Degradation and Recycling of Films Based on Biodegradable Polymers: A Short Review
Abstract
:1. Introduction
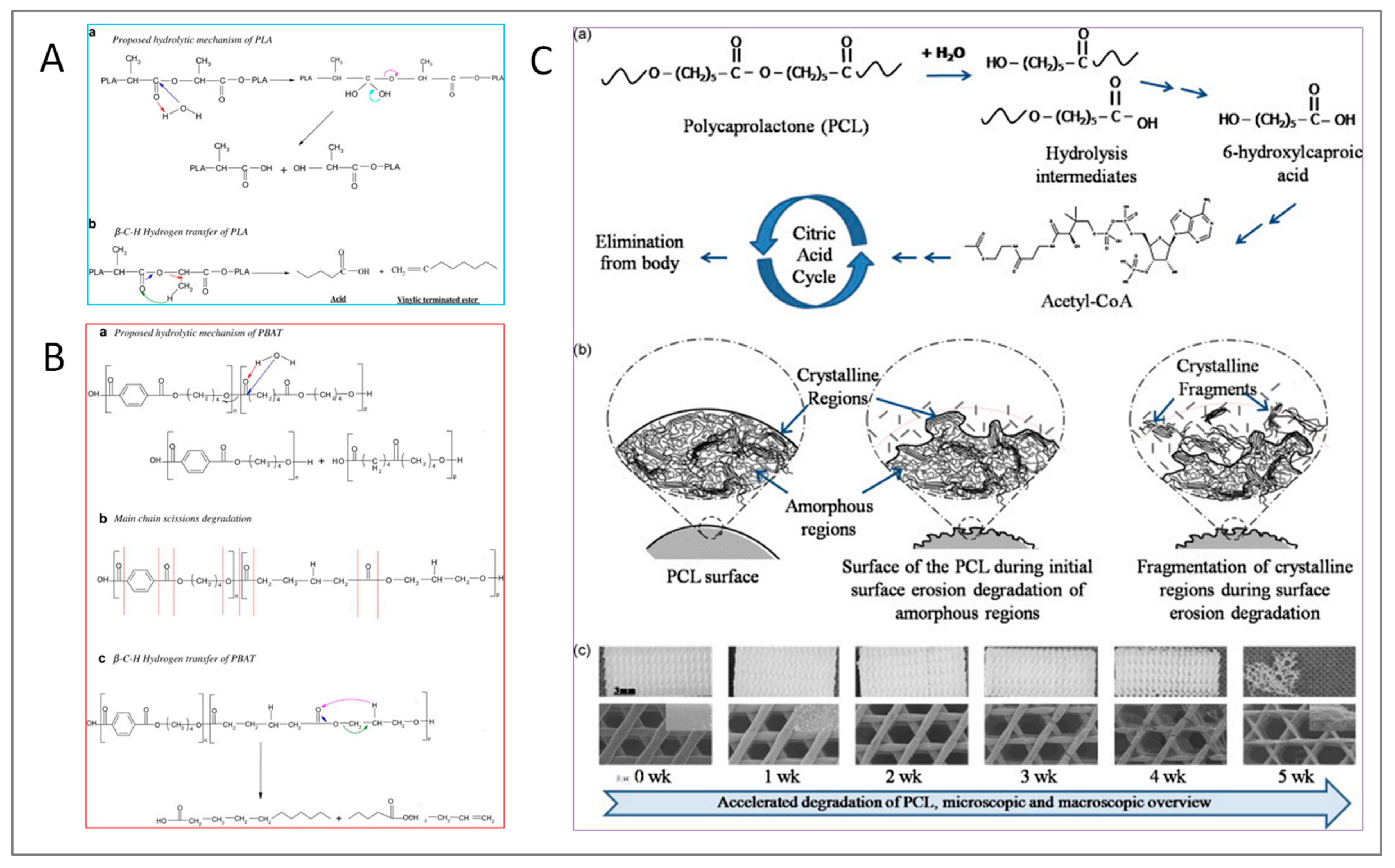
2. Degradation of Biodegradable Polymer-Based Systems
3. Recycling of Systems Based on Biodegradable Polymers
4. Processing of Films Based on Biodegradable Polymers
5. Properties of Biodegradable Polymer-Based Multilayer Films
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Scaffaro, R.; Maio, A. Optimization of two-step techniques engineered for the preparation of polyamide 6 graphene oxide nanocomposites. Compos. Part B Eng. 2019, 165, 55–64. [Google Scholar] [CrossRef]
- Edlund, U.; Lagerberg, T.; Ålander, E. Admicellar polymerization coating of CNF enhances integration in degradable nanocomposites. Biomacromolecules 2019, 20, 684–692. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Dintcheva, N.T.; Scaffaro, R.; Marino, R. Morphology and properties of polyethylene/clay nanocomposite drawn fibers. Macromol. Mater. Eng. 2008, 293, 83–91. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Marino, R.; Dintcheva, N.T. Morphology modification of polyethylene/clay nanocomposite samples under convergent flow. Macromol. Mater. Eng. 2009, 294, 575–581. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Arrigo, R.; Morreale, M. Effect of the orientation and rheological behaviour of biodegradable polymer nanocomposites. Eur. Polym. J. 2014, 54, 11–17. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Marino, R.; La Mantia, F.P. The role of the matrix-filler affinity on morphology and properties of polyethylene/clay and polyethylene/compatibilizer/clay nanocomposites drawn fibers. e-Polymers 2009, 9. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Burgos, N.; Peltzer, M.A.; López, J.; Peponi, L. Nanocellulose-Based Polymeric Blends for Food Packaging Applications. In Multifunctional Polymeric Nanocomposites Based on Cellulosic Reinforcements; Elsevier: Amsterdam, The Netherlands, 2016; pp. 205–252. [Google Scholar]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Kale, G.; Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S.E.; Singh, S.P. Compostability of bioplastic packaging materials: An overview. Macromol. Biosci. 2007, 7, 255–277. [Google Scholar] [CrossRef] [PubMed]
- Scaffaro, R.; Lopresti, F.; Maio, A.; Botta, L.; Rigogliuso, S.; Ghersi, G. Electrospun PCL/GO-g-PEG structures: Processing-morphology-properties relationships. Compos. Part A Appl. Sci. Manuf. 2017, 92, 97–107. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Lopresti, F. Physical properties of green composites based on poly-lactic acid or Mater-Bi® filled with Posidonia Oceanica leaves. Compos. Part A Appl. Sci. Manuf. 2018, 112, 315–327. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Gulino, E.F.; Megna, B. Structure-property relationship of PLA-Opuntia Ficus Indica biocomposites. Compos. Part B Eng. 2019, 167, 199–206. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Botta, L.; Maio, A. Mechanical behavior of polylactic acid/polycaprolactone porous layered functional composites. Compos. Part B Eng. 2016, 98, 70–77. [Google Scholar] [CrossRef]
- Scaffaro, R.; Botta, L.; Gallo, G. Photo-oxidative degradation of poly (ethylene-co-vinyl acetate)/nisin antimicrobial films. Polym. Degrad. Stab. 2012, 97, 653–660. [Google Scholar] [CrossRef]
- Ferreira, A.R.V.; Torres, C.A.V.; Freitas, F.; Sevrin, C.; Grandfils, C.; Reis, M.A.M.; Alves, V.D.; Coelhoso, I.M. Development and characterization of bilayer films of FucoPol and chitosan. Carbohydr. Polym. 2016, 147, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.; Heising, J.K.; Yuan, Y.; Karahan, H.E.; Wei, L.; Zhai, S.; Koh, J.X.; Htin, N.M.; Zhang, F.; Wang, R.; et al. Sandwich-architectured poly(lactic acid)-graphene composite food packaging films. ACS Appl. Mater. Interfaces 2016, 8, 9994–10004. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Fabra, M.J.; Castro-Mayorga, J.L.; Bourbon, A.I.; Pastrana, L.M.; Vicente, A.A.; Lagaron, J.M. Use of electrospinning to develop antimicrobial biodegradable multilayer systems: Encapsulation of cinnamaldehyde and their physicochemical characterization. Food Bioprocess Technol. 2016, 9, 1874–1884. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M. Development of expert system for biobased polymer material selection: Food packaging application. J. Food Sci. Technol. 2015, 52, 6445–6454. [Google Scholar] [CrossRef] [PubMed]
- Scaffaro, R.; Sutera, F.; Mistretta, M.C.; Botta, L.; Mantia, F.P. La Structure-properties relationships in melt reprocessed PLA/hydrotalcites nanocomposites. Express Polym. Lett. 2017, 11, 555–564. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Maio, A.; Sutera, F.; Botta, L. Development of polymeric functionally graded scaffolds: A brief review. J. Appl. Biomater. Funct. Mater. 2017, 15, 107–121. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Botta, L.; Maio, A.; Sutera, F.; Mistretta, M.C.; La Mantia, F.P. A facile and eco-friendly route to fabricate poly(lactic acid) scaffolds with graded pore size. J. Vis. Exp. 2016, 2016. [Google Scholar] [CrossRef]
- Gupta, A.; Simmons, W.; Schueneman, G.T.; Hylton, D.; Mintz, E.A. Rheological and thermo-mechanical properties of poly (lactic acid)/lignin-coated cellulose nanocrystal composites. ACS Sustain. Chem. Eng. 2017, 5, 1711–1720. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A. Integrated ternary bionanocomposites with superior mechanical performance via the synergistic role of graphene and plasma treated carbon nanotubes. Compos. Part B Eng. 2019, 168, 550–559. [Google Scholar] [CrossRef]
- Khalid, S.; Yu, L.; Feng, M.; Meng, L.; Bai, Y.; Ali, A.; Liu, H.; Chen, L. Development and characterization of biodegradable antimicrobial packaging films based on polycaprolactone, starch and pomegranate rind hybrids. Food Packag. Shelf Life 2018, 18, 71–79. [Google Scholar] [CrossRef]
- Xing, Q.; Buono, P.; Ruch, D.; Dubois, P.; Wu, L.; Wang, W.-J. Biodegradable UV-blocking films through core--shell lignin--melanin nanoparticles in poly (butylene adipate-co-terephthalate). ACS Sustain. Chem. Eng. 2019, 7, 4147–4157. [Google Scholar] [CrossRef]
- Witt, U.; Einig, T.; Yamamoto, M.; Kleeberg, I.; Deckwer, W.-D.; Müller, R.-J. Biodegradation of aliphatic--aromatic copolyesters: Evaluation of the final biodegradability and ecotoxicological impact of degradation intermediates. Chemosphere 2001, 44, 289–299. [Google Scholar] [CrossRef]
- Re, G.L.; Morreale, M.; Scaffaro, R.; La Mantia, F.P. Biodegradation paths of Mater-Bi®/kenaf biodegradable composites. J. Appl. Polym. Sci. 2013, 129, 3198–3208. [Google Scholar] [CrossRef]
- Scaffaro, R.; Morreale, M.; Lo Re, G.; La Mantia, F.P. Degradation of Mater-Bi®/wood flour biocomposites in active sewage sludge. Polym. Degrad. Stab. 2009, 94, 1220–1229. [Google Scholar] [CrossRef]
- Lo Re, G.; Morreale, M.; Scaffaro, R.; La Mantia, F.P. Kenaf-filled biodegradable composites: Rheological and mechanical behaviour. Polym. Int. 2012, 61, 1542–1548. [Google Scholar] [CrossRef]
- Alvarez, V.A.; Vázquez, A. Thermal degradation of cellulose derivatives/starch blends and sisal fibre biocomposites. Polym. Degrad. Stab. 2004, 84, 13–21. [Google Scholar] [CrossRef]
- Scaffaro, R.; Morreale, M.; Lo Re, G.; La Mantia, F.P. Effect of the processing techniques on the properties of ecocomposites based on vegetable oil-derived Mater-Bi® and wood flour. J. Appl. Polym. Sci. 2009, 114, 2855–2863. [Google Scholar] [CrossRef]
- Gregorová, A.; Riedl, E.; Sedlarik, V.; Stelzer, F. Effect of 4, 4′-methylenediphenyl diisocyanate on thermal and mechanical properties of Bioflex/lactic acid polycondensate blends. Asia-Pac. J. Chem. Eng. 2012, 7, S317–S323. [Google Scholar]
- Sedlarik, V.; Otgonzul, O.; Kitano, T.; Gregorova, A.; Hrabalova, M.; Junkar, I.; Cvelbar, U.; Mozetic, M.; Saha, P. Effect of phase arrangement on solid state mechanical and thermal properties of polyamide 6/polylactide based co-polyester blends. J. Macromol. Sci. Part B 2012, 51, 982–1001. [Google Scholar] [CrossRef]
- Kucharczyk, P.; Otgonzu, O.; Kitano, T.; Gregorova, A.; Kreuh, D.; Cvelbar, U.; Sedlarik, V.; Saha, P. Correlation of morphology and viscoelastic properties of partially biodegradable polymer blends based on polyamide 6 and polylactide copolyester. Polym. Plast. Technol. Eng. 2012, 51, 1432–1442. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Ceraulo, M.; Mistretta, M.C.; Morreale, M. Effect of hot drawing on the mechanical properties of biodegradable fibers. J. Polym. Environ. 2016, 24, 56–63. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Ceraulo, M.; Mistretta, M.C.; Morreale, M. Rheological behaviour, mechanical properties and processability of biodegradable polymer systems for film blowing. J. Polym. Environ. 2018, 26, 749–755. [Google Scholar] [CrossRef]
- Číhal, P.; Vopička, O.; Pilnáček, K.; Poustka, J.; Friess, K.; Hajšlová, J.; Dobiáš, J.; Dole, P. Aroma scalping characteristics of polybutylene succinate based films. Polym. Test. 2015, 46, 108–115. [Google Scholar] [CrossRef]
- Ferry, L.; Dorez, G.; Taguet, A.; Otazaghine, B.; Lopez-Cuesta, J.M. Chemical modification of lignin by phosphorus molecules to improve the fire behavior of polybutylene succinate. Polym. Degrad. Stab. 2015, 113, 135–143. [Google Scholar] [CrossRef]
- Oliveira, J.T.; Correlo, V.M.; Sol, P.C.; Costa-Pinto, A.R.; Malafaya, P.B.; Salgado, A.J.; Bhattacharya, M.; Charbord, P.; Neves, N.M.; Reis, R.L. Assessment of the suitability of chitosan/polybutylene succinate scaffolds seeded with mouse mesenchymal progenitor cells for a cartilage tissue engineering approach. Tissue Eng. Part A 2008, 14, 1651–1661. [Google Scholar] [CrossRef]
- Tyliszczak, B.; Drabczyk, A.; Kudłacik-Kramarczyk, S.; Sobczak-Kupiec, A. Sustainable Production of Chitosan. In Sustainable Production: Novel Trends in Energy, Environment and Material Systems; Królczyk, G.M., Wzorek, M., Król, A., Kochan, O., Su, J., Kacprzyk, J., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 45–60. ISBN 978-3-030-11274-5. [Google Scholar]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Improvement of thermal stability, rheological and mechanical properties of PLA, PBAT and their blends by reactive extrusion with functionalized epoxy. Polym. Degrad. Stab. 2012, 97, 1898–1914. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Vert, M. Degradable and bioresorbable polymers in surgery and in pharmacology: Beliefs and facts. J. Mater. Sci. Mater. Med. 2009, 20, 437–446. [Google Scholar] [CrossRef]
- Woodward, S.C.; Brewer, P.S.; Moatamed, F.; Schindler, A.; Pitt, C.G. The intracellular degradation of poly (epsilon-caprolactone). J. Biomed. Mater. Res. 1985, 19, 437–444. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, N. Mechanistic implications of plastic degradation. Polym. Degrad. Stab. 2008, 93, 561–584. [Google Scholar] [CrossRef]
- Ramasubramanian, G.; Madbouly, S. Thermal and Oxidative Degradation Behavior of Polymers and Nanocomposites. In Reactions and Mechanisms in Thermal Analysis of Advanced Materials; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 127–164. ISBN 9781119117711. [Google Scholar]
- Agustin-Salazar, S.; Gamez-Meza, N.; Medina-Juárez, L.Á.; Malinconico, M.; Cerruti, P. Stabilization of polylactic acid and polyethylene with nutshell extract: Efficiency assessment and economic evaluation. ACS Sustain. Chem. Eng. 2017, 5, 4607–4618. [Google Scholar] [CrossRef]
- Fukushima, K.; Feijoo, J.L.; Yang, M.-C. Comparison of abiotic and biotic degradation of PDLLA, PCL and partially miscible PDLLA/PCL blend. Eur. Polym. J. 2013, 49, 706–717. [Google Scholar] [CrossRef]
- Mofokeng, J.P.; Luyt, A.S. Morphology and thermal degradation studies of melt-mixed poly(lactic acid) (PLA)/poly(ε-caprolactone) (PCL) biodegradable polymer blend nanocomposites with TiO2 as filler. Polym. Test. 2015, 45, 93–100. [Google Scholar] [CrossRef]
- Castilla-Cortázar, I.; Más-Estellés, J.; Meseguer-Dueñas, J.M.; Ivirico, J.L.E.; Marí, B.; Vidaurre, A. Hydrolytic and enzymatic degradation of a poly(ε-caprolactone) network. Polym. Degrad. Stab. 2012, 97, 1241–1248. [Google Scholar] [CrossRef]
- Tsutsumi, C.; Hayase, N.; Nakagawa, K.; Tanaka, S.; Miyahara, Y. The enzymatic degradation of commercial biodegradable polymers by some lipases and chemical degradation of them. Macromol. Symp. 2003, 197, 431–442. [Google Scholar] [CrossRef]
- Nikolić, M.A.L.; Gauthier, E.; Colwell, J.M.; Halley, P.; Bottle, S.E.; Laycock, B.; Truss, R. The challenges in lifetime prediction of oxodegradable polyolefin and biodegradable polymer films. Polym. Degrad. Stab. 2017, 145, 102–119. [Google Scholar] [CrossRef]
- Touchaleaume, F.; Angellier-Coussy, H.; César, G.; Raffard, G.; Gontard, N.; Gastaldi, E. How performance and fate of biodegradable mulch films are impacted by field ageing. J. Polym. Environ. 2018, 26, 2588–2600. [Google Scholar] [CrossRef]
- Sangroniz, A.; Gonzalez, A.; Martin, L.; Irusta, L.; Iriarte, M.; Etxeberria, A. Miscibility and degradation of polymer blends based on biodegradable poly(butylene adipate-co-terephthalate). Polym. Degrad. Stab. 2018, 151, 25–35. [Google Scholar] [CrossRef]
- Müller, C.; Townsend, K.; Matschullat, J. Experimental degradation of polymer shopping bags (standard and degradable plastic, and biodegradable) in the gastrointestinal fluids of sea turtles. Sci. Total Env. 2012, 416, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Tuladhar, R.; Shi, F.; Shanks, R.A.; Combe, M.; Collister, T. Mechanical reprocessing of polyolefin waste: A review. Polym. Eng. Sci. 2015, 55, 2899–2909. [Google Scholar] [CrossRef]
- Scaffaro, R.; Botta, L.; Di Benedetto, G. Physical properties of virgin-recycled ABS blends: Effect of post-consumer content and of reprocessing cycles. Eur. Polym. J. 2012, 48, 637–648. [Google Scholar] [CrossRef]
- Luckachan, G.E.; Pillai, C.K.S. Biodegradable polymers-a review on recent trends and emerging perspectives. J. Polym. Environ. 2011, 19, 637–676. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; La Mantia, F.P.; Scaffaro, R.; Paci, M.; Acierno, D.; Camino, G. Reprocessing and restabilization of greenhouse films. Polym. Degrad. Stab. 2002, 75, 459–464. [Google Scholar] [CrossRef]
- Badia, J.D.; Strömberg, E.; Karlsson, S.; Ribes-Greus, A. Material valorisation of amorphous polylactide. Influence of thermo-mechanical degradation on the morphology, segmental dynamics, thermal and mechanical performance. Polym. Degrad. Stab. 2012, 97, 670–678. [Google Scholar] [CrossRef]
- Żenkiewicz, M.; Richert, J.; Rytlewski, P.; Moraczewski, K.; Stepczyńska, M.; Karasiewicz, T. Characterisation of multi-extruded poly (lactic acid). Polym. Test. 2009, 28, 412–418. [Google Scholar] [CrossRef]
- Peinado, V.; Castell, P.; García, L.; Fernández, Á. Effect of extrusion on the mechanical and rheological properties of a reinforced poly (lactic acid): Reprocessing and recycling of biobased materials. Materials 2015, 8, 7106–7117. [Google Scholar] [CrossRef]
- Scaffaro, R.; Morreale, M.; Mirabella, F.; La Mantia, F.P. Preparation and recycling of plasticized PLA. Macromol. Mater. Eng. 2011, 296. [Google Scholar] [CrossRef]
- Morreale, M.; Liga, A.; Mistretta, M.; Ascione, L.; Mantia, F. Mechanical, thermomechanical and reprocessing behavior of green composites from biodegradable polymer and wood flour. Materials 2015, 8, 7536–7548. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Botta, L.; Morreale, M.; Scaffaro, R. Effect of small amounts of poly(lactic acid) on the recycling of poly(ethyleneterephtalate) bottles. Polym. Degrad. Stab. 2012, 97, 21–24. [Google Scholar]
- La Mantia, F.P.; Scaffaro, R.; Bastioli, C. Recycling of a starch-based biodegradable polymer. Macromol. Symp. 2002, 180, 133–140. [Google Scholar] [CrossRef]
- Van de Velde, K.; Kiekens, P. Biopolymers: Overview of several properties and consequences on their applications. Polym. Test. 2002, 21, 433–442. [Google Scholar] [CrossRef]
- Paola, S.; Luciano, D.M.; Loredana, I. Recent advances and migration issues in biodegradable polymers from renewable sources for food packaging. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Niaounakis, M. (Ed.) Biopolymers Reuse, Recycling, and Disposal; Plastics Design Library; William Andrew Publishing: Oxford, UK, 2013; ISBN 978-1-4557-3145-9. [Google Scholar]
- Badia, J.D.; Ribes-Greus, A. Mechanical recycling of polylactide, upgrading trends and combination of valorization techniques. Eur. Polym. J. 2016, 84, 22–39. [Google Scholar] [CrossRef]
- Morreale, M.; Scaffaro, R.; Maio, A.; La Mantia, F.P. Effect of adding wood flour to the physical properties of a biodegradable polymer. Compos. Part A Appl. Sci. Manuf. 2008, 39, 503–513. [Google Scholar] [CrossRef]
- Morreale, M.; Scaffaro, R.; Maio, A.; Mantia, F.P. La Mechanical behaviour of Mater-Bi®/wood flour composites: A statistical approach. Compos. Part A Appl. Sci. Manuf. 2008, 39, 1537–1546. [Google Scholar] [CrossRef]
- Beltrán, F.R.; Lorenzo, V.; Acosta, J.; de la Orden, M.U.; Urreaga, J.M. Effect of simulated mechanical recycling processes on the structure and properties of poly(lactic acid). J. Environ. Manag. 2018, 216, 25–31. [Google Scholar] [CrossRef]
- Beltrán, F.R.; Lorenzo, V.; de la Orden, M.U.; Martínez-Urreaga, J. Effect of different mechanical recycling processes on the hydrolytic degradation of poly(l-lactic acid). Polym. Degrad. Stab. 2016, 133, 339–348. [Google Scholar] [CrossRef]
- Amorin, N.S.Q.S.; Rosa, G.; Alves, J.F.; Gonçalves, S.P.C.; Franchetti, S.M.M.; Fechine, G.J.M. Study of thermodegradation and thermostabilization of poly(lactide acid) using subsequent extrusion cycles. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Mistretta, M.C.; Morreale, M. Recycling and thermomechanical degradation of LDPE/modified clay nanocomposites. Macromol. Mater. Eng. 2014, 299, 96–103. [Google Scholar] [CrossRef]
- Scaffaro, R.; Botta, L.; La Mantia, F.P. Preparation and Characterization of polyolefin-based nanocomposite blown films for agricultural applications. Macromol. Mater. Eng. 2009, 294, 445–454. [Google Scholar] [CrossRef]
- Scaffaro, R.; Botta, L.; Mistretta, M.C.; La Mantia, F.P. Preparation and characterization of polyamide 6/polyethylene blend-clay nanocomposites in the presence of compatibilisers and stabilizing system. Polym. Degrad. Stab. 2010, 95, 2547–2554. [Google Scholar] [CrossRef]
- Tesfaye, M.; Patwa, R.; Gupta, A.; Kashyap, M.J.; Katiyar, V. Recycling of poly (lactic acid)/silk based bionanocomposites films and its influence on thermal stability, crystallization kinetics, solution and melt rheology. Int. J. Biol. Macromol. 2017, 101, 580–594. [Google Scholar] [CrossRef]
- Rojas-González, A.F.; Carrero-Mantilla, J.I. Cinética de degradación térmica de poliácido láctico en múltiples extrusiones. Ingenieria y Universidad 2015, 19, 189–206. [Google Scholar] [CrossRef]
- Nascimento, L.; Gamez-Perez, J.; Santana, O.O.; Velasco, J.I.; Maspoch, M.L.; Franco-Urquiza, E. Effect of the recycling and annealing on the mechanical and fracture properties of poly(lactic acid). J. Polym. Environ. 2010, 18, 654–660. [Google Scholar] [CrossRef]
- Zhou, Q.; Xanthos, M. Nanoclay and crystallinity effects on the hydrolytic degradation of polylactides. Polym. Degrad. Stab. 2008, 93, 1450–1459. [Google Scholar] [CrossRef]
- Pandey, J.K.; Reddy, K.R.; Kumar, A.P.; Singh, R.P. An overview on the degradability of polymer nanocomposites. Polym. Degrad. Stab. 2005, 88, 234–250. [Google Scholar] [CrossRef]
- Puccini, M.; Seggiani, M.; Vitolo, S. Polyethylene and hydrolyzed collagen blend films produced by blown extrusion. Chem. Eng. Trans. 2015, 43, 1705–1710. [Google Scholar]
- Li, X.; Yan, X.; Yang, J.; Pan, H.; Gao, G.; Zhang, H.; Dong, L. Improvement of compatibility and mechanical properties of the poly (lactic acid)/poly (butylene adipate-co-terephthalate) blends and films by reactive extrusion with chain extender. Polym. Eng. Sci. 2018, 58, 1868–1878. [Google Scholar] [CrossRef]
- Li, X.; Ai, X.; Pan, H.; Yang, J.; Gao, G.; Zhang, H.; Yang, H.; Dong, L. The morphological, mechanical, rheological, and thermal properties of PLA/PBAT blown films with chain extender. Polym. Adv. Technol. 2018, 29, 1706–1717. [Google Scholar] [CrossRef]
- Mallegni, N.; Phuong, T.V.; Coltelli, M.-B.; Cinelli, P.; Lazzeri, A. Poly (lactic acid)(PLA) Based Tear Resistant and Biodegradable Flexible Films by Blown Film Extrusion. Materials 2018, 11, 148. [Google Scholar] [CrossRef]
- Li, J.; Lai, L.; Wu, L.; Severtson, S.J.; Wang, W.-J. Enhancement of water vapor barrier properties of biodegradable poly (butylene adipate-co-terephthalate) films with highly oriented organomontmorillonite. ACS Sustain. Chem. Eng. 2018, 6, 6654–6662. [Google Scholar] [CrossRef]
- Ruellan, A.; Ducruet, V.; Gratia, A.; Saelices Jimenez, L.; Guinault, A.; Sollogoub, C.; Chollet, G.; Domenek, S. Palm oil deodorizer distillate as toughening agent in polylactide packaging films. Polym. Int. 2016, 65, 683–690. [Google Scholar] [CrossRef]
- Schneider, J.; Manjure, S.; Narayan, R. Reactive modification and compatibilization of poly (lactide) and poly (butylene adipate-co-terephthalate) blends with epoxy functionalized-poly (lactide) for blown film applications. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Cunha, M.; Fernandes, B.; Covas, J.A.; Vicente, A.A.; Hilliou, L. Film blowing of PHBV blends and PHBV-based multilayers for the production of biodegradable packages. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Thunwall, M.; Kuthanová, V.; Boldizar, A.; Rigdahl, M. Film blowing of thermoplastic starch. Carbohydr. Polym. 2008, 71, 583–590. [Google Scholar] [CrossRef]
- Sun, S.; Liu, P.; Ji, N.; Hou, H.; Dong, H. Effects of low polyhydroxyalkanoate content on the properties of films based on modified starch acquired by extrusion blowing. Food Hydrocoll. 2017, 72, 81–89. [Google Scholar] [CrossRef]
- Botta, L.; Scaffaro, R.; Sutera, F.; Mistretta, M.C. Reprocessing of PLA/graphene nanoplatelets nanocomposites. Polymers 2018, 10, 18. [Google Scholar] [CrossRef]
- Maio, A.; Scaffaro, R.; Lentini, L.; Palumbo Piccionello, A.; Pibiri, I. Perfluorocarbons–graphene oxide nanoplatforms as biocompatible oxygen reservoirs. Chem. Eng. J. 2018, 334, 54–65. [Google Scholar] [CrossRef]
- Maio, A.; Giallombardo, D.; Scaffaro, R.; Palumbo Piccionello, A.; Pibiri, I. Synthesis of a fluorinated graphene oxide--silica nanohybrid: Improving oxygen affinity. RSC Adv. 2016, 6, 46037–46047. [Google Scholar] [CrossRef]
- Maio, A.; Agnello, S.; Khatibi, R.; Botta, L.; Alessi, A.; Piazza, A.; Buscarino, G.; Mezzi, A.; Pantaleo, G.; Scaffaro, R. A rapid and eco-friendly route to synthesize graphene-doped silica nanohybrids. J. Alloy. Compd. 2016, 664, 428–438. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Lopresti, F.; Giallombardo, D.; Botta, L.; Bondì, M.L.; Agnello, S. Synthesis and self-assembly of a PEGylated-graphene aerogel. Compos. Sci. Technol. 2016, 128, 193–200. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Arrigo, R.; Gambarotti, C.; Carroccio, S.; Filippone, G.; Cicogna, F.; Guenzi, M. α-Tocopherol-induced radical scavenging activity in carbon nanotubes for thermo-oxidation resistant ultra-high molecular weight polyethylene-based nanocomposites. Carbon N. Y. 2014, 74, 14–21. [Google Scholar] [CrossRef]
- Li, J.-H.; Miao, J.; Wu, J.-L.; Chen, S.-F.; Zhang, Q.-Q. Preparation and characterization of active gelatin-based films incorporated with natural antioxidants. Food Hydrocoll. 2014, 37, 166–173. [Google Scholar] [CrossRef]
- Pagno, C.H.; de Farias, Y.B.; Costa, T.M.H.; Rios, A.O.; Flôres, S.H. Synthesis of biodegradable films with antioxidant properties based on cassava starch containing bixin nanocapsules. J. Food Sci. Technol. 2016, 53, 3197–3205. [Google Scholar] [CrossRef]
- Scaffaro, R.; Botta, L.; Passaglia, E.; Oberhauser, W.; Frediani, M.; Di Landro, L. Comparison of different processing methods to prepare poly (lactid acid)--hydrotalcite composites. Polym. Eng. Sci. 2014, 54, 1804–1810. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Ceraulo, M.; Mistretta, M.C.; Sutera, F.; Ascione, L.; Nasillo, G. Effect of elongational flow and polarity of organomodified clay on morphology and mechanical properties of a PLA based nanobiocomposite. Int. Polym. Process. 2016, 31, 541–547. [Google Scholar] [CrossRef]
- Scaffaro, R.; Botta, L.; Lopresti, F.; Maio, A.; Sutera, F. Polysaccharide nanocrystals as fillers for PLA based nanocomposites. Cellulose 2016, 24, 447–478. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Lo Re, G.; Parisi, A.; Busacca, A. Advanced piezoresistive sensor achieved by amphiphilic nanointerfaces of graphene oxide and biodegradable polymer blends. Compos. Sci. Technol. 2018, 156, 166–176. [Google Scholar] [CrossRef]
- Maio, A.; Fucarino, R.; Khatibi, R.; Rosselli, S.; Bruno, M.; Scaffaro, R. A novel approach to prevent graphene oxide re-aggregation during the melt compounding with polymers. Compos. Sci. Technol. 2015, 119, 131–137. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A. A green method to prepare nanosilica modified graphene oxide to inhibit nanoparticles re-aggregation during melt processing. Chem. Eng. J. 2017, 308, 1034–1047. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Lopresti, F. Effect of graphene and fabrication technique on the release kinetics of carvacrol from polylactic acid. Compos. Sci. Technol. 2019, 169, 60–69. [Google Scholar] [CrossRef]
- Scaffaro, R.; Maio, A.; Botta, L.; Gulino, E.F.; Gulli, D. Tunable release of chlorhexidine from polycaprolactone-based filaments containing graphene nanoplatelets. Eur. Polym. J. 2019, 110, 221–232. [Google Scholar] [CrossRef]
- Tian, H.; Wang, Y.; Zhang, L.; Quan, C.; Zhang, X. Improved flexibility and water resistance of soy protein thermoplastics containing waterborne polyurethane. Ind. Crop. Prod. 2010, 32, 13–20. [Google Scholar] [CrossRef]
- Botta, L.; Mistretta, M.C.; Palermo, S.; Fragalà, M.; Pappalardo, F. Characterization and processability of blends of polylactide acid with a new biodegradable medium-chain-length polyhydroxyalkanoate. J. Polym. Environ. 2015, 23, 478–486. [Google Scholar] [CrossRef]
- Sanyang, M.L.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R.; Sahari, J. Development and characterization of sugar palm starch and poly (lactic acid) bilayer films. Carbohydr. Polym. 2016, 146, 36–45. [Google Scholar] [CrossRef]
- González, A.; Alvarez Igarzabal, C.I. Soy protein—Poly (lactic acid) bilayer films as biodegradable material for active food packaging. Food Hydrocoll. 2013, 33, 289–296. [Google Scholar] [CrossRef]
- Kurek, M.; Galus, S.; Debeaufort, F. Surface, mechanical and barrier properties of bio-based composite films based on chitosan and whey protein. Food Packag. Shelf Life 2014, 1, 56–67. [Google Scholar] [CrossRef]
- Messin, T.; Follain, N.; Guinault, A.; Miquelard-Garnier, G.; Sollogoub, C.; Delpouve, N.; Gaucher, V.; Marais, S. Confinement effect in PC/MXD6 multilayer films: Impact of the microlayered structure on water and gas barrier properties. J. Membr. Sci. 2017, 525, 135–145. [Google Scholar] [CrossRef]
- Belyamani, I.; Prochazka, F.; Assezat, G. Production and characterization of sodium caseinate edible films made by blown-film extrusion. J. Food Eng. 2014, 121, 39–47. [Google Scholar] [CrossRef]
- Botta, L.; Scaffaro, R.; La Mantia, F.P.; Dintcheva, N.T. Effect of different matrices and nanofillers on the rheological behavior of polymer-clay nanocomposites. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 344–355. [Google Scholar] [CrossRef]
- Nostro, A.; Scaffaro, R.; Ginestra, G.; D’Arrigo, M.; Botta, L.; Marino, A.; Bisignano, G. Control of biofilm formation by poly-ethylene-co-vinyl acetate films incorporating nisin. Appl. Microbiol. Biotechnol. 2010, 87, 729–737. [Google Scholar] [CrossRef]

| Bioplastic(s) | Composition/Layout | Additives | Processing | Main Outcomes | Ref. |
|---|---|---|---|---|---|
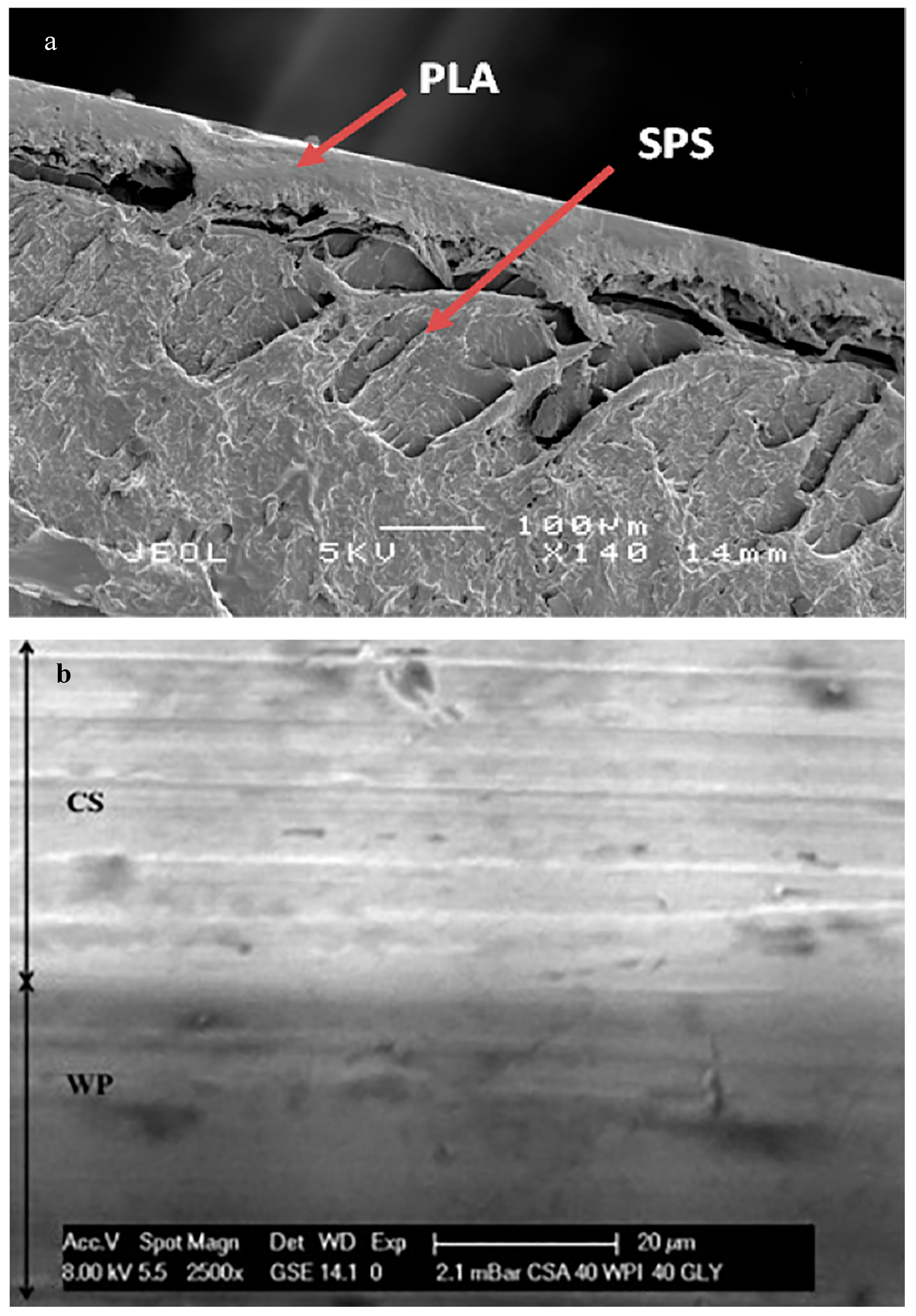
| Sugar-palm starch (SPS) and polylactic acid (PLA) | Bilayer | Solvent-casting | Lack of interface adhesion between the two layers | [114] | |
| PLA-soy protein (SPI) | bilayer | Solvent-casting | Adequate layer-layer adhesion | [115] | |
| Chitosan (CS) and whey protein (WP) | bilayer | Solution coating | Good transparency, improved mechanical performance, better water barrier properties | [116] | |
| Poly(hydroxybutyrate-co-valerate) (PHBV)/zein/PHBV PHBV/zein/alginate | Three-layer | CNMA | Hot pressing +electrospinning | Good interlayer adhesion; reduced transparency | [17] |
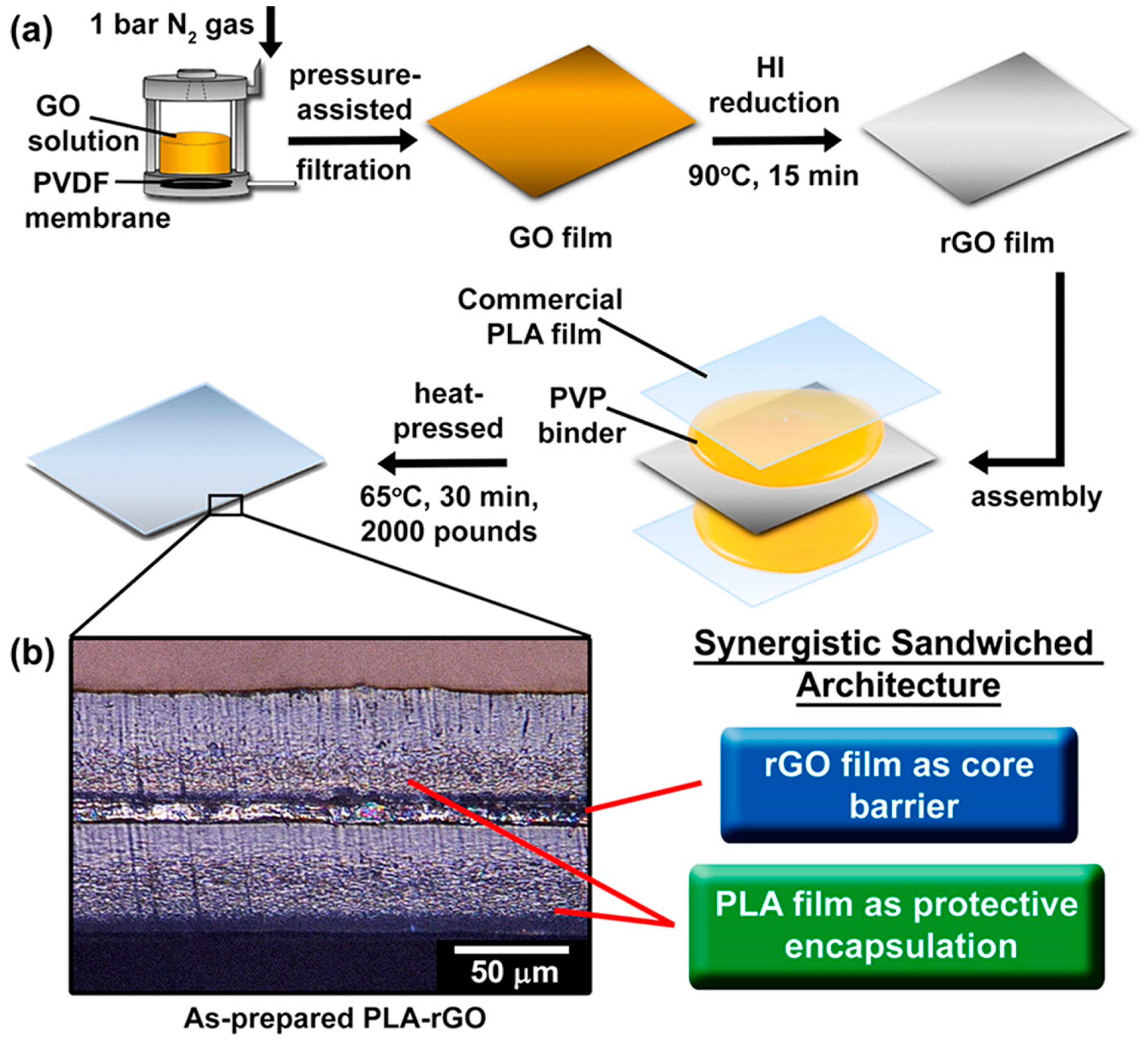
| PLA/polyvinyl pyrrolidone (PVP)/GO/PVP/PLA | multilayer | Hot pressing, GO layer obtained by solvent method | Excellent barrier properties to H2O and O2 | [16] | |
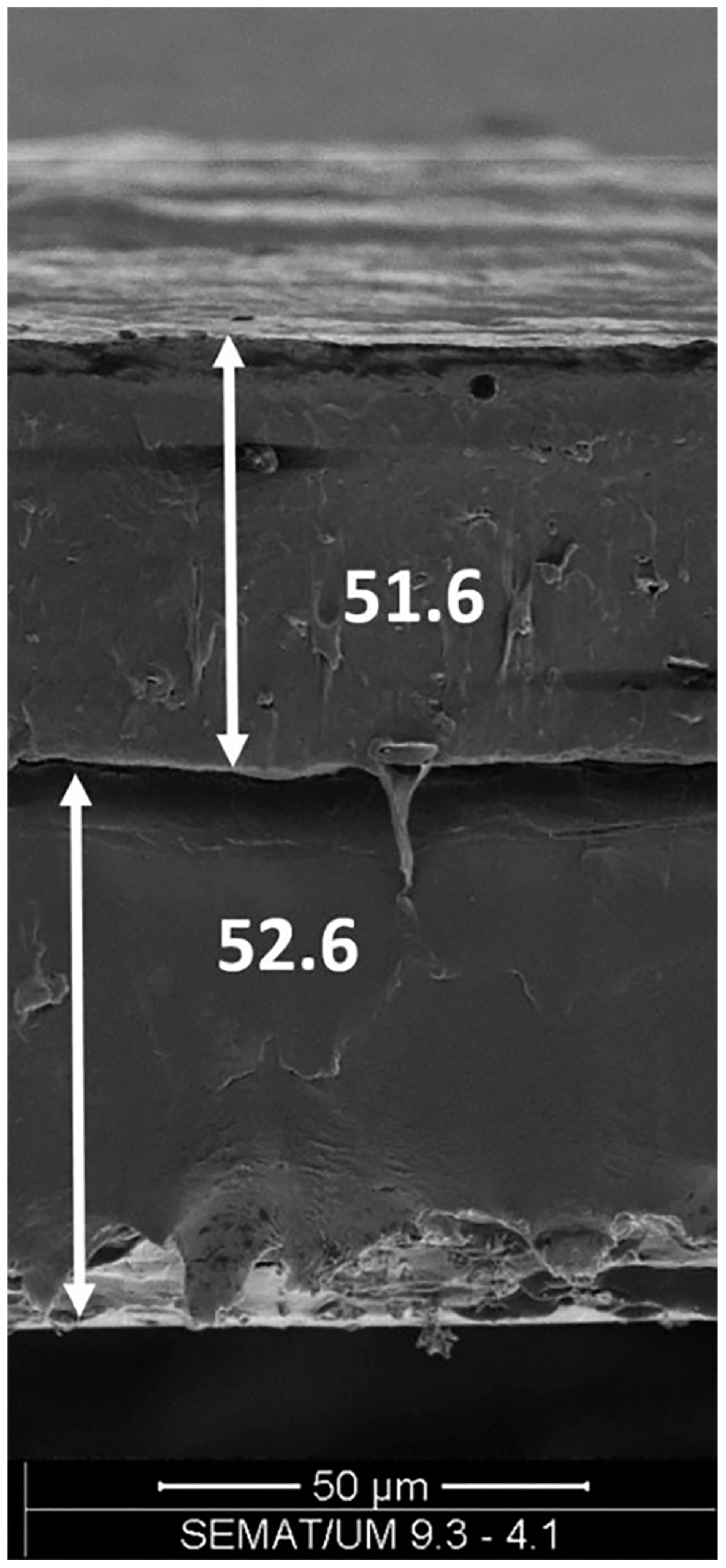
| PHBV/PBAT | bilayer | Co-extrusion | Good mechanical properties; poor interfacial adhesion | [93] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scaffaro, R.; Maio, A.; Sutera, F.; Gulino, E.F.; Morreale, M. Degradation and Recycling of Films Based on Biodegradable Polymers: A Short Review. Polymers 2019, 11, 651. https://doi.org/10.3390/polym11040651
Scaffaro R, Maio A, Sutera F, Gulino EF, Morreale M. Degradation and Recycling of Films Based on Biodegradable Polymers: A Short Review. Polymers. 2019; 11(4):651. https://doi.org/10.3390/polym11040651
Chicago/Turabian StyleScaffaro, Roberto, Andrea Maio, Fiorenza Sutera, Emmanuel Fortunato Gulino, and Marco Morreale. 2019. "Degradation and Recycling of Films Based on Biodegradable Polymers: A Short Review" Polymers 11, no. 4: 651. https://doi.org/10.3390/polym11040651
APA StyleScaffaro, R., Maio, A., Sutera, F., Gulino, E. F., & Morreale, M. (2019). Degradation and Recycling of Films Based on Biodegradable Polymers: A Short Review. Polymers, 11(4), 651. https://doi.org/10.3390/polym11040651








