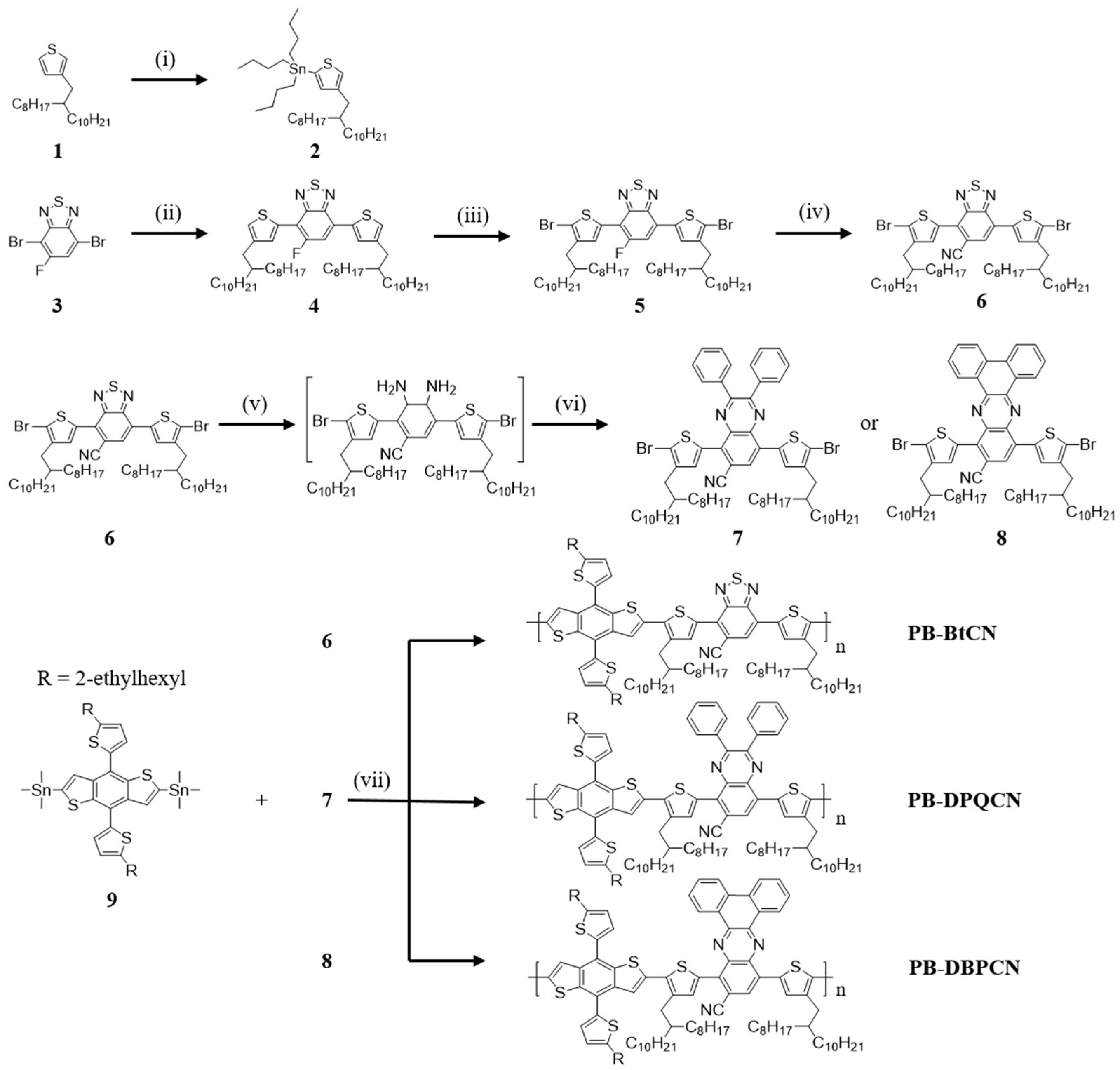
2.2. Syntheses of Monomers and Polymers
2.2.1. Synthesis of (4-(2-Octyldodecyl)thiophen-2-yl)tributylstannane (2)
2.2.2. Synthesis of 5-Fluoro-4,7-bis(4-(2-octyldodecyl)thiophen-2-yl)benzo[c][1,2,5]thiadiazole (4)
2.2.3. Synthesis of 4,7-Bis(5-bromo-4-(2-octyldodecyl)thiophen-2-yl)-5- fluorobenzo[c][1,2,5]thiadiazole (5)
2.2.4. Synthesis of 4,7-Bis(5-bromo-4-(2-octyldodecyl)thiophen-2-yl)benzo[c][1,2,5]thiadiazole-5-carbonitrile (6)
2.2.5. Synthesis of 5,8-Bis(5-bromo-4-(2-octyldodecyl)thiophen-2-yl)-2,3-diphenylquinoxaline-6- carbonitrile (7)
2.2.6. Synthesis of 10,13-Bis(5-bromo-4-(2-octyldodecyl)thiophen-2-yl)dibenzo[a,c]phenazine-11-carbonitrile (8)
2.2.7. Synthesis of PB-BtCN Via Palladium-Catalyzed Stille Reaction
2.2.8. Synthesis of PB–DPQCN
2.2.9. Synthesis of PB–DBPCN
3. Results and Discussion
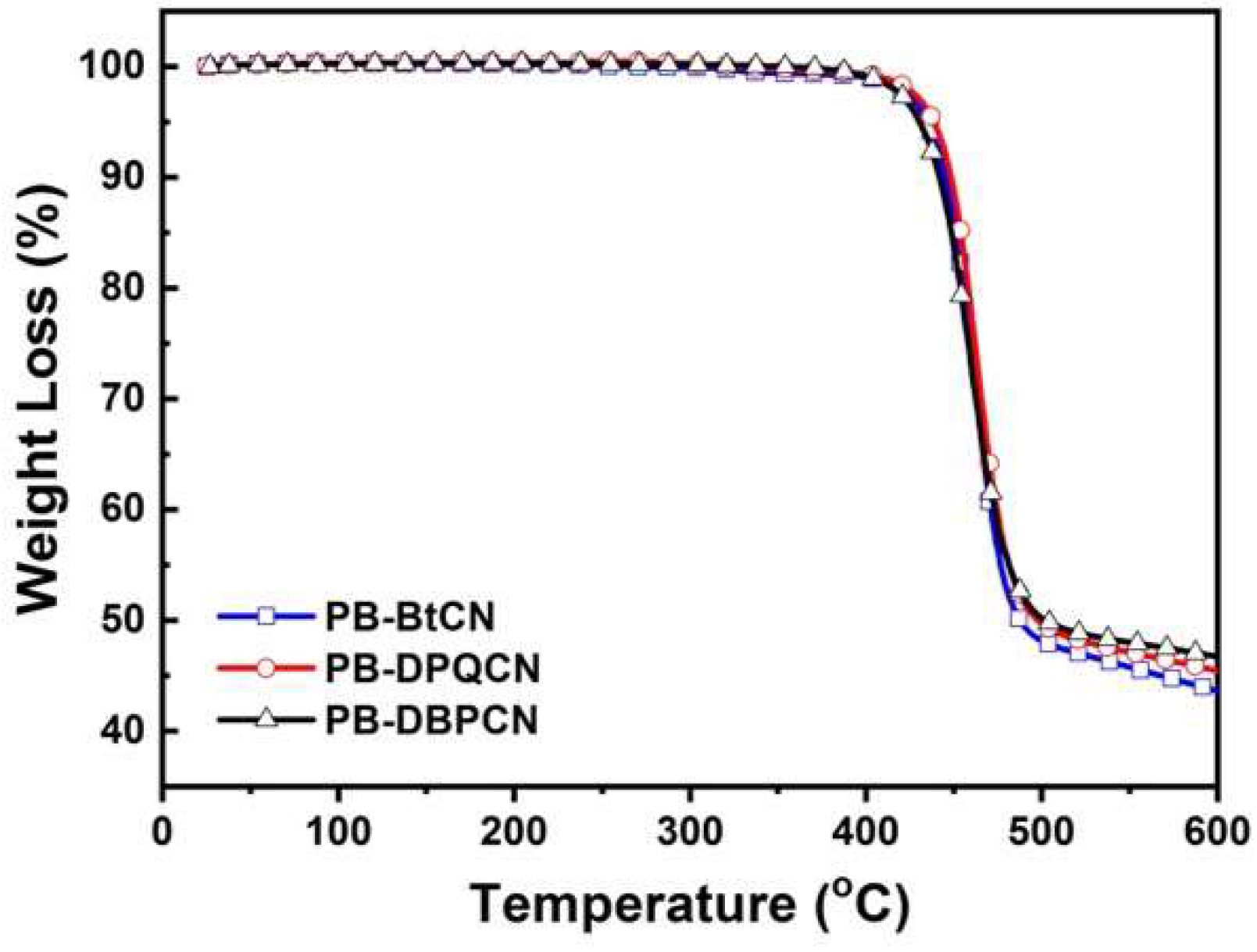
3.1. Synthesis and Thermal Properties
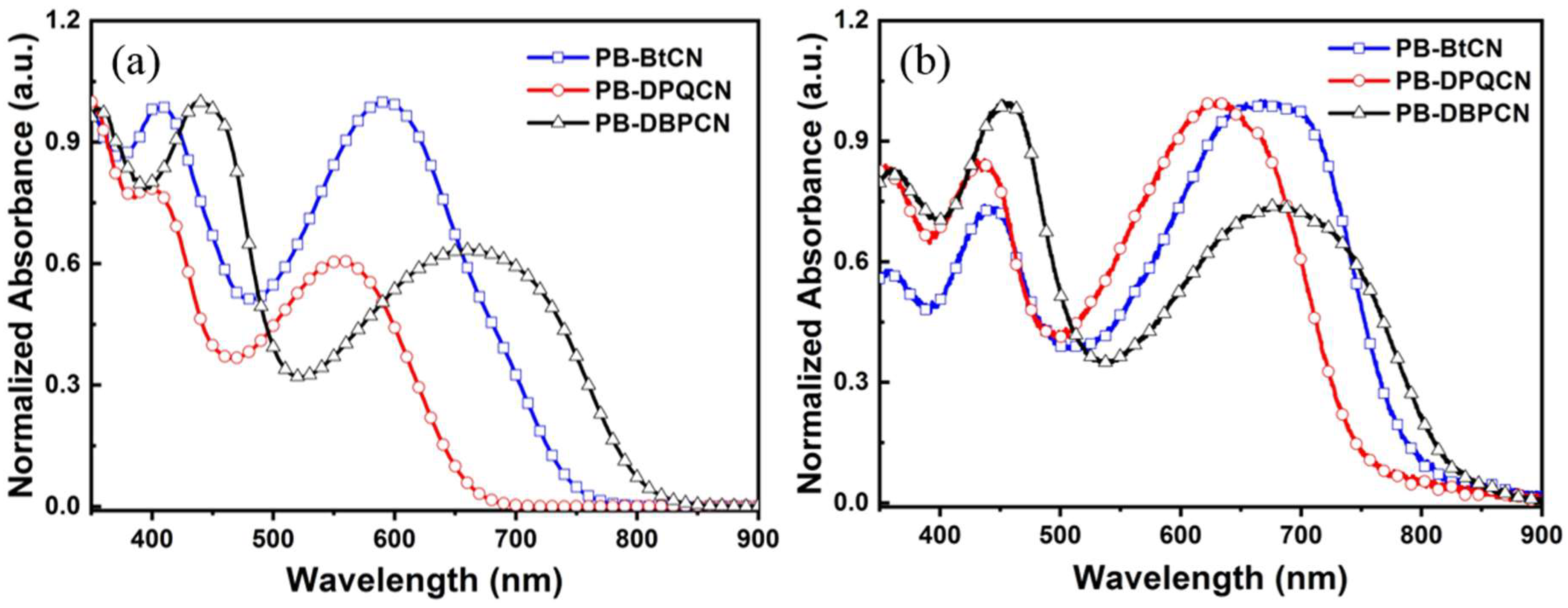
3.2. Optical and Electrochemical Properties
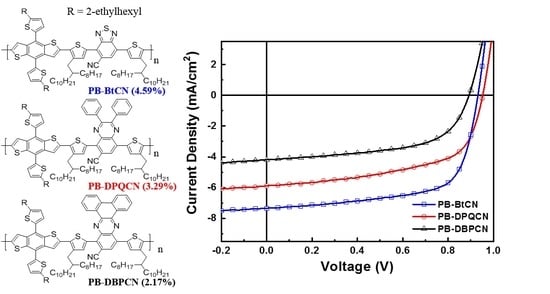
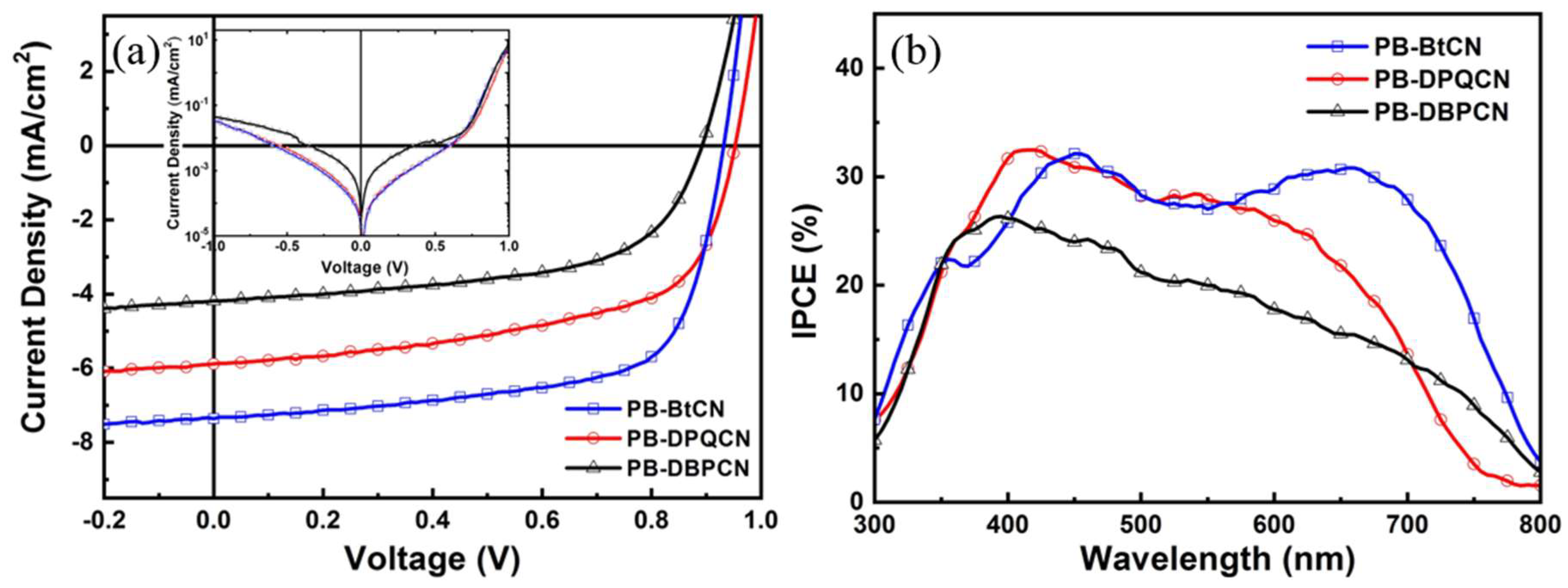
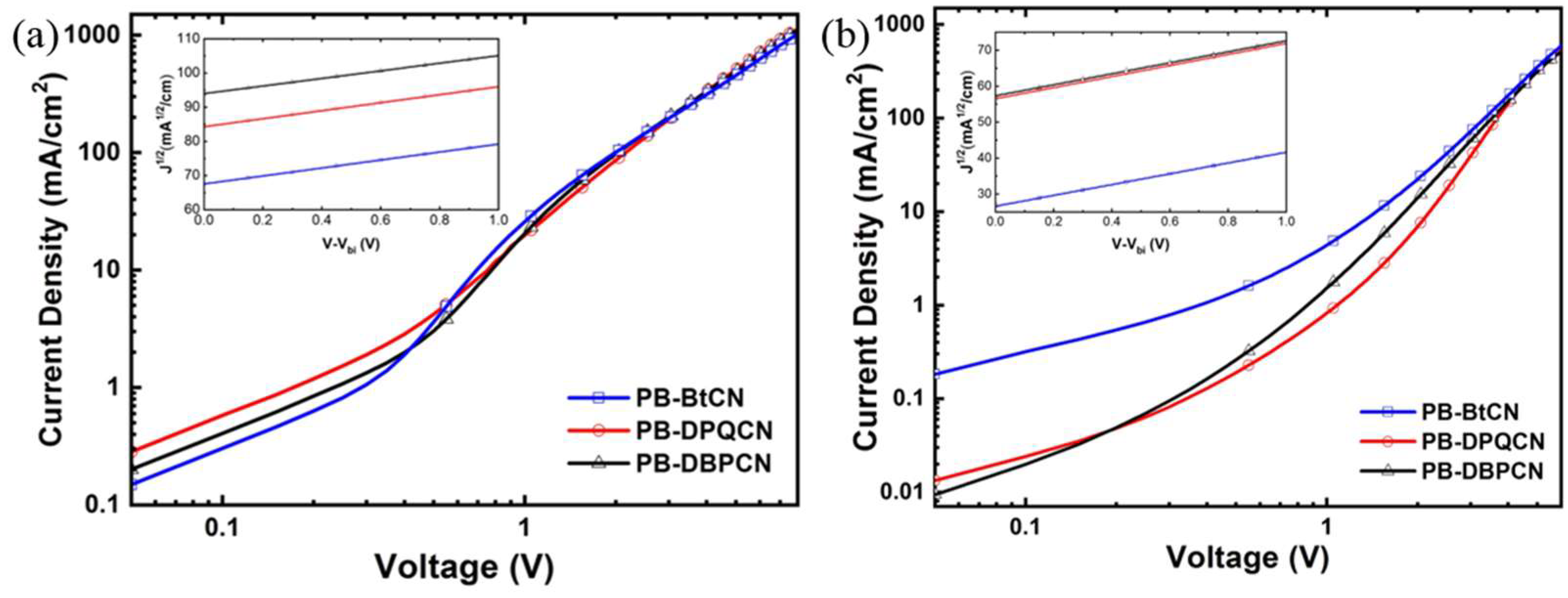
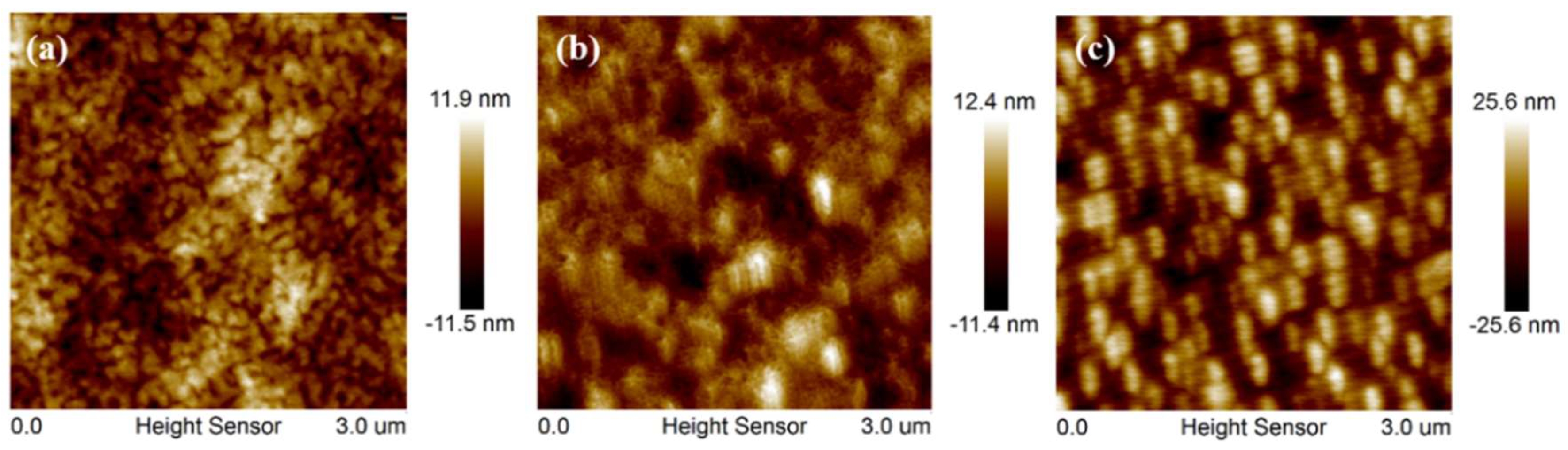
3.3. Photovoltaic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huang, Y.; Ye, L.; Wu, F.; Mei, S.; Chen, H.; Tan, S. Synthesis and photovoltaic properties of two-dimensional copolymers based on novel benzothiadiazole and quinoxaline acceptors with conjugated dithienylbenzothiadiazole pendants. J. Polym. Sci. A Polym. Chem. 2016, 54, 668–677. [Google Scholar] [CrossRef]
- Hou, W.; Xiao, Y.; Han, G.; Lin, J.-Y. The applications of polymers in solar cells: A review. Polymers 2019, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Melkonyan, F.S.; Facchetti, A.; Marks, T.J. All-polymer solar cells: Recent progress, challenges, and prospects. Angew. Chem. Int. Ed. 2019, 58, 4129–4142. [Google Scholar] [CrossRef]
- Xue, R.; Zhang, J.; Li, Y.; Li, Y. Organic solar cell materials toward commercialization. Small 2018, 14, 1801793. [Google Scholar] [CrossRef]
- Xu, X.; Li, Z.; Wang, Z.; Li, K.; Feng, K.; Peng, Q. 10.20% Efficiency polymer solar cells via employing bilaterally hole-cascade diazaphenanthrobisthiadiazole polymer donors and electron-cascade indene-C70 bisadduct acceptor. Nano Energy 2016, 25, 170–183. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Yang, G.; Jiang, K.; Lin, H.; Ade, H.; Ma, W.; Yan, H. Efficient organic solar cells processed from hydrocarbon solvents. Nat. Energy 2016, 1, 15027. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, Z.; Dong, S.; Zheng, N.; Ying, L.; Jiang, X.F.; Liu, F.; Huang, F.; Cao, Y. A novel naphtho[1,2-c:5,6-c′]-bis-([1,2,5]thiadiazole)-based narrow-bandgap π-conjugated polymer with power conversion efficiency over 10%. Adv. Mater. 2016, 28, 9811–9818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Qin, Y.; Zhu, J.; Hou, J. Over 14% efficiency in polymer solar cells enabled by a chlorinated polymer donor. Adv. Mater. 2018, 30, 1800868. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zeng, D.; Gao, X.; Li, P.; Zhang, Q.; Peng, X. Non-fullerene polymer acceptors based on perylene diimides in all-polymer solar cells. Sol. Energy Mater. Sol. Cells 2019, 189, 103–117. [Google Scholar] [CrossRef]
- Blom, P.W.; Mihailetchi, V.D.; Koster, L.J.A.; Markov, D.E. Device physics of polymer: Fullerene bulk heterojunction solar cells. Adv. Mater. 2007, 19, 1551–1566. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, Y.; Zhang, Q.; Gao, X. Non-fullerene small molecule acceptors based on perylene diimides. J. Mater. Chem. A 2016, 4, 17604–17622. [Google Scholar] [CrossRef]
- Lu, L.; Zheng, T.; Wu, Q.; Schneider, A.M.; Zhao, D.; Yu, L. Recent advances in bulk heterojunction polymer solar cells. Chem. Rev. 2015, 115, 12666–12731. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yang, L.; You, W. Rational design of high performance conjugated polymers for organic solar cells. Macromolecules 2012, 45, 607–632. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, Z.-G.; Li, Y.; Chen, X.; Qin, J. Thiophene-fused benzothiadiazole: A strong electron-acceptor unit to build D–A copolymer for highly efficient polymer solar cells. Chem. Mater. 2014, 26, 3495–3501. [Google Scholar] [CrossRef]
- Gedefaw, D.; Tessarolo, M.; Zhuang, W.; Kroon, R.; Wang, E.; Bolognesi, M.; Seri, M.; Muccini, M.; Andersson, M.R. Conjugated polymers based on benzodithiophene and fluorinated quinoxaline for bulk heterojunction solar cells: Thiophene versus thieno [3,2-b] thiophene as π-conjugated spacers. Polym. Chem. 2014, 5, 2083–2093. [Google Scholar] [CrossRef]
- Pina, J.; de Melo, J.S.; Breusov, D.; Scherf, U. Donor–acceptor–donor thienyl/bithienyl-benzothiadiazole/quinoxaline model oligomers: Experimental and theoretical studies. Phys. Chem. Chem. Phys. 2013, 15, 15204–15213. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, J.; Yip, H.-L.; Chen, K.-S.; Zeigler, D.F.; Sun, Y.; Jen, A.K.-Y. Indacenodithiophene and quinoxaline-based conjugated polymers for highly efficient polymer solar cells. Chem. Mater. 2011, 23, 2289–2291. [Google Scholar] [CrossRef]
- Lee, Y.; Nam, Y.M.; Jo, W.H. Enhanced device performance of polymer solar cells by planarization of quinoxaline derivative in a low-bandgap polymer. J. Mater. Chem. 2011, 21, 8583–8590. [Google Scholar] [CrossRef]
- Huang, J.; Lin, Z.; Feng, W.; Wang, W. Synthesis of bithiophene-based D-A1-D-A2 terpolymers with different A2 moieties for polymer solar cells via direct arylation. Polymers 2019, 11, 55. [Google Scholar] [CrossRef]
- Tong, J.; An, L.; Lv, J.; Guo, P.; Wang, X.; Yang, C.; Xia, Y. Enhanced photovoltaic performance in D-π-A copolymers containing triisopropylsilylethynyl-substituted dithienobenzodithiophene by modulating the electron-deficient units. Polymers 2019, 11, 12. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, Y.; Dong, J.; Yang, M.; Liu, M.; Zhang, Y.; Wen, J.; Ma, H.; Gao, X.; Chen, W. Chlorinated wide-bandgap donor polymer enabling annealing free nonfullerene solar cells with the efficiency of 11.5%. J. Phys. Chem. Lett. 2018, 9, 6955–6962. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Wu, Y.; Wang, Y.; Li, Y.; Liu, L.; Jiang, H.; Liu, Z.; Yang, R. Pyran-bridged indacenodithiophene as a building block for constructing efficient A–D–A-type nonfullerene acceptors for polymer solar cells. ChemSusChem 2018, 11, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Stuart, A.C.; Tumbleston, J.R.; Zhou, H.; Li, W.; Liu, S.; Ade, H.; You, W. Fluorine substituents reduce charge recombination and drive structure and morphology development in polymer solar cells. J. Am. Chem. Soc. 2013, 135, 1806–1815. [Google Scholar] [CrossRef]
- Price, S.C.; Stuart, A.C.; Yang, L.; Zhou, H.; You, W. Fluorine substituted conjugated polymer of medium band gap yields 7% efficiency in polymer−fullerene solar cells. J. Am. Chem. Soc. 2011, 133, 4625–4631. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-P.; Li, Y.; Luo, M.-M.; Peng, Q. Recent progress towards fluorinated copolymers for efficient photovoltaic applications. Chin. Chem. Lett. 2016, 27, 1241–1249. [Google Scholar] [CrossRef]
- Chen, J.; Liao, Q.; Wang, G.; Yan, Z.; Wang, H.; Wang, Y.; Zhang, X.; Tang, Y.; Facchetti, A.; Marks, T.J. Enhancing polymer photovoltaic performance via optimized intramolecular ester-based noncovalent Sulfur–Oxygen interactions. Macromolecules 2018, 51, 3874–3885. [Google Scholar] [CrossRef]
- Shi, S.; Liao, Q.; Tang, Y.; Guo, H.; Zhou, X.; Wang, Y.; Yang, T.; Liang, Y.; Cheng, X.; Liu, F. Head-to-head linkage containing bithiophene-based polymeric semiconductors for highly efficient polymer solar cells. Adv. Mater. 2016, 28, 9969–9977. [Google Scholar] [CrossRef]
- Putri, S.K.; Kim, Y.H.; Whang, D.R.; Kim, J.H.; Chang, D.W. Synthesis of trifluoromethylated quinoxaline-based polymers for photovoltaic applications. Macromol. Rapid Commun. 2018, 39, 1800260. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Huo, L.; Zhang, S.; Guo, X.; Han, C.C.; Li, Y.; Hou, J. Sulfonyl: A new application of electron-withdrawing substituent in highly efficient photovoltaic polymer. Chem. Commun. 2011, 47, 8904–8906. [Google Scholar] [CrossRef]
- Casey, A.; Dimitrov, S.D.; Shakya-Tuladhar, P.; Fei, Z.; Nguyen, M.; Han, Y.; Anthopoulos, T.D.; Durrant, J.R.; Heeney, M. Effect of systematically tuning conjugated donor polymer lowest unoccupied molecular orbital levels via cyano substitution on organic photovoltaic device performance. Chem. Mater. 2016, 28, 5110–5120. [Google Scholar] [CrossRef]
- Liu, X.; Li, M.; He, R.; Shen, W. Theoretical investigations on fluorinated and cyano copolymers for improvements of photovoltaic performances. Phys. Chem. Chem. Phys. 2014, 16, 311–323. [Google Scholar] [CrossRef]
- Li, W.; Yan, L.; Zhou, H.; You, W. A general approach toward electron deficient triazole units to construct conjugated polymers for solar cells. Chem. Mater. 2015, 27, 6470–6476. [Google Scholar] [CrossRef]
- Efrem, A.; Lei, Y.; Wu, B.; Wang, M.; Ng, S.C.; Ong, B.S. Dithienobenzochalcogenodiazole-based electron donor–acceptor polymers for organic electronics. Dyes Pigments 2016, 129, 90–99. [Google Scholar] [CrossRef]
- Yu, C.-Y.; Hsu, C.-C.; Weng, H.-C. Synthesis, characterization, aggregation-induced emission, solvatochromism and mechanochromism of fluorinated benzothiadiazole bonded to tetraphenylethenes. RSC Adv. 2018, 8, 12619–12627. [Google Scholar] [CrossRef]
- Huo, L.; Zhang, S.; Guo, X.; Xu, F.; Li, Y.; Hou, J. Replacing alkoxy groups with alkylthienyl groups: A feasible approach to improve the properties of photovoltaic polymers. Angew. Chem. It. Ed. 2011, 123, 9871–9876. [Google Scholar] [CrossRef]
- Putri, S.K.; Kim, Y.H.; Whang, D.R.; Lee, M.S.; Kim, J.H.; Chang, D.W. Step-by-step improvement in photovoltaic properties of fluorinated quinoxaline-based low-band-gap polymers. Org. Electron. 2017, 47, 14–23. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Choi, H.; Ko, S.-J.; Uddin, M.A.; Walker, B.; Yum, S.; Jeong, J.-E.; Yun, M.; Shin, T.; Hwang, S. Semi-crystalline photovoltaic polymers with efficiency exceeding 9% in a ~300 nm thick conventional single-cell device. Energy Environ. Sci. 2014, 7, 3040–3051. [Google Scholar] [CrossRef]
- Kim, J.; Yun, M.H.; Kim, G.-H.; Lee, J.; Lee, S.M.; Ko, S.-J.; Kim, Y.; Dutta, G.K.; Moon, M.; Park, S.Y. Synthesis of PCDTBT-based fluorinated polymers for high open-circuit voltage in organic photovoltaics: Towards an understanding of relationships between polymer energy levels engineering and ideal morphology control. ACS Appl. Mater. Interfaces 2014, 6, 7523–7534. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.W.; Lee, H.J.; Kim, J.H.; Park, S.Y.; Park, S.-M.; Dai, L.; Baek, J.-B. Novel quinoxaline-based organic sensitizers for dye-sensitized solar cells. Org. Lett. 2011, 13, 3880–3883. [Google Scholar] [CrossRef]
- Ko, S.J.; Lee, W.; Choi, H.; Walker, B.; Yum, S.; Kim, S.; Shin, T.J.; Woo, H.Y.; Kim, J.Y. Improved performance in polymer solar cells using mixed PC61BM/PC71BM acceptors. Adv. Energy Mater. 2015, 5, 1401687. [Google Scholar] [CrossRef]
- Boland, P.; Lee, K.; Namkoong, G. Device optimization in PCPDTBT: PCBM plastic solar cells. Sol. Energy Mater. Sol. Cells 2010, 94, 915–920. [Google Scholar] [CrossRef]
- Bagui, A.; Iyer, S.S.K. Increase in hole mobility in poly (3-hexylthiophene-2,5-diyl) films annealed under electric field during the solvent drying step. Org. Electron. 2014, 15, 1387–1395. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, L.; Shao, M.; Wu, Y.; Zeng, D.; Cai, X.; Duan, J.; Zhang, X.; Gao, X. Fine-tuning the quasi-3D geometry: Enabling efficient nonfullerene organic solar cells based on perylene diimides. ACS Appl. Mater. Interfaces 2017, 10, 762–768. [Google Scholar] [CrossRef] [PubMed]


| Polymer | ||||
|---|---|---|---|---|
| PB-BtCN | 406, 594, (442, 670) | −5.37 | −3.57 | 1.80 |
| PB-DPQCN | 391, 557, (434, 629) | −5.41 | −3.53 | 1.88 |
| PB-DBPCN | 441, 662, (452, 682) | −5.30 | −3.57 | 1.73 |
| Polymer | Blend Ratio | Jsc (mA/cm2) | Rs a (Ω cm2) | |||
|---|---|---|---|---|---|---|
| PB–BtCN | 3:6 | 7.36 (7.24 ± 0.18) | 0.93 (0.94 ± 0.00) | 67.1 (65.1 ± 1.51) | 4.59 (4.40 ± 0.10) | 3.75 |
| PB–DPQCN | 3:5 | 5.89 (5.89 ± 0.09) | 0.95 (0.94 ± 0.01) | 58.8 (57.2 ± 0.95) | 3.29 (3.17 ± 0.07) | 4.34 |
| PB–DBPCN | 3:4 | 4.19 (4.09 ± 0.13) | 0.89 (0.89 ± 0.01) | 58.3 (56.9 ± 0.72) | 2.17 (2.07 ± 0.08) | 9.96 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.H.; Jin, H.C.; Kim, J.H.; Chang, D.W. Synthesis of Cyano-Substituted Conjugated Polymers for Photovoltaic Applications. Polymers 2019, 11, 746. https://doi.org/10.3390/polym11050746
Yang MH, Jin HC, Kim JH, Chang DW. Synthesis of Cyano-Substituted Conjugated Polymers for Photovoltaic Applications. Polymers. 2019; 11(5):746. https://doi.org/10.3390/polym11050746
Chicago/Turabian StyleYang, Mun Ho, Ho Cheol Jin, Joo Hyun Kim, and Dong Wook Chang. 2019. "Synthesis of Cyano-Substituted Conjugated Polymers for Photovoltaic Applications" Polymers 11, no. 5: 746. https://doi.org/10.3390/polym11050746
APA StyleYang, M. H., Jin, H. C., Kim, J. H., & Chang, D. W. (2019). Synthesis of Cyano-Substituted Conjugated Polymers for Photovoltaic Applications. Polymers, 11(5), 746. https://doi.org/10.3390/polym11050746