Chemically Reduced Graphene Oxide-Reinforced Poly(Lactic Acid)/Poly(Ethylene Glycol) Nanocomposites: Preparation, Characterization, and Applications in Electromagnetic Interference Shielding
Abstract
1. Introduction
2. Experimental Details
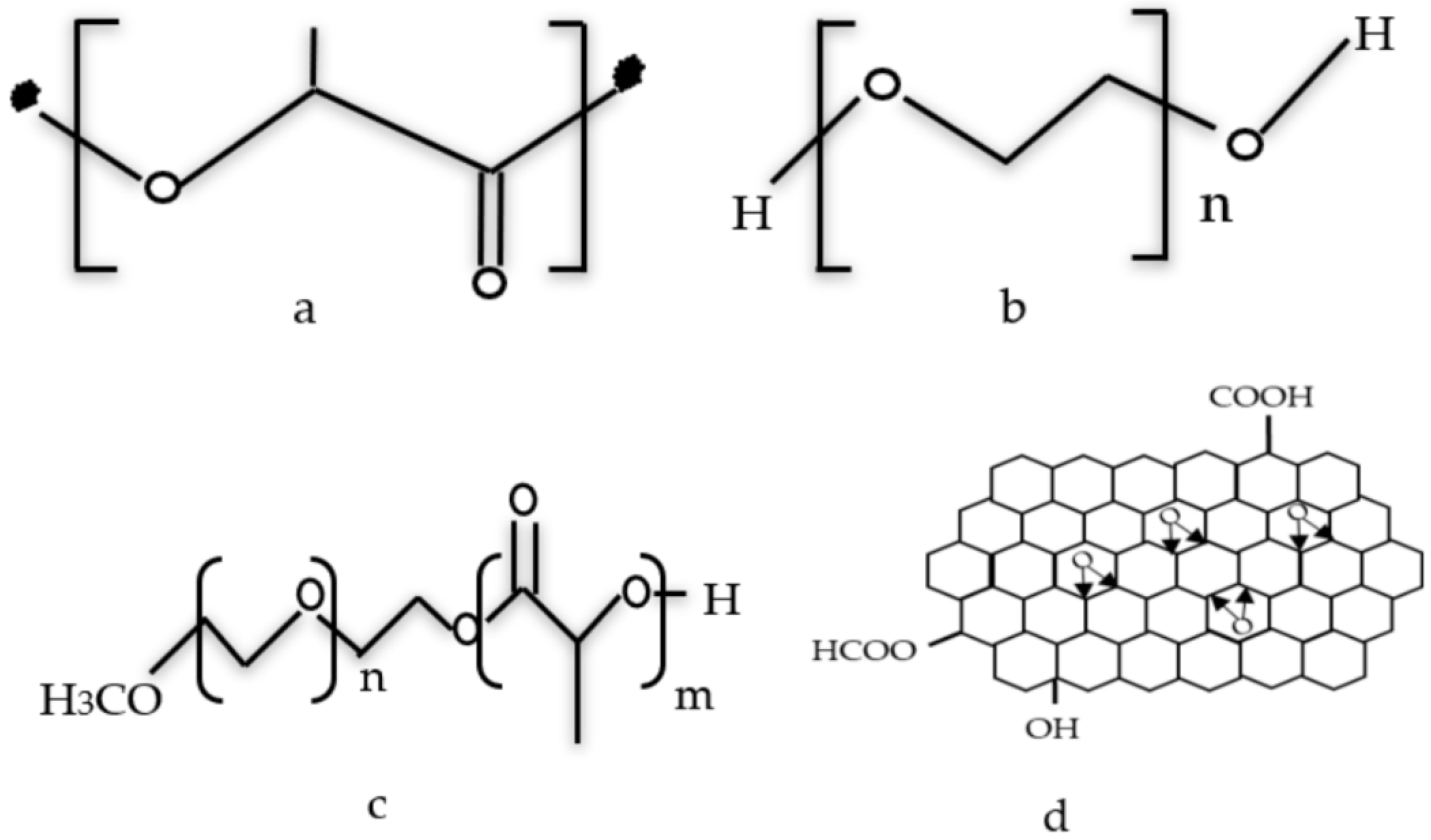
2.1. Materials
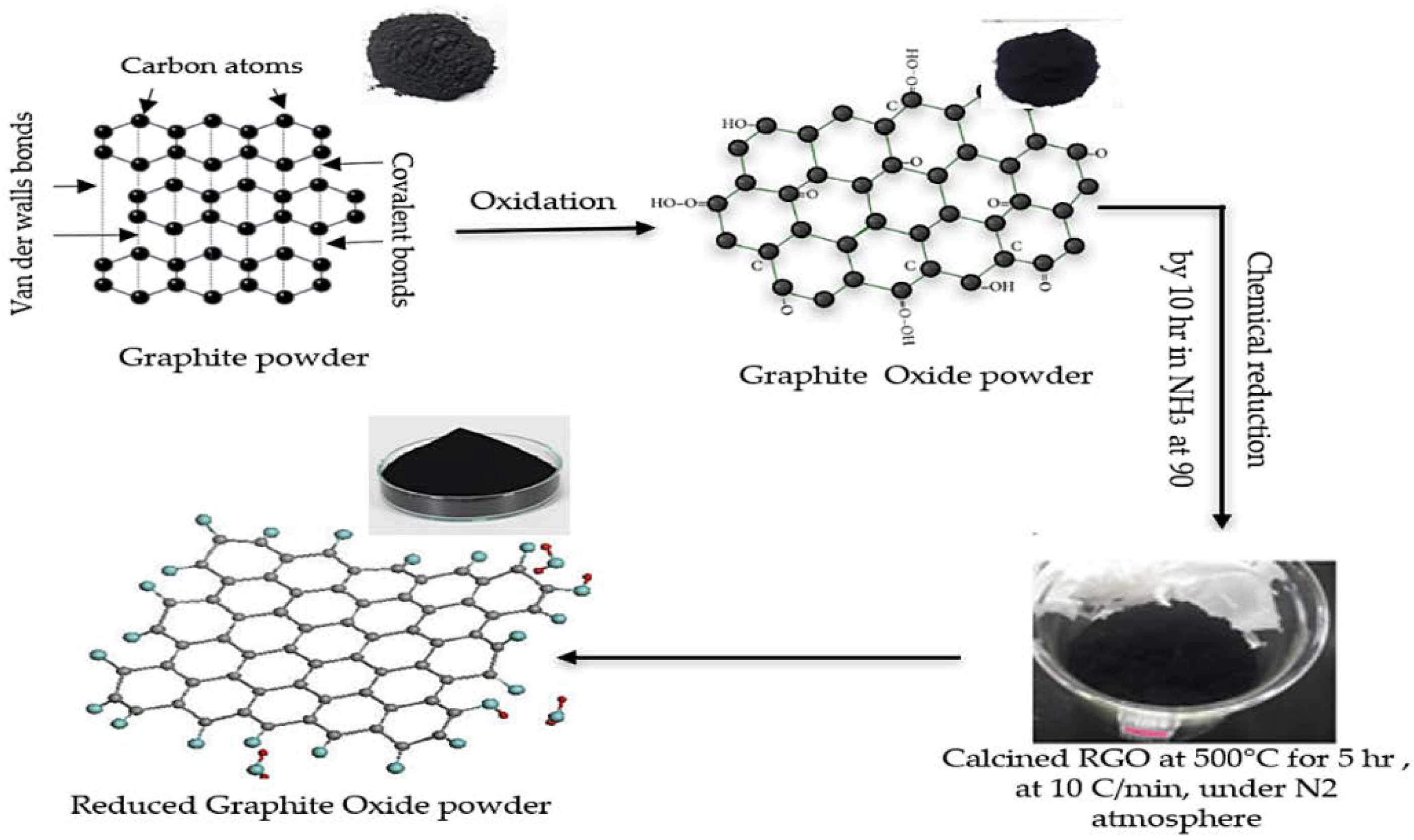
2.2. Preparation of Reduced Graphene Oxide (RGO)
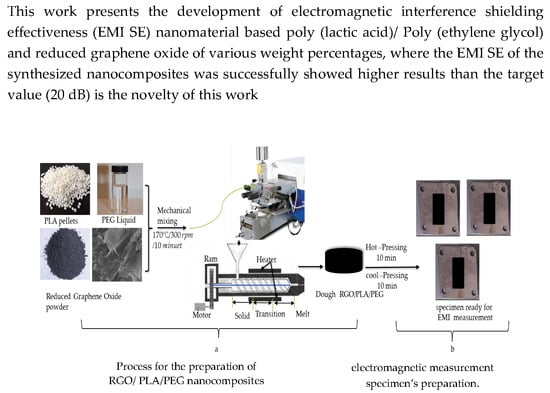
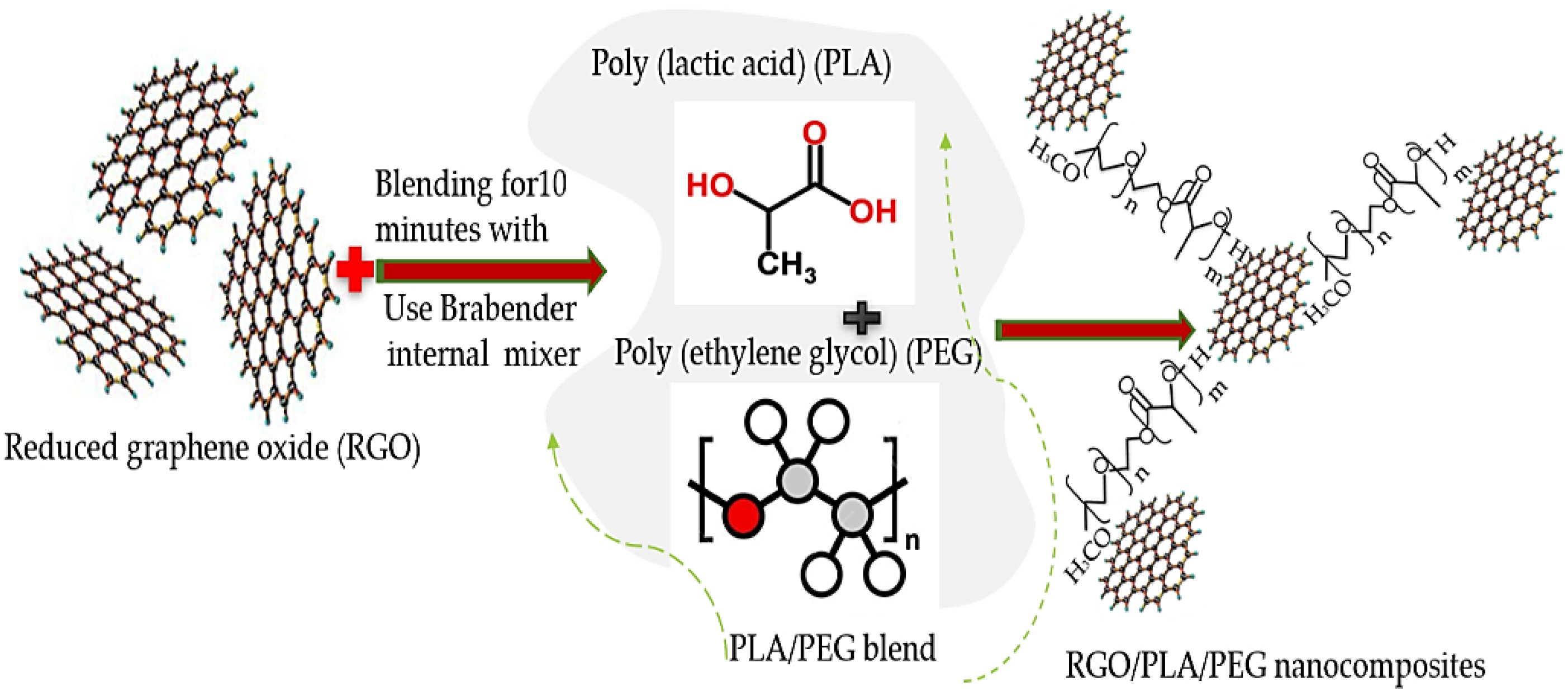
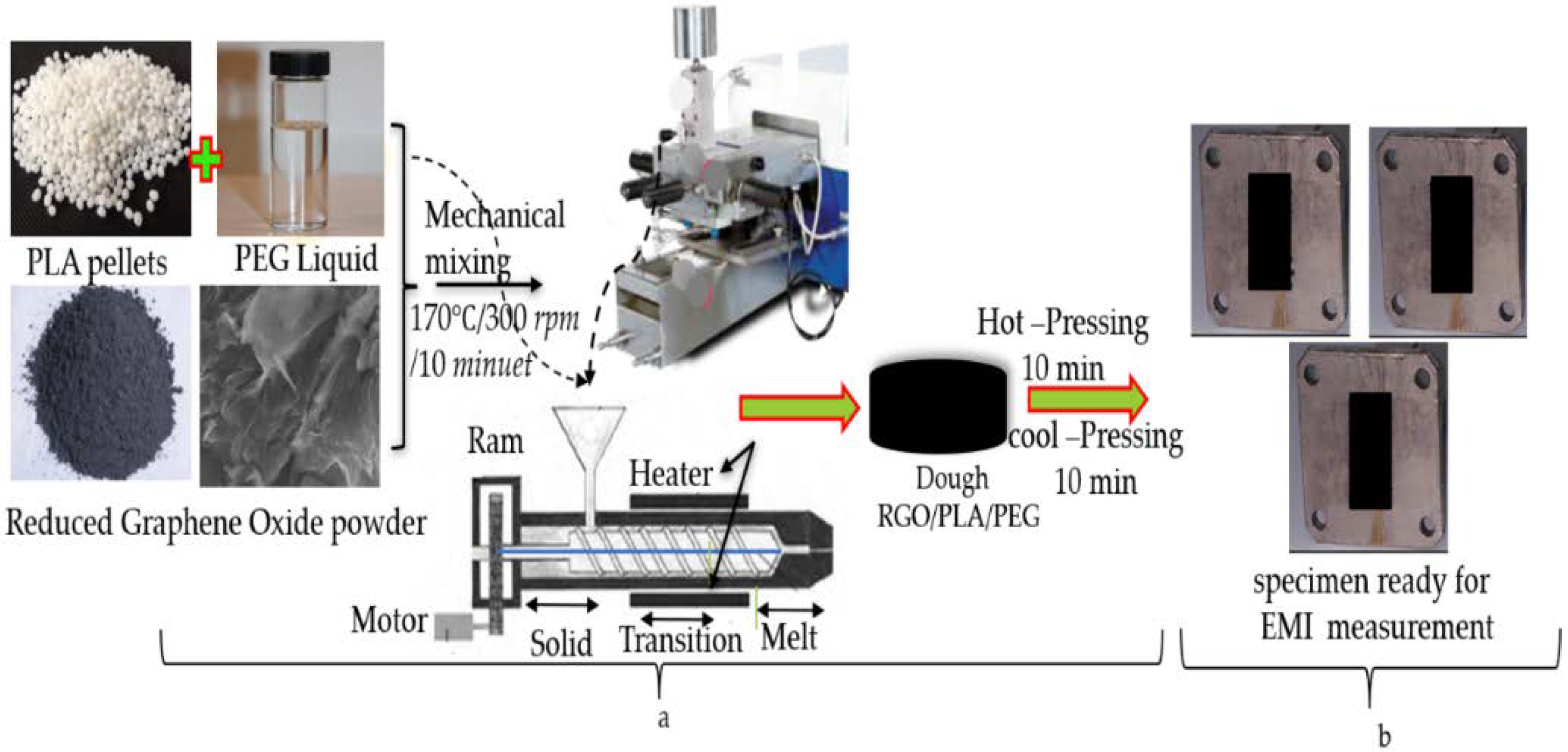
2.3. Preparation of RGO/PLA/PEG Nanocomposites
2.4. Characterisation
2.4.1. Morphological Characterization
2.4.2. X-Ray Diffraction
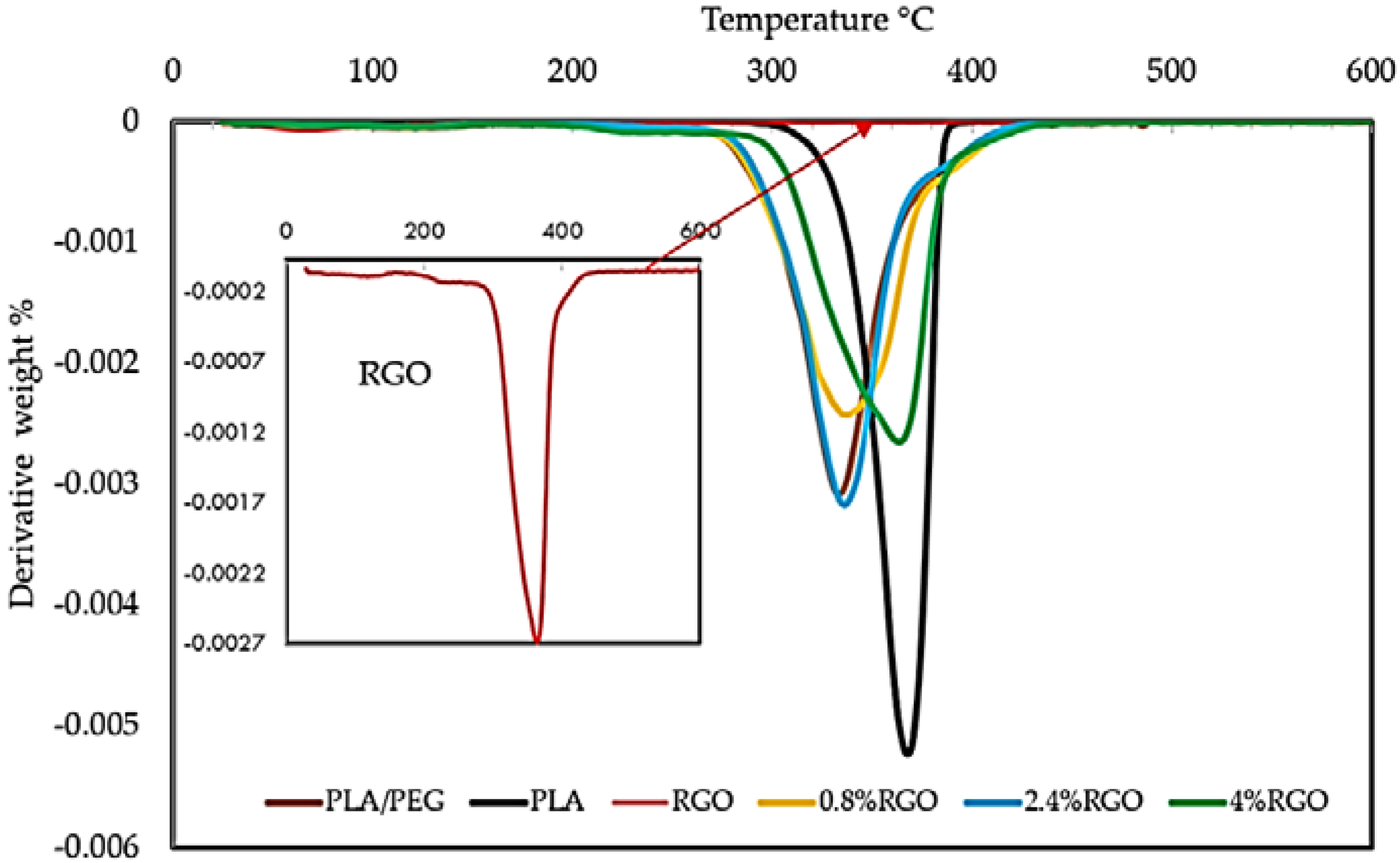
2.4.3. Thermal Stability (TGA and DTG) Properties
2.4.4. Fourier Transform Infrared (FT-IR) Analysis
2.4.5. Electromagnetic Interference (EMI) Properties
Electrical Properties
EMI Shielding Effectiveness (EMI SE)
3. Results and Discussion
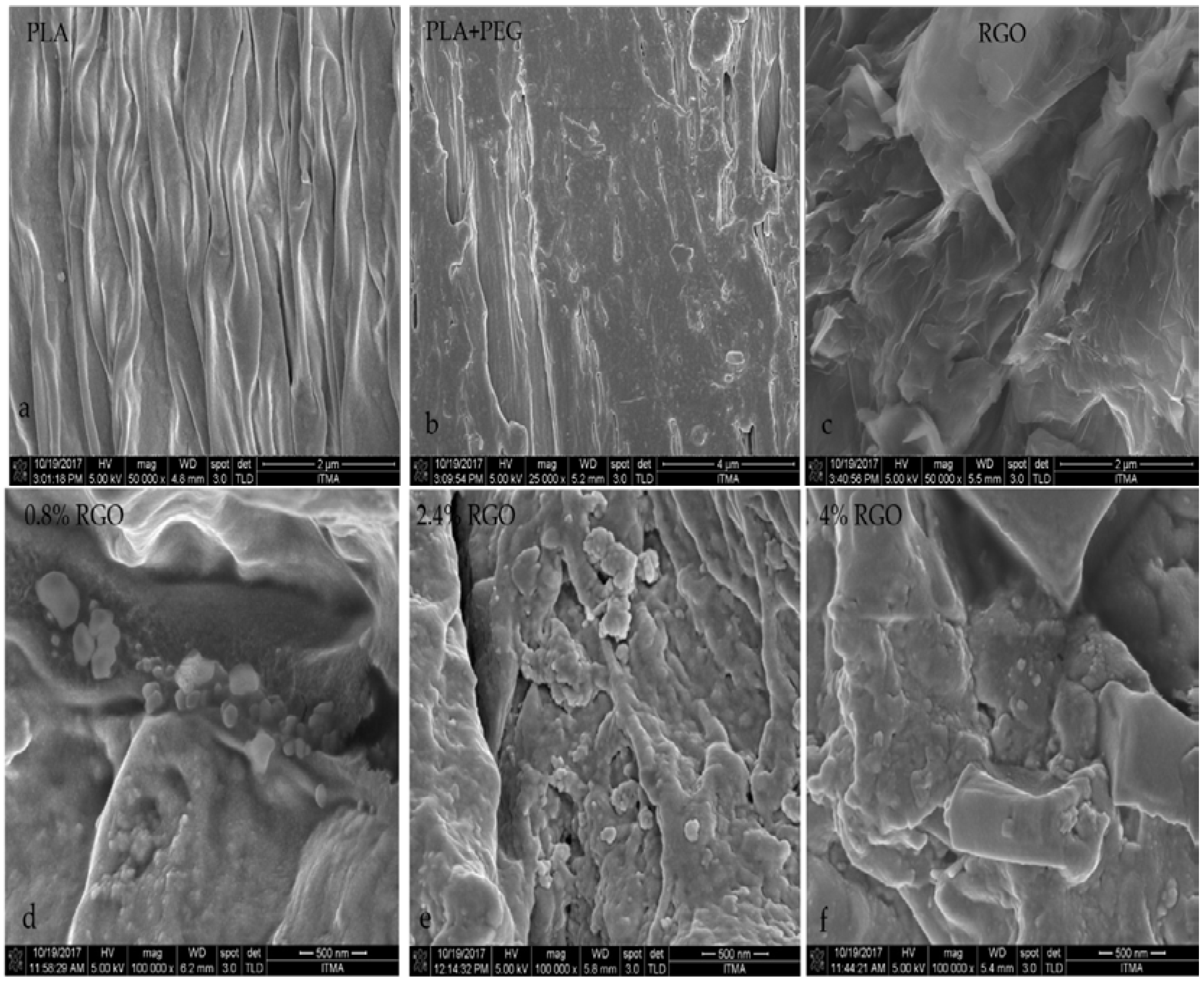
3.1. Field Emission-Scanning Electron Microscopy Results
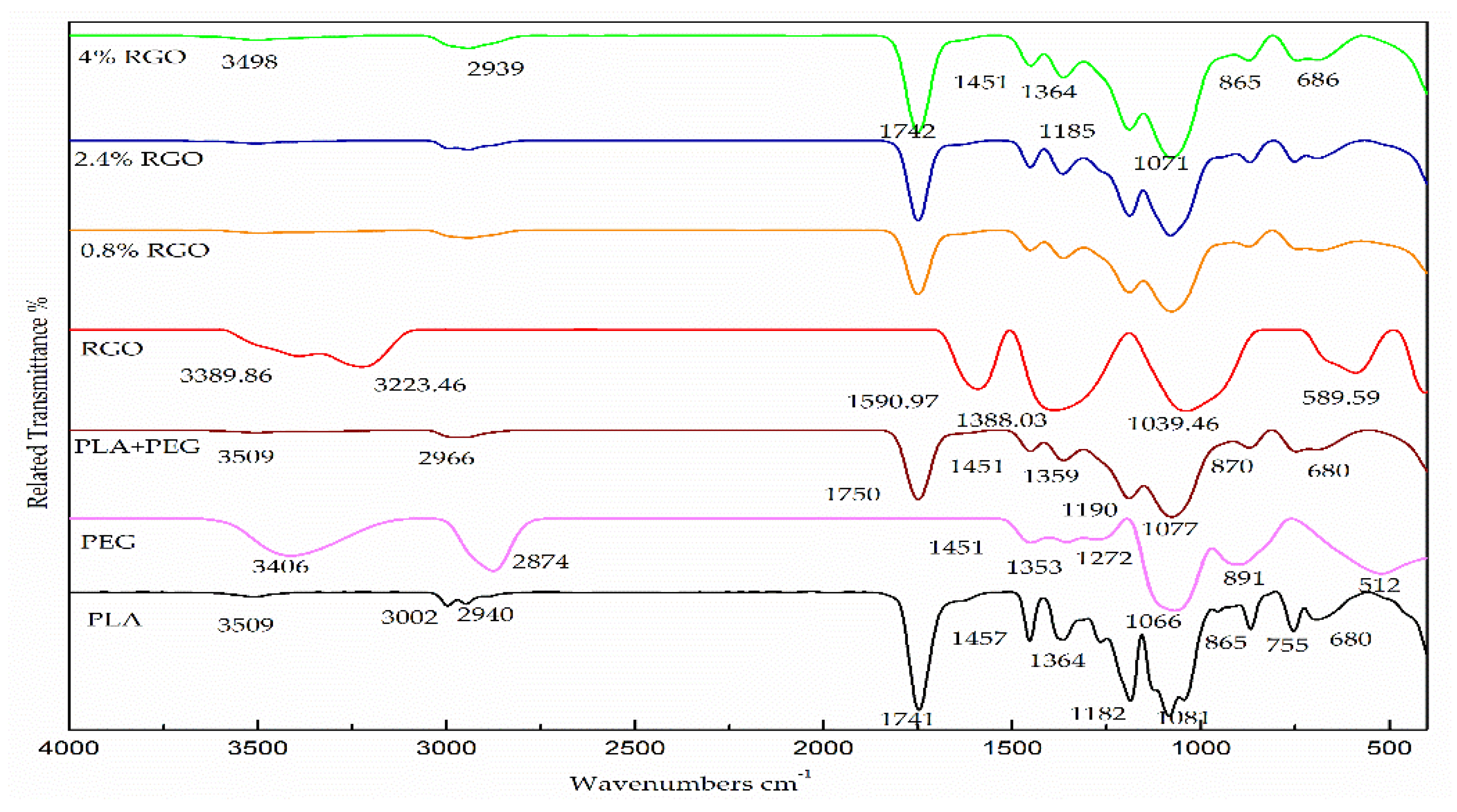
3.2. Fourier Transform Infrared (FT-IR) Analysis
3.3. X-Ray Diffraction
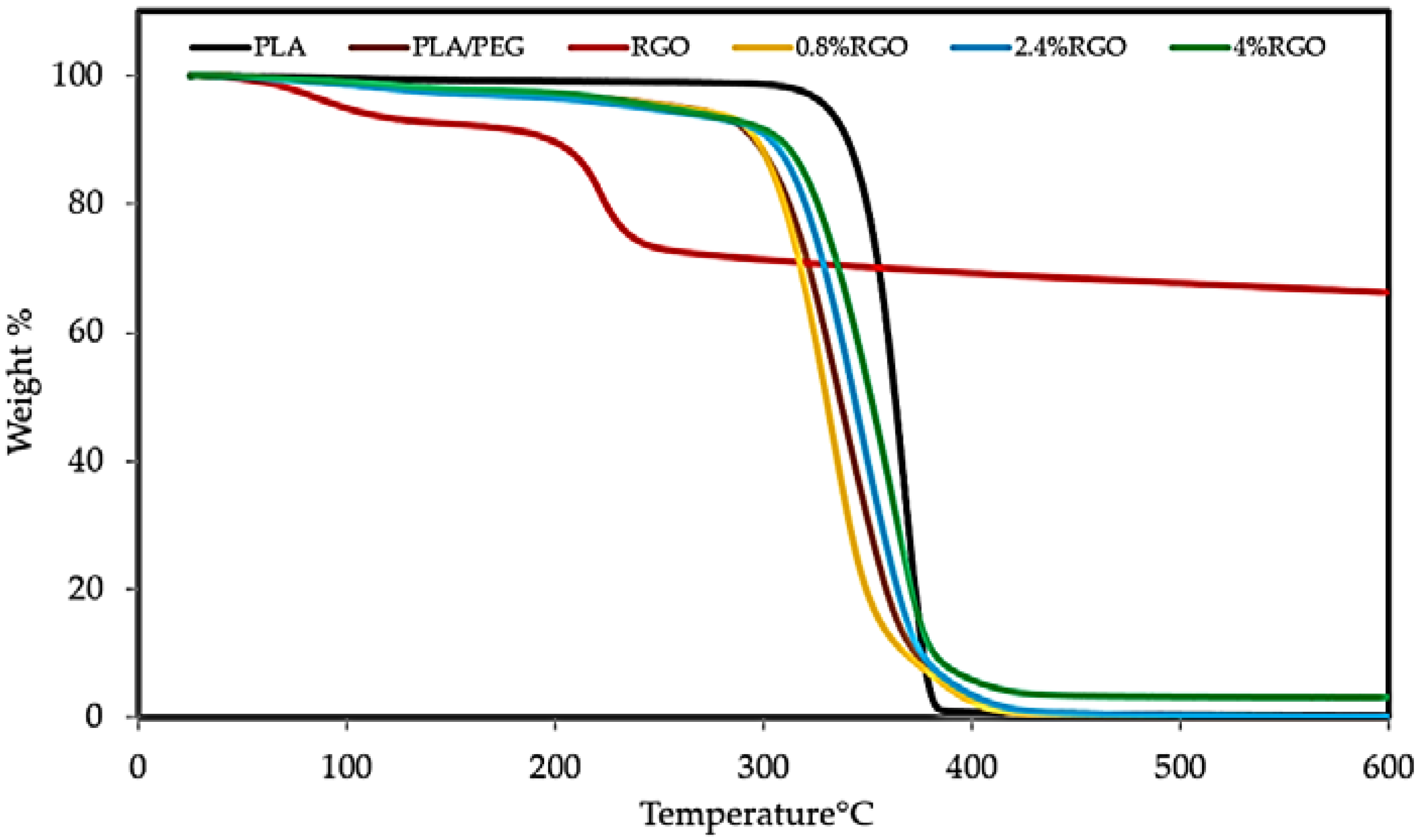
3.4. Thermogravimetric Analysis (TGA)
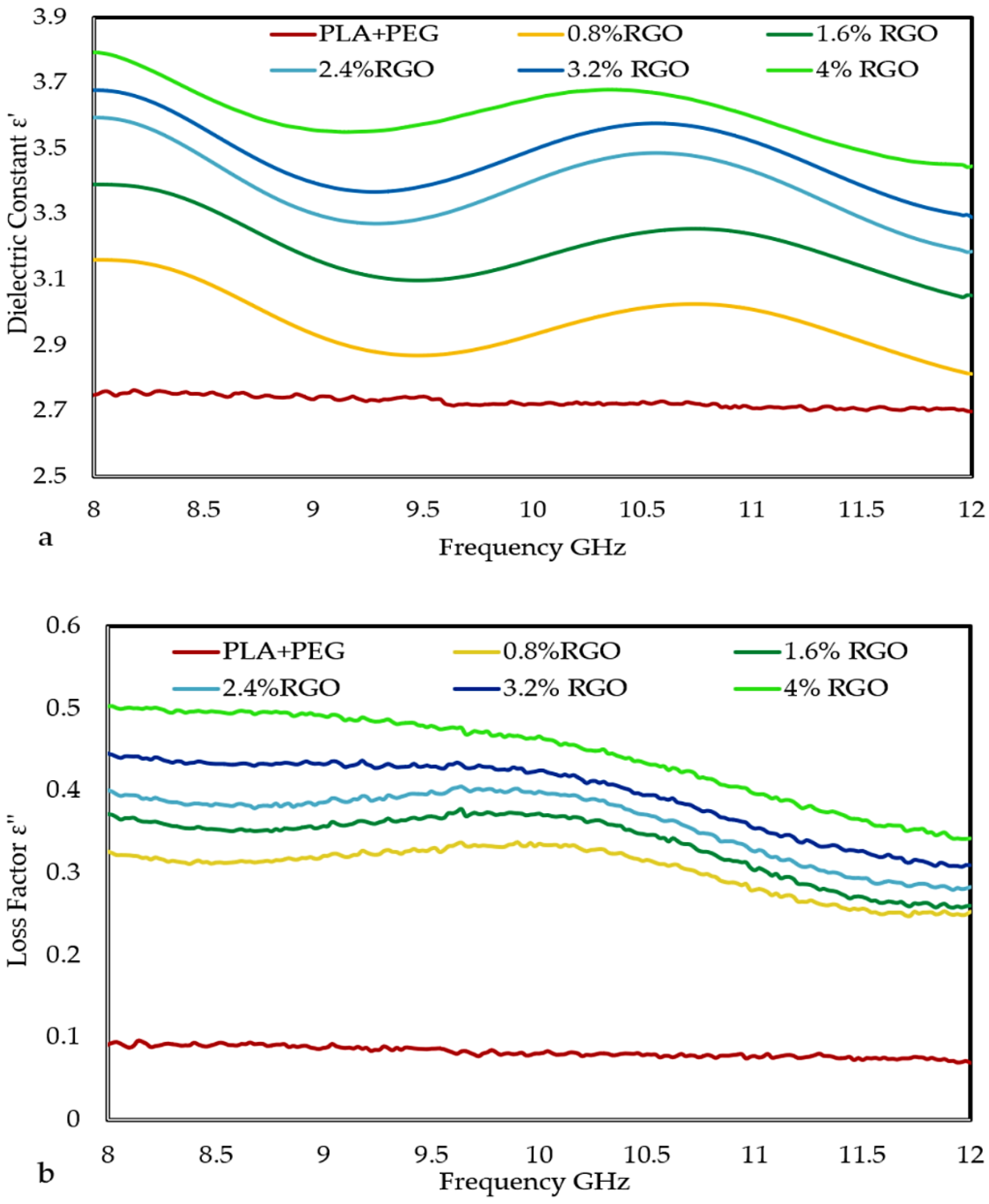
3.5. The Dielectric Properties of the Composites
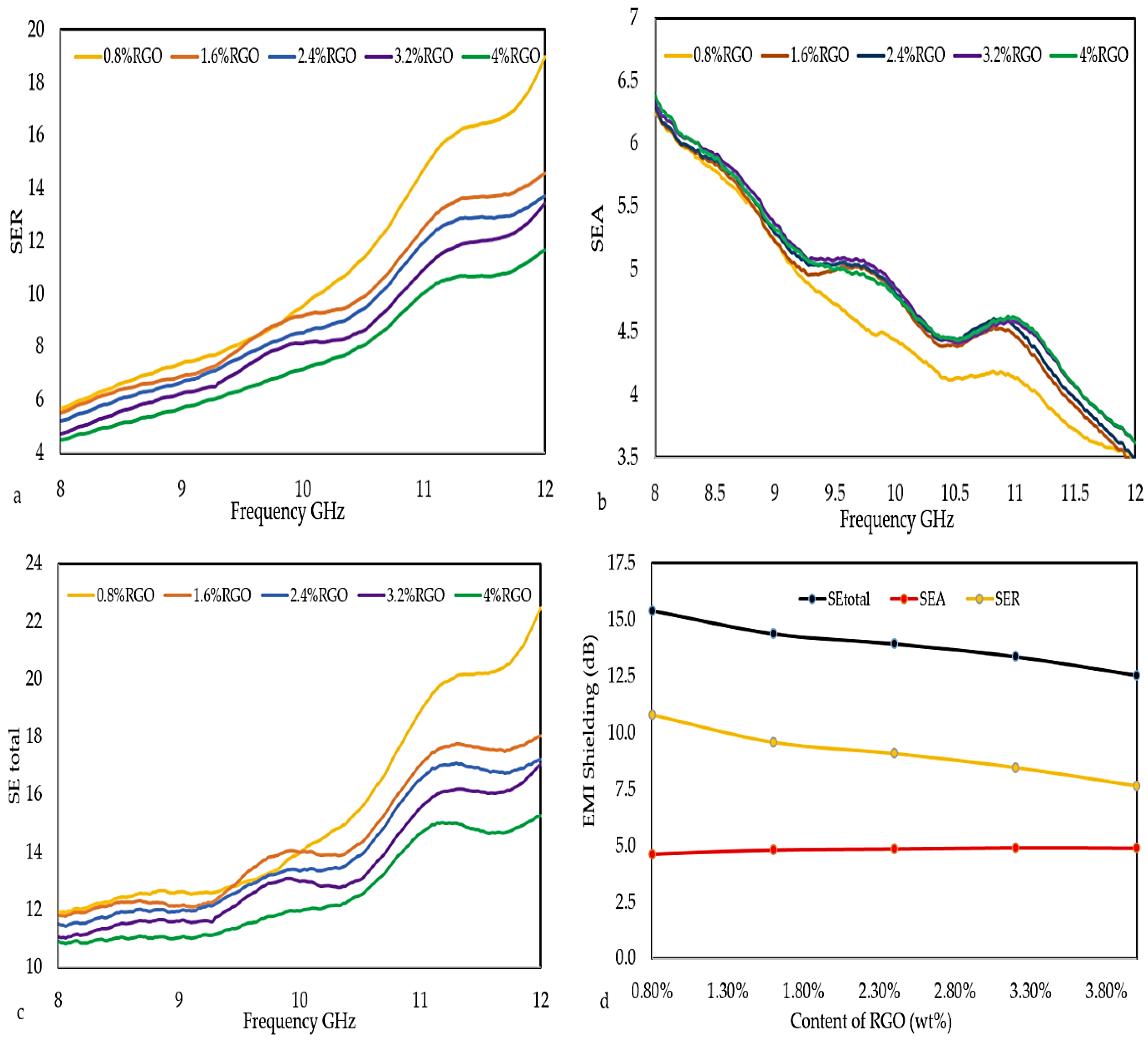
3.6. EMI Shielding Effectiveness
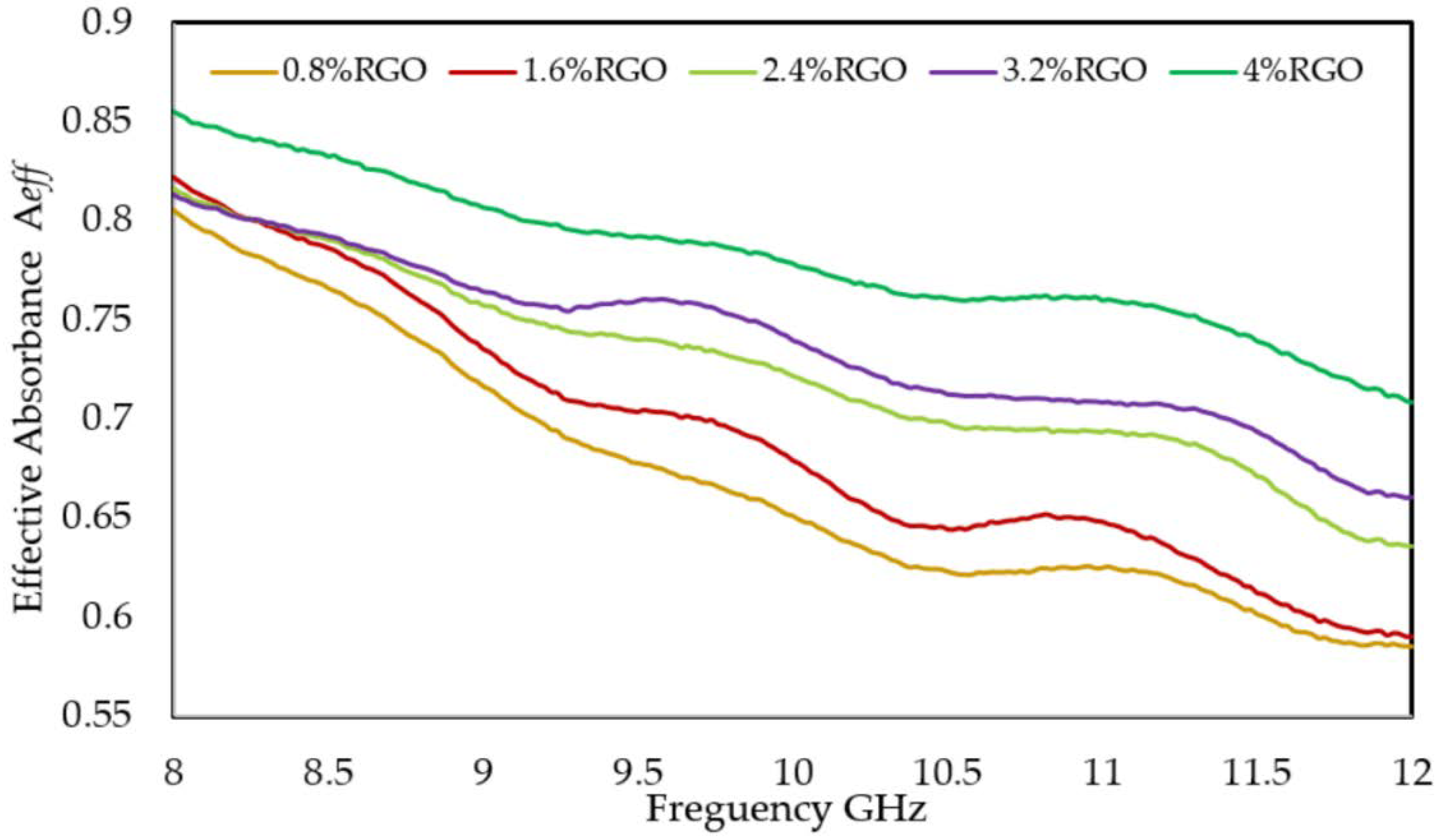
3.7. Power Loss and Affective Absorbance Aeff
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kashi, S.; Gupta, R.K.; Baum, T.; Kao, N.; Bhattacharya, S.N. Morphology, electromagnetic properties and electromagnetic interference shielding performance of Polylactide/graphene nanoplatelet nanocomposites. Mater. Des. 2016, 95, 119–126. [Google Scholar] [CrossRef]
- Ahmad, A.F.; Abbas, Z.; Obaiys, S.J.; Abdalhadi, D.M. Attenuation performance of polymer composites incorporating NZF Filler for electromagnetic interference shielding at microwave frequencies. J. Mater. Sci Eng. 2016, 5. [Google Scholar] [CrossRef]
- Thomassin, J.M.; Jerome, C.; Pardoen, T.; Bailly, C.; Huynen, I.; Detrembleur, C. Polymer/carbon-based composites as electromagnetic interference (EMI) shielding materials. Mater. Sci. Eng. R 2013, 74, 211–322. [Google Scholar] [CrossRef]
- Phang, S.W.; Tadokoro, M.; Watanabe, J.; Kuramoto, N. Synthesis, characterization and microwave absorption property of doped polyaniline nanocomposites containing TiO2 nanoparticles and carbon nanotubes. Synth. Met. 2008, 158, 251–258. [Google Scholar] [CrossRef]
- Yang, Y.; Gupta, M.C.; Dudley, K.L. Towards cost-efficient EMI shielding materials using carbon nanostructure-based nanocomposites. Nanotechnology 2007, 34, 345701. [Google Scholar] [CrossRef]
- Al-Saleh, M.H.; Saadeh, W.H.; Sundararaj, U. EMI shielding effectiveness of carbon based nanostructured polymeric materials: A comparative study. Carbon 2013, 60, 146–156. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Li, J.T.; Zhang, S.; Ni, Y.; Cai, S.; Huang, J. Enhanced dielectric constant for efficient electromagnetic shielding based on carbon-nanotube-added styrene acrylic emulsion-based composite. Nanoscale Res. 2010, 7, 1170. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, B.; Zhang, J. Properties of Poly(lactic acid)/Poly(butylene adipate-co-terephthalate)/nanoparticle ternary composites. Ind. Eng. Chem. Res. 2009, 48, 7594–7602. [Google Scholar] [CrossRef]
- Lin, S.; Guo, W.; Chen, C.; Ma, J.; Wang, B. Mechanical properties and morphology of biodegradable Poly(lactic acid)/Poly(butylene adipate-co-terephthalate) blends compatibilized by transesterification. Mater. Des. 2012, 36, 604–608. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Improvement of thermal stability, rheological and mechanical properties of PLA, PBAT and their blends by reactive extrusion with functionalized epoxy. Polym. Degrad. Stab. 2012, 97, 1898–1914. [Google Scholar] [CrossRef]
- Notta, C.D.; Odent, J.; Delille, R.; Murariu, M.; Lauro, F.; Raquez, J.M.; Bennani, B.; Dubois, P. Tailoring polylactide (PLA) properties for automotive applications: Effect of addition of designed additives on main mechanical properties. Polym. Test. 2014, 36, 1–9. [Google Scholar] [CrossRef]
- Silverajah, V.S.; Ibrahim, N.A.; Yunus, W.M.; Hassan, H.A.; Woei, C.B. A comparative study on the mechanical, thermal and morphological characterization of Poly(lactic acid)/epoxidized palm oil blend. Int. J. Mol. Sci. 2012, 13, 5878–5898. [Google Scholar] [CrossRef]
- Chiang, M.F.; Wu, T.M. Synthesis and characterization of biodegradable Poly(l-lactide)/layered double hydroxide nanocomposites. Compos. Sci. Technol. 2010, 70, 110–115. [Google Scholar] [CrossRef]
- Wu, C.S.; Liao, H.T. Study on the preparation and characterization of biodegradable polylactide/multi-walled carbon nanotubes nanocomposites. Polymer 2007, 48, 4449–4458. [Google Scholar] [CrossRef]
- Gumus, S.; Ozkoc, G.; Aytac, A. Plasticized and unplasticized PLA/organoclay nanocomposites: Short-and long-term thermal properties, morphology, and nonisothermal crystallization behavior. J. Appl. Polym. Sci. 2012, 123, 2837–2848. [Google Scholar] [CrossRef]
- Sungsanit, K.; Kao, N.; Bhattacharya, S.N. Properties of linear Poly(lactic acid)/polyethylene glycol blends. Polym. Eng. Sci. 2012, 52, 108–116. [Google Scholar] [CrossRef]
- Hassouna, F.; Raquez, J.M.; Addiego, F.; Dubois, P.; Toniazzo, V.; Ruch, D. New approach on the development of plasticized polylactide (PLA): Grafting of Poly(ethylene glycol) (PEG) via reactive extrusion. Eur. Polym. J. 2011, 47, 2134–2144. [Google Scholar] [CrossRef]
- Hu, Y.; Rogunova, M.; Topolkaraev, V.; Hiltner, A.; Baer, E. Aging of Poly(lactide)/Poly(ethylene glycol) blends. Part 1. Poly(lactide) with low stereoregularity. Polymer 2003, 44, 5701–5710. [Google Scholar] [CrossRef]
- Frackowiak, S.; Ludwiczak, J.; Leluk, K.; Orzechowski, K.; Kozlowski, M. Foamed Poly(lactic acid) composites with carbonaceous fillers for electromagnetic shielding. Mater. Des. 2015, 65, 749–756. [Google Scholar] [CrossRef]
- Tang, G.; Jiang, Z.G.; Li, X.; Zhang, H.B.; Dasari, A.; Yu, Z.Z. Three-dimensional graphene aerogels and their electrically conductive composites. Carbon 2014, 77, 592–609. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Z.; Lv, W.; Zhang, M.; Yang, S.; Yin, L.; Hong, J.; Han, D.; Chen, C.; Swarts, S.; et al. Interleukin 11 protects bone marrow mitochondria from radiation damage. Adv. Exp. Med. Biol. 2013, 789, 257–264. [Google Scholar]
- Krueger, Q.J.; King, J.A. Synergistic effects of carbon fillers on shielding effectiveness in conductive nylon 6, 6-and polycarbonate-based resins. Adv. Polym. Technol. J. Polym Process Inst. 2003, 22, 96–111. [Google Scholar] [CrossRef]
- Thomassin, J.M.; Vuluga, D.; Alexandre, M.; Jérôme, C.; Molenberg, I.; Huynen, I.; Detrembleur, C. A convenient route for the dispersion of carbon nanotubes in polymers: Application to the preparation of electromagnetic interference (EMI) absorbers. Polymer 2012, 53, 169–174. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, C.; Ma, C.; Ren, W.; Cheng, H.M. Lightweight and flexible graphene foam composites for high-performance electromagnetic interference shielding. Adv. Mater. 2013, 25, 1296–1300. [Google Scholar] [CrossRef]
- Vallés, C.; Abdelkader, A.M.; Young, R.J.; Kinloch, I.A. Few layers graphene–polypropylene nanocomposites: The role of flake diameter. Faraday Discuss. 2014, 173, 379–390. [Google Scholar] [CrossRef]
- Yao, H.; Hawkins, S.A.; Sue, H.J. Preparation of epoxy nanocomposites containing well-dispersed graphene nanosheets. Compos. Sci. Technol. 2017, 146, 161–168. [Google Scholar] [CrossRef]
- Berger, C.; Song, Z.; Li, X.; Wu, X.; Brown, N.; Naud, C.; Mayou, D.; Li, T.; Hass, J.; Marchenkov, A.N.; Conrad, E.H. Electronic confinement and coherence in patterned epitaxial graphene. Science 2006, 312, 1191–1196. [Google Scholar] [CrossRef]
- Kang, J.; Kim, D.; Kim, Y.; Choi, J.B.; Hong, B.H.; Kim, S.W. High-performance near-field electromagnetic wave attenuation in ultra-thin and transparent graphene films. 2D Materials 2017, 4, 025003. [Google Scholar] [CrossRef]
- Cai, W.; Moore, A.L.; Zhu, Y.; Li, X.; Chen, S.; Shi, L.; Ruoff, R.S. Thermal transport in suspended and supported monolayer graphene grown by chemical vapor deposition. Nano Lett. 2010, 10, 1645–1651. [Google Scholar] [CrossRef]
- Poulin, P.; SASIKALA, S.P.; Aymonier, C.; Centre National de la Recherche Scientifique CNRS. Method and System for Producing Polymer/Graphene Oxide Nanocomposites Reduced by in situ Reduction of Graphene Oxide. U.S. Patent 15/749,749, 25 October 2018. [Google Scholar]
- Xu, J.; Wang, Y.; Hu, S. Nanocomposites of graphene and graphene oxides: Synthesis, molecular functionalization and application in electrochemical sensors and biosensors. Mikrochim. Acta 2017, 184, 1–44. [Google Scholar] [CrossRef]
- Qiu, J.; Wang, D.; Geng, H.; Guo, J.; Qian, S.; Liu, X. How oxygen-containing groups on graphene influence the antibacterial behaviors. Adv. Mater. Interfaces 2017, 15, 1700228. [Google Scholar] [CrossRef]
- Wen, B.; Wang, X.X.; Cao, W.Q.; Shi, H.L.; Lu, M.M.; Wang, G.; Jin, H.B.; Wang, W.Z.; Yuan, J.; Cao, M.S. Reduced graphene oxides: The thinnest and most lightweight materials with highly efficient microwave attenuation performances of the carbon world. Nanoscale 2014, 11, 5754–5761. [Google Scholar] [CrossRef]
- Zhang, X.F.; Guan, P.F.; Dong, X.L. Multidielectric polarizations in the core/shell Co/graphite nanoparticles. Appl. Phys. Lett. 2010, 22, 223111. [Google Scholar] [CrossRef]
- Ahmad, A.; Sidek, A.A.; Abbas, Z.; Obaiys, S.; Khamis, A.; Hussain, I.; Zaid, M. Preparation of a chemically reduced graphene oxide reinforced epoxy resin polymer as a composite for electromagnetic interference shielding and microwave-absorbing applications. Polymers 2018, 10, 1180. [Google Scholar] [CrossRef]
- Gu, S.Y.; Zhang, K.; Ren, J.; Zhan, H. Melt rheology of polylactide/Poly(butylene adipate-co-terephthalate) blends. Carbohydr. Polym. 2008, 74, 79–85. [Google Scholar] [CrossRef]
- Fahad, A.A.; Abbas, Z.; Shaari, A.H.; Suzan, J.O.; Sa’ad, A.U. Synthesis, thermal, dielectric, and microwave reflection loss properties of nickel oxide filler with natural fiber-reinforced polymer composite. J. Appl. Polym. Sci. 2018, 46998. [Google Scholar] [CrossRef]
- Agilent Technology. Agilent 85701B Software Calibration Kit; Agilent Technology: Santa Clara, CA, USA, 2010. [Google Scholar]
- Ahmad, A.F.; Abbas, Z.; Ab, A.S.; Obaiys, S.J.; Zainuddin, M.F. Synthesis and characterisation of nickel oxide reinforced with polycaprolactone composite for dielectric applications by controlling nickel oxide as a filler. Results Phys. 2018, 11, 427–435. [Google Scholar] [CrossRef]
- Mondal, S.; Ganguly, S.; Rahaman, M.; Aldalbahi, A.; Chaki, T.K.; Khastgir, D.; Das, N.C. A strategy to achieve enhanced electromagnetic interference shielding at low concentration with a new generation of conductive carbon black in a chlorinated polyethylene elastomeric matrix. Phys. Chem. Chem. Phys. 2016, 35, 24591–24599. [Google Scholar] [CrossRef]
- Lyu, J.; Zhao, X.; Hou, X.; Zhang, Y.; Li, T.; Yan, Y. Electromagnetic interference shielding based on a high strength polyaniline-aramid nanocomposite. Compos. Sci. Technol. 2017, 149, 159–165. [Google Scholar] [CrossRef]
- Al-Saleh, M.H.; Sundararaj, U. X-band EMI shielding mechanisms and shielding effectiveness of high structure carbon black/polypropylene composites. J. Phys. D Appl. Phys. 2012, 46, 035304. [Google Scholar] [CrossRef]
- Wang, H.; Xiang, L.; Wei, W.; An, J.; He, J.; Gong, C.; Hou, Y. Efficient and lightweight electromagnetic wave absorber derived from metal organic framework-encapsulated cobalt nanoparticles. ACS Appl. Mater. Interfaces 2017, 48, 42102–42110. [Google Scholar] [CrossRef]
- Karteri, I.; Altun, M.; Gunes, M. Electromagnetic interference shielding performance and electromagnetic properties of wood-plastic nanocomposite with graphene nanoplatelets. J. Mater. Sci. Mater. Electron. 2017, 28, 6704–6711. [Google Scholar] [CrossRef]
- Awale, R.; Ali, F.; Azmi, A.; Puad, N.; Anuar, H.; Hassan, A. Enhanced Flexibility of Biodegradable Polylactic Acid/Starch Blends Using Epoxidized Palm Oil as Plasticizer. Polymers 2018, 10, 977. [Google Scholar] [CrossRef]
- Du, J.; Cheng, H.M. The fabrication, properties, and uses of graphene/polymer composites. Macromol. Chem. Phys. 2012, 213, 1060–1077. [Google Scholar] [CrossRef]
- Verma, M.; Singh, A.P.; Sambyal, P.; Singh, B.P.; Dhawan, S.K.; Choudhary, V. Barium ferrite decorated reduced graphene oxide nanocomposite for effective electromagnetic interference shielding. PCCP 2015, 17, 1610–1618. [Google Scholar] [CrossRef]
- Shameli, K.; Bin, A.M.; Jazayeri, S.D.; Sedaghat, S.; Shabanzadeh, P.; Jahangirian, H.; Mahdavi, M.; Abdollahi, Y. Synthesis and characterization of polyethylene glycol mediated silver nanoparticles by the green method. Int. J. Mol. Sci. 2012, 13, 6639–6650. [Google Scholar] [CrossRef]
- Kamatchi, R.; Venkatachalapathy, S.; Srinivas, B.A. Synthesis, stability, transport properties, and surface wettability of reduced graphene oxide/water nanofluids. Int. J. Therm. Sci 2015, 97, 17–25. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Hussein, M.Z. Poly(lactic acid)/Poly(ethylene glycol) polymer nanocomposites: Effects of graphene nanoplatelets. Polymers 2013, 6, 93–104. [Google Scholar] [CrossRef]
- Chhetri, S.; Samanta, P.; Murmu, N.C.; Srivastava, S.K.; Kuila, T. Electromagnetic interference shielding and thermal properties of non-covalently functionalized reduced graphene oxide/epoxy composites. AIMS Mater. Sci. 2016, 21, 61–74. [Google Scholar] [CrossRef]
- Bijarimi, M.; Ahmad, S.; Rasid, R.; Khushairi, M.A.; Zakir, M. Poly(lactic acid)/Poly(ethylene glycol) blends: Mechanical, thermal and morphological properties. AIP Conf. Proc. 2016, 21, 020002. [Google Scholar] [CrossRef]
- Daniels, P.H. A brief overview of theories of PVC plasticization and methods used to evaluate PVC-plasticizer interaction. J. Vinyl. Addit. Techn. 2009, 15, 219–223. [Google Scholar] [CrossRef]
- Kim, I.H.; Jeong, Y.G. Polylactide/exfoliated graphite nanocomposites with enhanced thermal stability, mechanical modulus, and electrical conductivity. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 850–858. [Google Scholar] [CrossRef]
- Wang, X.; Yang, H.; Song, L.; Hu, Y.; Xing, W.; Lu, H. Morphology, mechanical and thermal properties of graphene-reinforced Poly(butylene succinate) nanocomposites. Compos. Sci. Technol. 2011, 72, 1–6. [Google Scholar] [CrossRef]
- Phang, S.W.; Tadokoro, M.; Watanabe, J.; Kuramoto, N. Microwave absorption behaviors of polyaniline nanocomposites containing TiO2 nanoparticles. Curr. Appl. Phys. 2008, 8, 391–394. [Google Scholar] [CrossRef]
- Ameli, A.; Jung, P.U.; Park, C.B. Electrical properties and electromagnetic interference shielding effectiveness of polypropylene/carbon fiber composite foams. Carbon 2013, 60, 379–391. [Google Scholar] [CrossRef]
- Liang, J.; Wang, Y.; Huang, Y.; Ma, Y.; Liu, Z.; Cai, J.; Zhang, C.; Gao, H.; Chen, Y. Electromagnetic interference shielding of graphene/epoxy composites. Carbon 2009, 47, 922–925. [Google Scholar] [CrossRef]
- Li, C.; Yang, G.; Deng, H.; Wang, K.; Zhang, Q.; Chen, F.; Fu, Q. The preparation and properties of polystyrene/functionalized graphene nanocomposite foams using supercritical carbon dioxide. Polym. Int. 2013, 62, 1077–1084. [Google Scholar] [CrossRef]
- Huang, Y.; Li, N.; Ma, Y.; Du, F.; Li, F.; He, X.; Lin, X.; Gao, H.; Chen, Y. The influence of single-walled carbon nanotube structure on the electromagnetic interference shielding efficiency of its epoxy composites. Carbon 2007, 45, 1614–1621. [Google Scholar] [CrossRef]
- Yang, Y.; Gupta, M.C.; Dudley, K.L.; Lawrence, R.W. A comparative study of EMI shielding properties of carbon nanofiber and multi-walled carbon nanotube filled polymer composites. J. Nanosci. Nanotechnol. 2005, 5, 927–931. [Google Scholar] [CrossRef]
- Mondal, S.; Nayak, L.; Rahaman, M.; Aldalbahi, A.; Chaki, T.K.; Khastgir, D.; Das, N.C. An effective strategy to enhance mechanical, electrical, and electromagnetic shielding effectiveness of chlorinated polyethylene-carbon nanofiber nanocomposites. Compos. Part. B 2017, 15, 155–169. [Google Scholar] [CrossRef]
- Rohini, R.; Bose, S. Extraordinary Improvement in Mechanical Properties and Absorption-Driven Micro-wave Shielding through Epoxy-Grafted Graphene “Interconnects”. ACS Omega. 2018, 3, 3200–3210. [Google Scholar] [CrossRef]
- Zhan, Y.; Wang, J.; Zhang, K.; Li, Y.; Meng, Y.; Yan, N.; Wei, W.; Peng, F.; Xia, H. Fabrication of a flexible electromagnetic interference shielding Fe3O4@reduced graphene oxide/natural rubber composite with segregated network. Chem. Eng. J. 2018, 344, 184–193. [Google Scholar] [CrossRef]
- Al-Saleh, M.H.; Sundararaj, U. Electromagnetic interference shielding mechanisms of CNT/polymer composites. Carbon 2009, 47, 1738–1746. [Google Scholar] [CrossRef]
- Choudary, V.; Dhawan, S.K.; Saini, P. Polymer based nanocomposites for electromagnetic interference (EMI) shielding. In EMI Shielding Theory and Development of New Materials; Research Signpost: Kerala, India, 2012. [Google Scholar]



| Sample | Weight of PLA | Weight of PEG | Filler Contents of RGO | Mass (g) RGO/PLA/PEG | |||
|---|---|---|---|---|---|---|---|
| RGO/PLA/PEG | g | % | g | % | g | % | 25 gm |
| 22.5 | 90.00 | 2.5 | 10 | 0 | 0 | ||
| 22.32 | 89.28 | 2.48 | 9.92 | 0.2 | 0.8 | ||
| 22.14 | 88.56 | 2.46 | 9.84 | 0.4 | 1.6 | ||
| 21.96 | 87.84 | 2.44 | 9.76 | 0.6 | 2.4 | ||
| 21.78 | 87.12 | 2.42 | 9.68 | 0.8 | 3.2 | ||
| 21.6 | 86.4 | 2.4 | 9.60 | 1 | 4 | ||
| Samples | T50% °C | Td-max °C | Loss Weight (%) Td-mix |
|---|---|---|---|
| PLA | 365 | 387.33 | 97 |
| PLA/PEG | 335 | 372.0 | 95 |
| RGO | 227 | 241.83 | 27 |
| 0.8% RGO | 330 | 359.12 | 92 |
| 2.4% RGO | 340 | 378.167 | 90 |
| 4% RGO | 350 | 386 | 87 |
| Percentage of RGO Filler | SEtotal | SEA | SER |
|---|---|---|---|
| 0.8 | 15.41 | 4.62 | 10.78 |
| 1.6 | 14.38 | 4.81 | 9.57 |
| 2.4 | 13.94 | 4.85 | 9.08 |
| 3.2 | 13.37 | 4.90 | 8.47 |
| 4 | 12.55 | 4.89 | 7.66 |
| Composites | Filler Content | Mixing Procedure | Thickness (mm) | Frequency (GHz) | EMI SEtotal (dB) | Reference |
|---|---|---|---|---|---|---|
| Epoxy/reduced graphene oxide | 15 wt % | Solution | 2 | 8.2–12.4 | 21 | [57] |
| Polystyrene/reduced graphene oxide | 10 wt % | Solution | 2.8 | 8.2–12.4 | 18 | [58] |
| Epoxy/Single walled carbon nanotube | 15 wt% | Solution | 2 | 8.2–12.4 | 23 | [59] |
| Polystyrene/Vapor grown carbon nanofiber | 7 wt % | Solution | 1 | 10 | 8 | [60] |
| Multi walled carbon nanotube/Cellulose Triacetate | 40 wt % | Solution | 6 | 8.2–12.4 | 30 | [61] |
| Carbon paste electrode/carbon nanofibers | 10 wt % | Melt | 1 | 8.2–12.4 | 22.5 | [62] |
| Reduced graphene oxide/cured epoxy | 5% wt % | Solution | 6 | 8.2–12.4 | 25.748 | [35] |
| Sulfanilic acid azocromotrop/reduced graphene oxide/epoxy | 0.5 wt % | Solution | ------- | 2–8 | 22.6 | [63] |
| Reduced graphene oxide/poly(lactic acid)/poly(ethylene glycol) | 0.8 wt % | Melt | 6 | 8.2–12.4 | 22.5 | Current work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, A.F.; Aziz, S.A.; Abbas, Z.; Obaiys, S.J.; Matori, K.A.; Zaid, M.H.M.; Raad, H.K.; Aliyu, U.S. Chemically Reduced Graphene Oxide-Reinforced Poly(Lactic Acid)/Poly(Ethylene Glycol) Nanocomposites: Preparation, Characterization, and Applications in Electromagnetic Interference Shielding. Polymers 2019, 11, 661. https://doi.org/10.3390/polym11040661
Ahmad AF, Aziz SA, Abbas Z, Obaiys SJ, Matori KA, Zaid MHM, Raad HK, Aliyu US. Chemically Reduced Graphene Oxide-Reinforced Poly(Lactic Acid)/Poly(Ethylene Glycol) Nanocomposites: Preparation, Characterization, and Applications in Electromagnetic Interference Shielding. Polymers. 2019; 11(4):661. https://doi.org/10.3390/polym11040661
Chicago/Turabian StyleAhmad, Ahmad Fahad, Sidek Ab Aziz, Zulkifly Abbas, Suzan Jabbar Obaiys, Khamirul Amin Matori, Mohd Hafiz Mohd Zaid, Haider K. Raad, and Umar Sa’ad Aliyu. 2019. "Chemically Reduced Graphene Oxide-Reinforced Poly(Lactic Acid)/Poly(Ethylene Glycol) Nanocomposites: Preparation, Characterization, and Applications in Electromagnetic Interference Shielding" Polymers 11, no. 4: 661. https://doi.org/10.3390/polym11040661
APA StyleAhmad, A. F., Aziz, S. A., Abbas, Z., Obaiys, S. J., Matori, K. A., Zaid, M. H. M., Raad, H. K., & Aliyu, U. S. (2019). Chemically Reduced Graphene Oxide-Reinforced Poly(Lactic Acid)/Poly(Ethylene Glycol) Nanocomposites: Preparation, Characterization, and Applications in Electromagnetic Interference Shielding. Polymers, 11(4), 661. https://doi.org/10.3390/polym11040661