Luminescent Organic Barcode Nanowires for Effective Chemical Sensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Luminescent Organic Barcode NWs
2.2. Measurement of Nanoscale Optical Properties and Structural Characteristics
3. Results
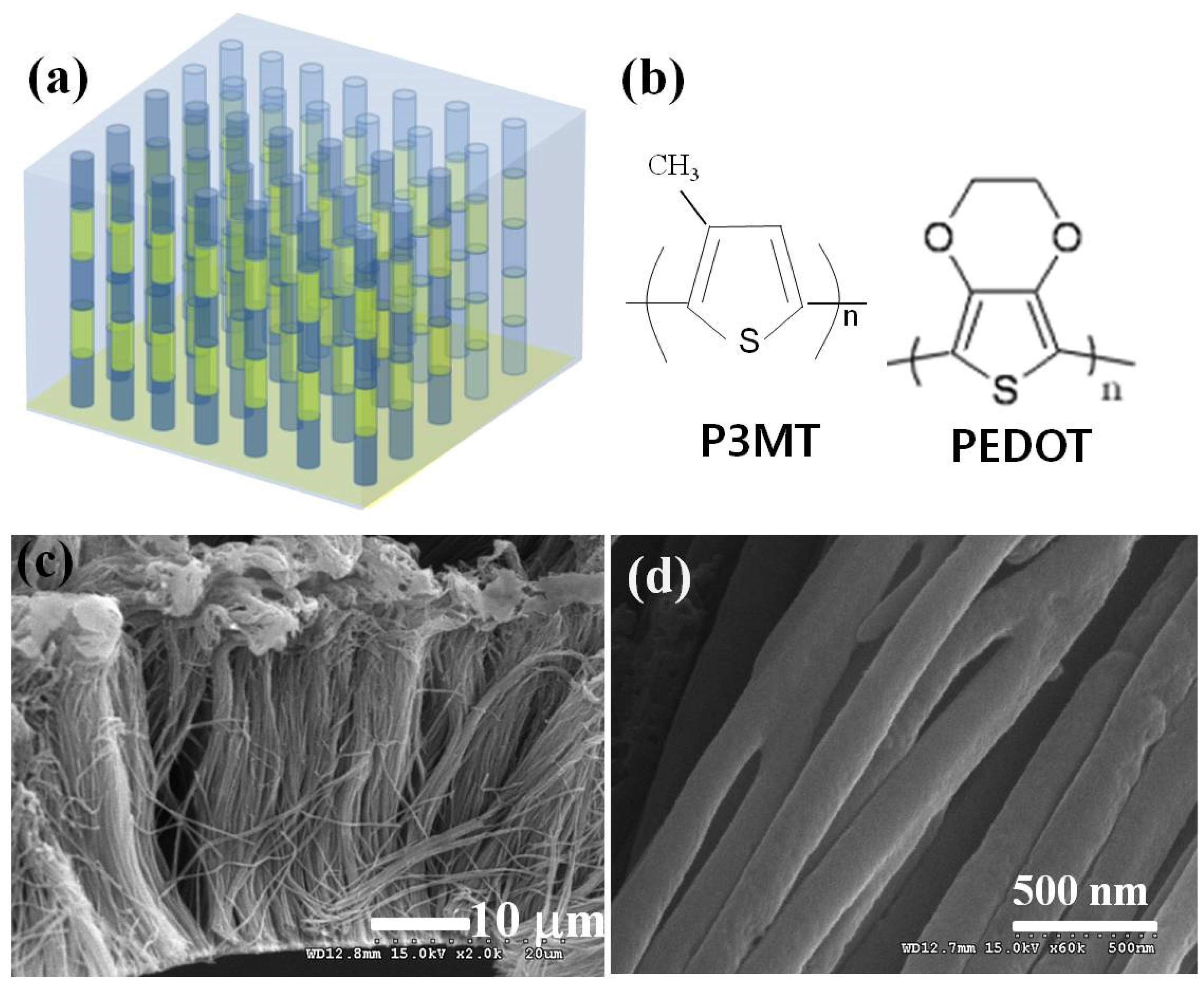
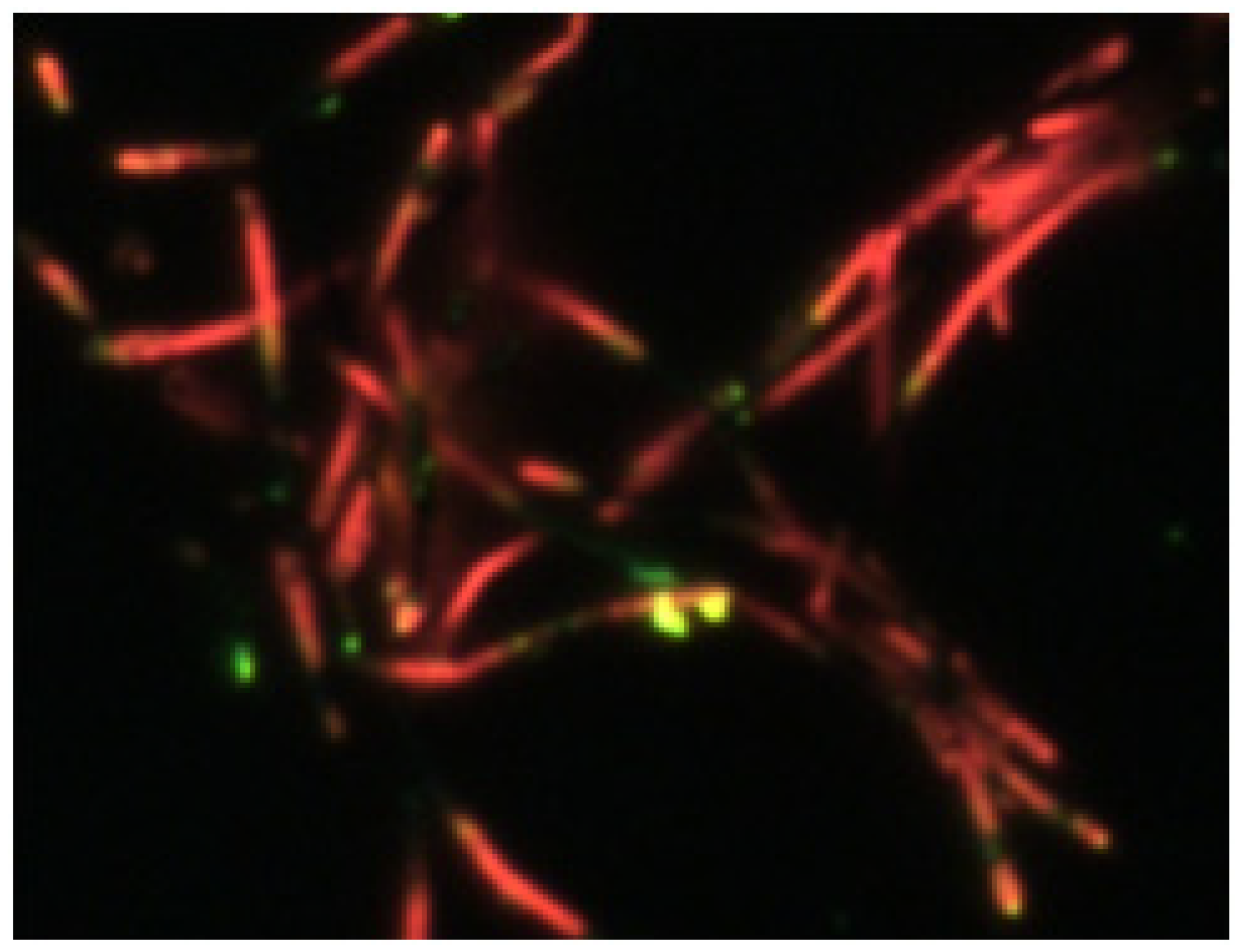
3.1. Formation of Organic Barcode NWs of P3MT/PEDOT
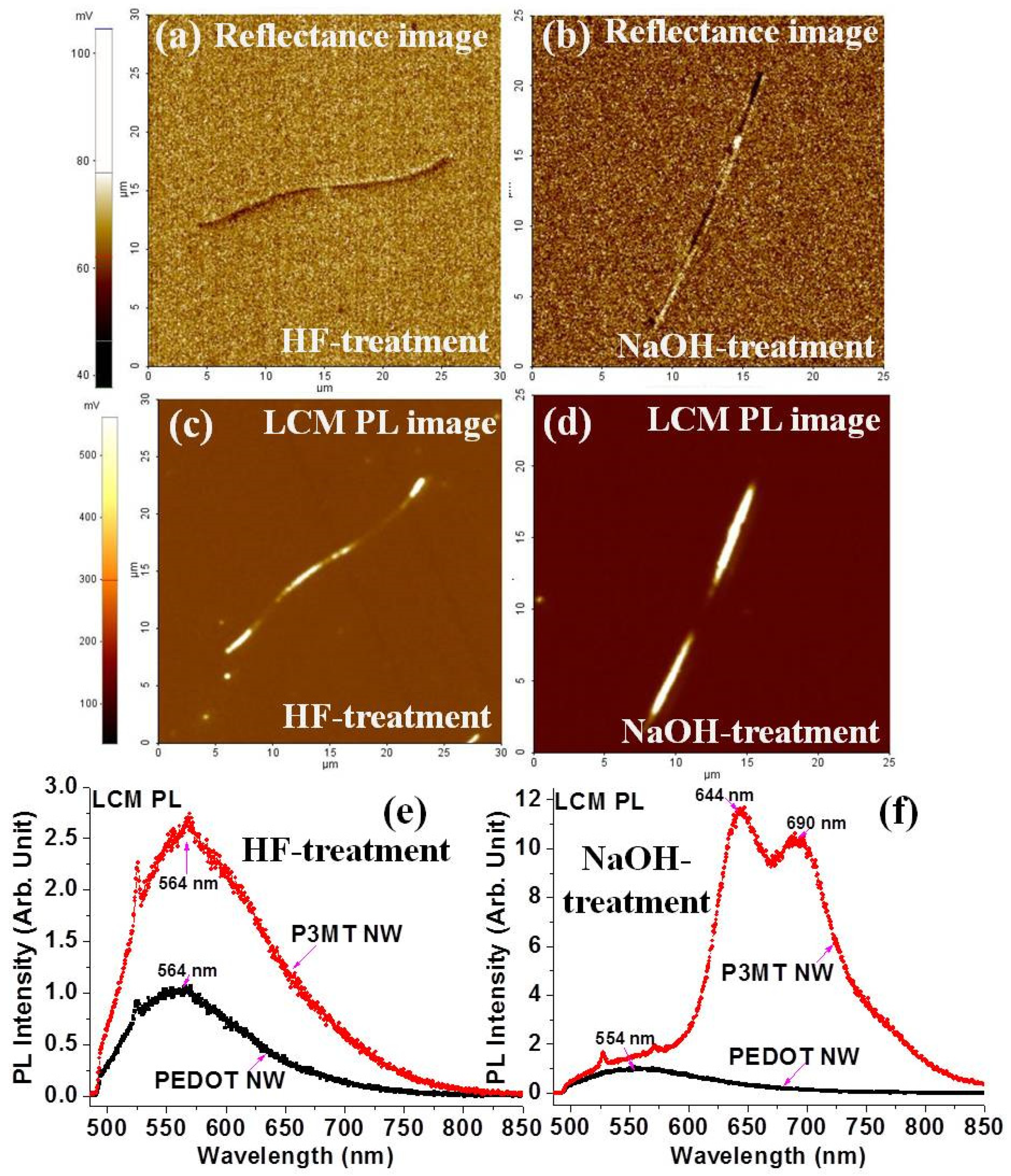
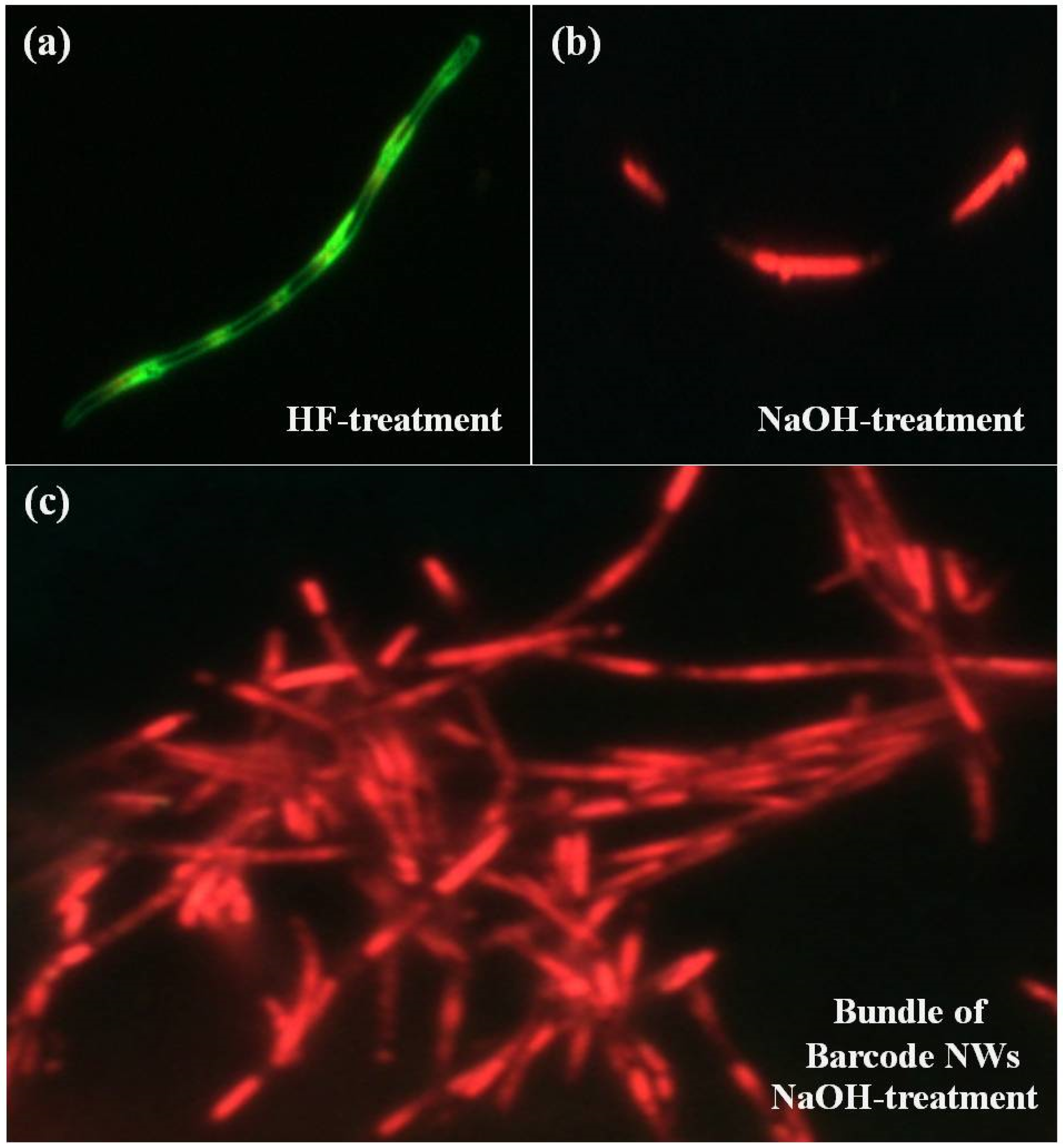
3.2. Luminescence Characteristics of Organic Barcode NWs
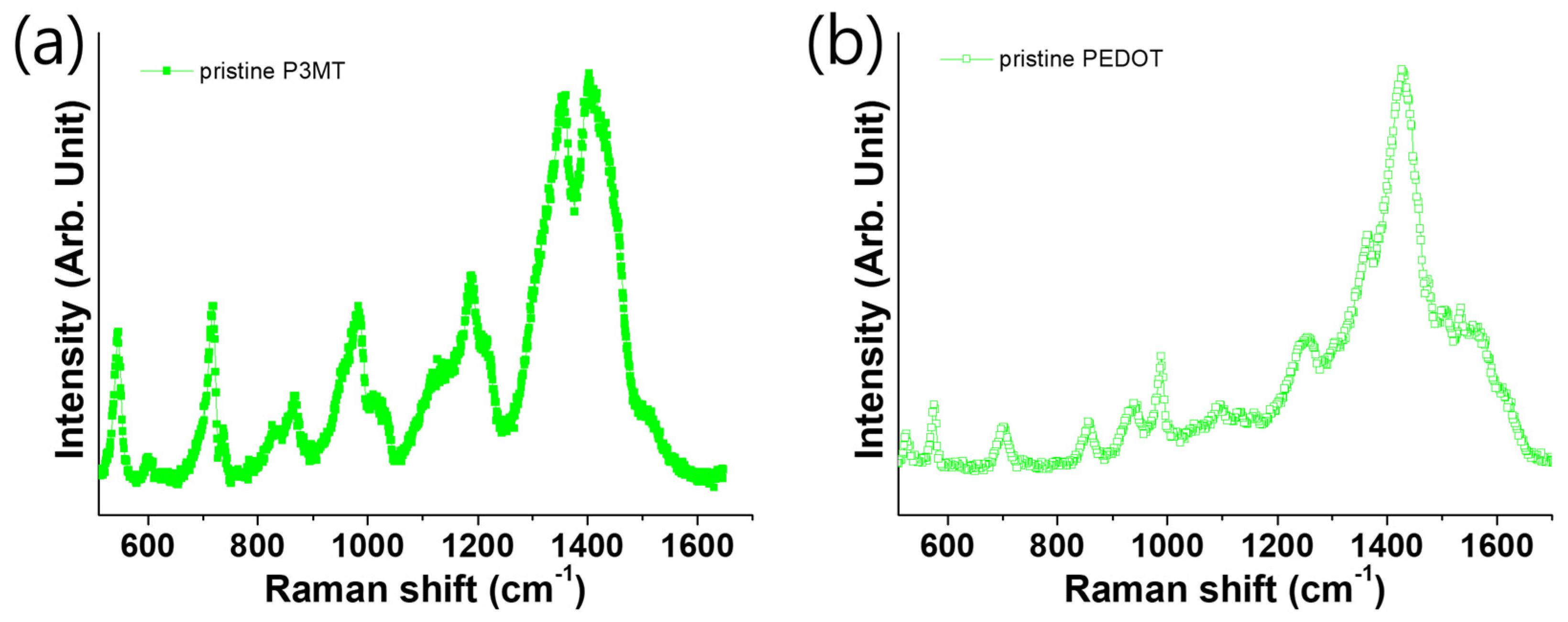
3.3. Raman Scattering of P3MT and PEDOT with Difference pH Scale
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Huang, Y.; Duan, X.; Cui, Y.; Lauhon, L.J.; Kim, K.; Lieber, C.M. Logic Gates and Computation from Assembled Nanowire Building Blocks. Science 2001, 294, 1313–1317. [Google Scholar] [CrossRef]
- Hayden, O.; Agarwal, R.; Lieber, C.M. Nanoscale Avalanche Photodiodes for Highly Sensitive and Spatially Resolved Photon Detection. Nat. Mater. 2006, 5, 352. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Lu, W.; Hu, Y.; Wu, Y.; Yan, H.; Lieber, C.M. Ge/Si Nanowire Heterostructures as High-Performance Field-Effect Transistors. Nature 2006, 441, 489. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Zheng, X.; Kempa, T.J.; Fang, Y.; Yu, N.; Yu, G.; Huang, J.; Lieber, C.M. Coaxial Silicon Nanowires as Solar Cells and Nanoelectronic Power Sources. Nature 2007, 449, 885. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.; Chu, H.Y.; Lee, J. Controlling the Optical Efficiency of the Transparent Organic Light-Emitting Diode using Capping Layers. J. Inf. Disp. 2013, 14, 57–60. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, S.Y.; Choi, K.C. Analysis and Structure Optimization of Nanostructure-Embedded Organic Light-Emitting Diodes. J. Inf. Disp. 2013, 14, 73–77. [Google Scholar] [CrossRef]
- Huang, J.; Choi, J.; Lee, G.; Chen, F.; Cui, C.; Jin, L.; Park, D. Photoluminescence Enhancement of Poly (3-Methylthiophene) Nanowires upon Length Variable DNA Hybridization. Polymers 2018, 10, 100. [Google Scholar] [CrossRef]
- Rasmussen, S.C.; Evenson, S.J.; McCausland, C.B. Fluorescent Thiophene-Based Materials and their Outlook for Emissive Applications. Chem. Commun. 2015, 51, 4528–4543. [Google Scholar] [CrossRef]
- Santhanam, K.; Sangoi, R.; Fuller, L. A Chemical Sensor for Chloromethanes using a Nanocomposite of Multiwalled Carbon Nanotubes with Poly (3-Methylthiophene). Sens. Actuators B Chem. 2005, 106, 766–771. [Google Scholar]
- Bronstein, H.; Chen, Z.; Ashraf, R.S.; Zhang, W.; Du, J.; Durrant, J.R.; Shakya Tuladhar, P.; Song, K.; Watkins, S.E.; Geerts, Y. Thieno [3, 2-B] Thiophene—Diketopyrrolopyrrole-Containing Polymers for High-Performance Organic Field-Effect Transistors and Organic Photovoltaic Devices. J. Am. Chem. Soc. 2011, 133, 3272–3275. [Google Scholar] [CrossRef] [PubMed]
- Perepichka, I.F.; Perepichka, D.F.; Meng, H.; Wudl, F. Light-emitting Polythiophenes. Adv. Mater. 2005, 17, 2281–2305. [Google Scholar] [CrossRef]
- Hong, Y.K.; Park, D.H.; Jo, S.G.; Koo, M.H.; Kim, D.; Kim, J.; Kim, J.; Jang, S.; Joo, J. Fine Characteristics Tailoring of Organic and Inorganic Nanowires using Focused Electron-Beam Irradiation. Angew. Chem. Int. Ed. 2011, 50, 3734–3738. [Google Scholar] [CrossRef]
- Park, D.H.; Hong, Y.K.; Cho, E.H.; Kim, M.S.; Kim, D.; Bang, J.; Kim, J.; Joo, J. Light-Emitting Color Barcode Nanowires using Polymers: Nanoscale Optical Characteristics. ACS Nano 2010, 4, 5155–5162. [Google Scholar] [CrossRef]
- Lu, W.; Fadeev, A.G.; Qi, B.; Smela, E.; Mattes, B.R.; Ding, J.; Spinks, G.M.; Mazurkiewicz, J.; Zhou, D.; Wallace, G.G.; et al. Use of Ionic Liquids for π-Conjugated Polymer Electrochemical Devices. Science 2002, 297, 983–987. [Google Scholar] [CrossRef]
- Park, D.H.; Kim, H.S.; Jeong, M.Y.; Lee, Y.B.; Kim, H.J.; Kim, D.C.; Kim, J.; Joo, J. Significantly Enhanced Photoluminescence of Doped Polymer-Metal Hybrid Nanotubes. Adv. Funct. Mater. 2008, 18, 2526–2534. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, D.H.; Lee, Y.B.; Kim, D.C.; Kim, H.J.; Kim, J.; Joo, J. Doped and de-doped polypyrrole nanowires by using a BMIMPF6 ionic liquid. Synth. Met. 2007, 157, 910–913. [Google Scholar] [CrossRef]
- Park, D.H.; Hong, Y.K.; Kim, M.S.; Cho, E.H.; Choi, W.J.; Kim, K.H.; Park, Q.; Kim, D.; Song, H.; Kim, J. Nanoscale Photoluminescence of Light-Emitting Poly (3-Methylthiophene) Nanotubes Hybridized with Au Nanoparticles. Synth. Met. 2010, 160, 604–608. [Google Scholar] [CrossRef]
- Hong, Y.K.; Park, D.H.; Park, S.K.; Song, H.; Kim, D.; Kim, J.; Han, Y.H.; Park, O.K.; Lee, B.C.; Joo, J. Tuning and Enhancing Photoluminescence of Light-Emitting Polymer Nanotubes through Electron-Beam Irradiation. Adv. Funct. Mater. 2009, 19, 567–572. [Google Scholar] [CrossRef]
- Han, Y.; Chang, M.; Huang, W.; Pan, H.; Ho, K.; Hsieh, T.; Pan, S. Improved Performance of Polymer Solar Cells Featuring One-Dimensional PEDOT Nanorods in a Modified Buffer Layer. J. Electrochem. Soc. 2011, 158, K88–K93. [Google Scholar] [CrossRef]
- Horikawa, M.; Fujiki, T.; Shirosaki, T.; Ryu, N.; Sakurai, H.; Nagaoka, S.; Ihara, H. The Development of a Highly Conductive PEDOT System by Doping with Partially Crystalline Sulfated Cellulose and its Electric Conductivity. J. Mater. Chem. C 2015, 3, 8881–8887. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.S.; Park, Y.D. Doped PEDOT: PSS Electrodes, Patterned through Wettability Control, and their Effects on the Electrical Properties of Polymer Thin Film Transistors. Org. Electron. 2016, 30, 296–301. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.; Kim, S.; Park, J.W.; Lee, S.H.; Seo, Y.J.; Park, D.H. Luminescent Organic Barcode Nanowires for Effective Chemical Sensors. Polymers 2019, 11, 662. https://doi.org/10.3390/polym11040662
Choi J, Kim S, Park JW, Lee SH, Seo YJ, Park DH. Luminescent Organic Barcode Nanowires for Effective Chemical Sensors. Polymers. 2019; 11(4):662. https://doi.org/10.3390/polym11040662
Chicago/Turabian StyleChoi, Jinho, Seokho Kim, Jung Woon Park, Seung Hwan Lee, Young Ju Seo, and Dong Hyuk Park. 2019. "Luminescent Organic Barcode Nanowires for Effective Chemical Sensors" Polymers 11, no. 4: 662. https://doi.org/10.3390/polym11040662
APA StyleChoi, J., Kim, S., Park, J. W., Lee, S. H., Seo, Y. J., & Park, D. H. (2019). Luminescent Organic Barcode Nanowires for Effective Chemical Sensors. Polymers, 11(4), 662. https://doi.org/10.3390/polym11040662



