Lightweight Poly(ε-Caprolactone) Composites with Surface Modified Hollow Glass Microspheres for Use in Rotational Molding: Thermal, Rheological and Mechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
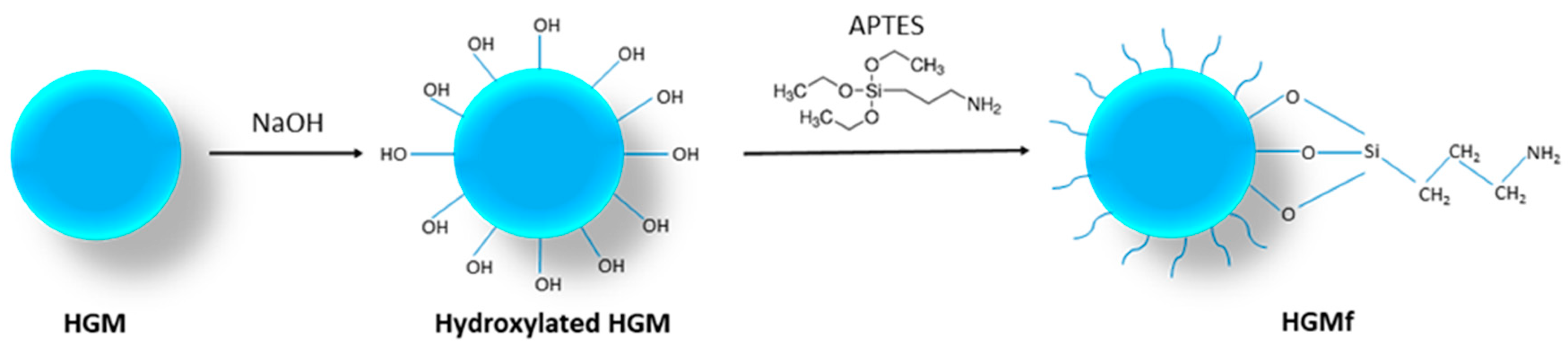
2.2. Hollow Glass Microspheres Surface Treatment
2.3. Composite Preparation
2.4. Methods
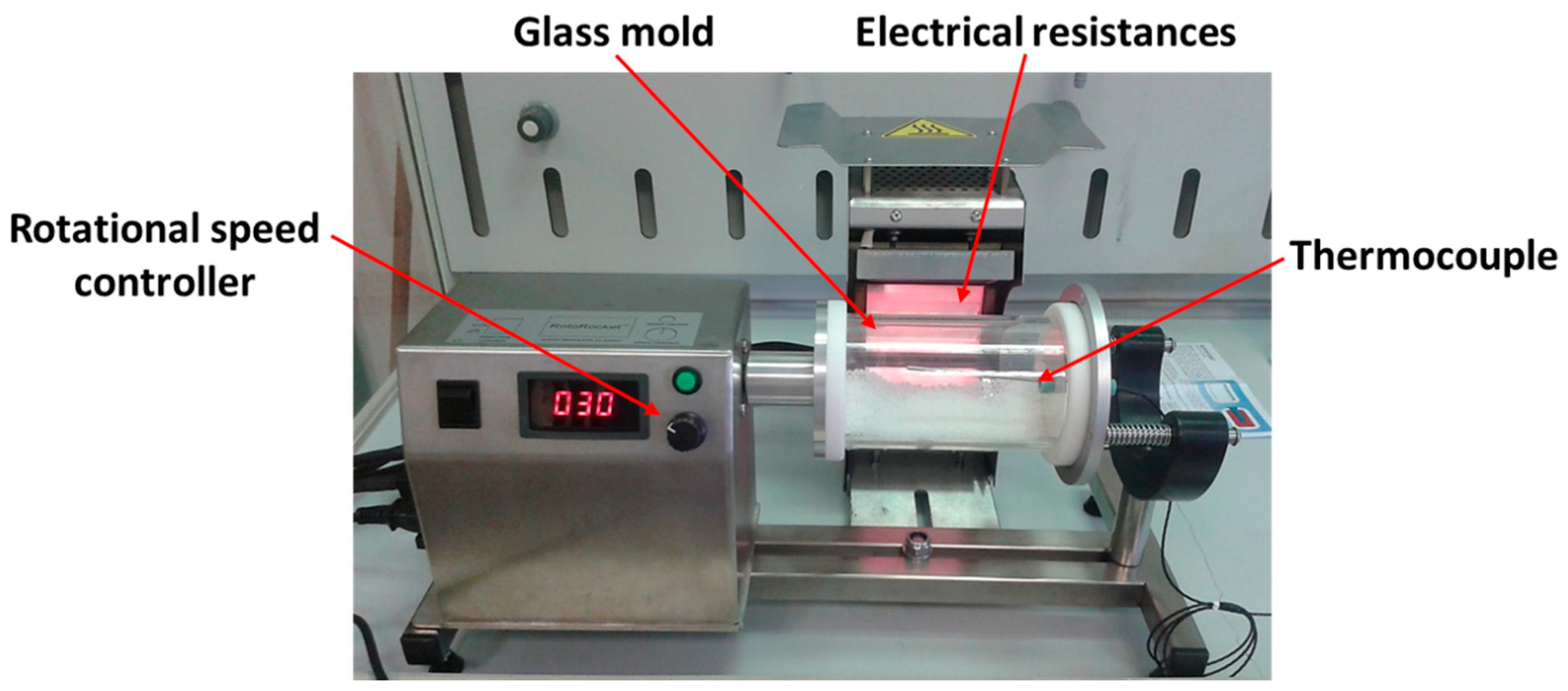
2.5. Rotational Molding Tests
3. Results and Discussion
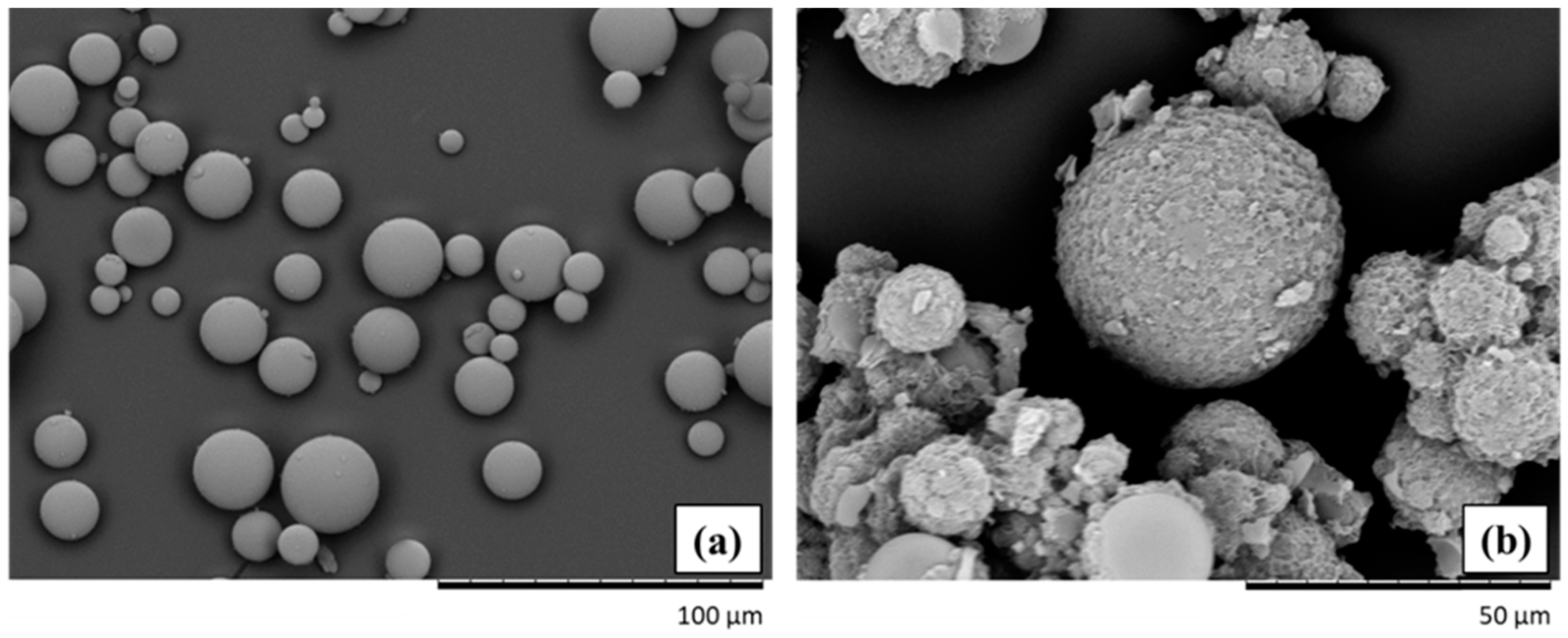
3.1. Characterization of Hollow Glass Microspheres
3.2. Composite Characterization and Properties
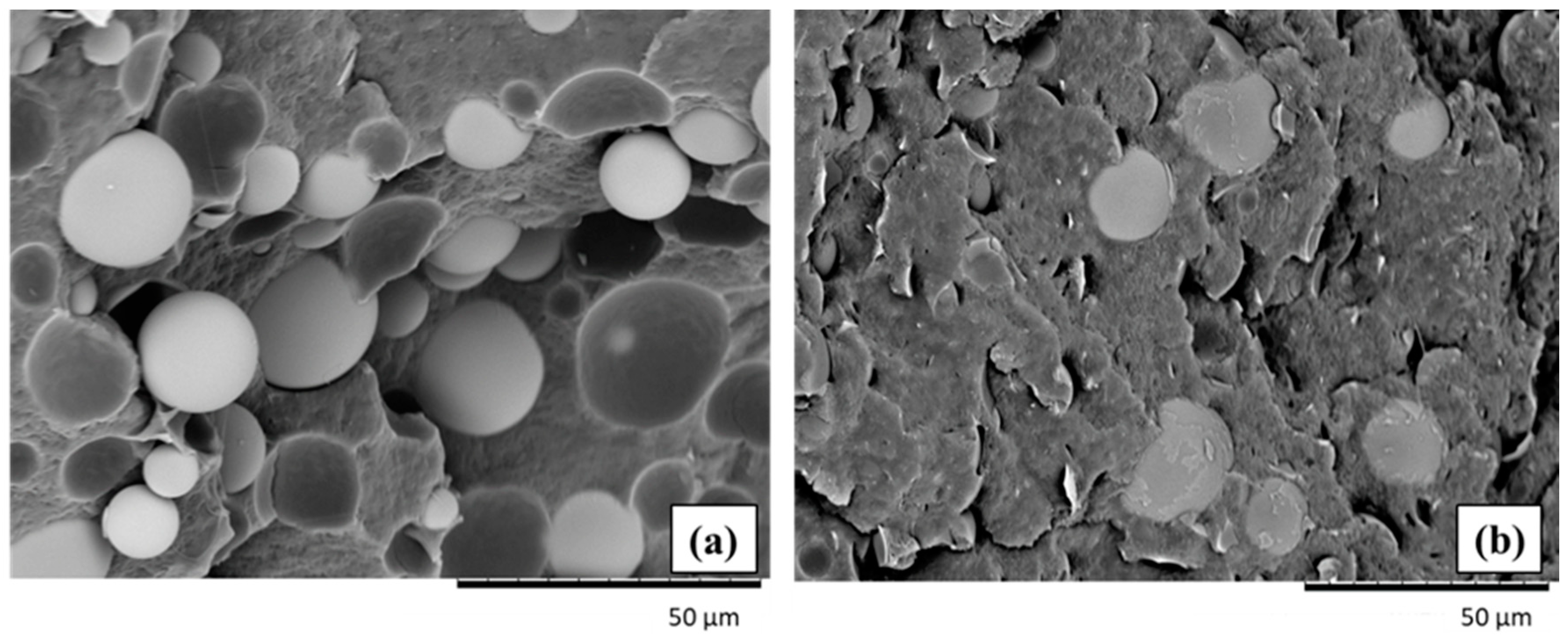
3.2.1. Molecular Characterization and Morphology
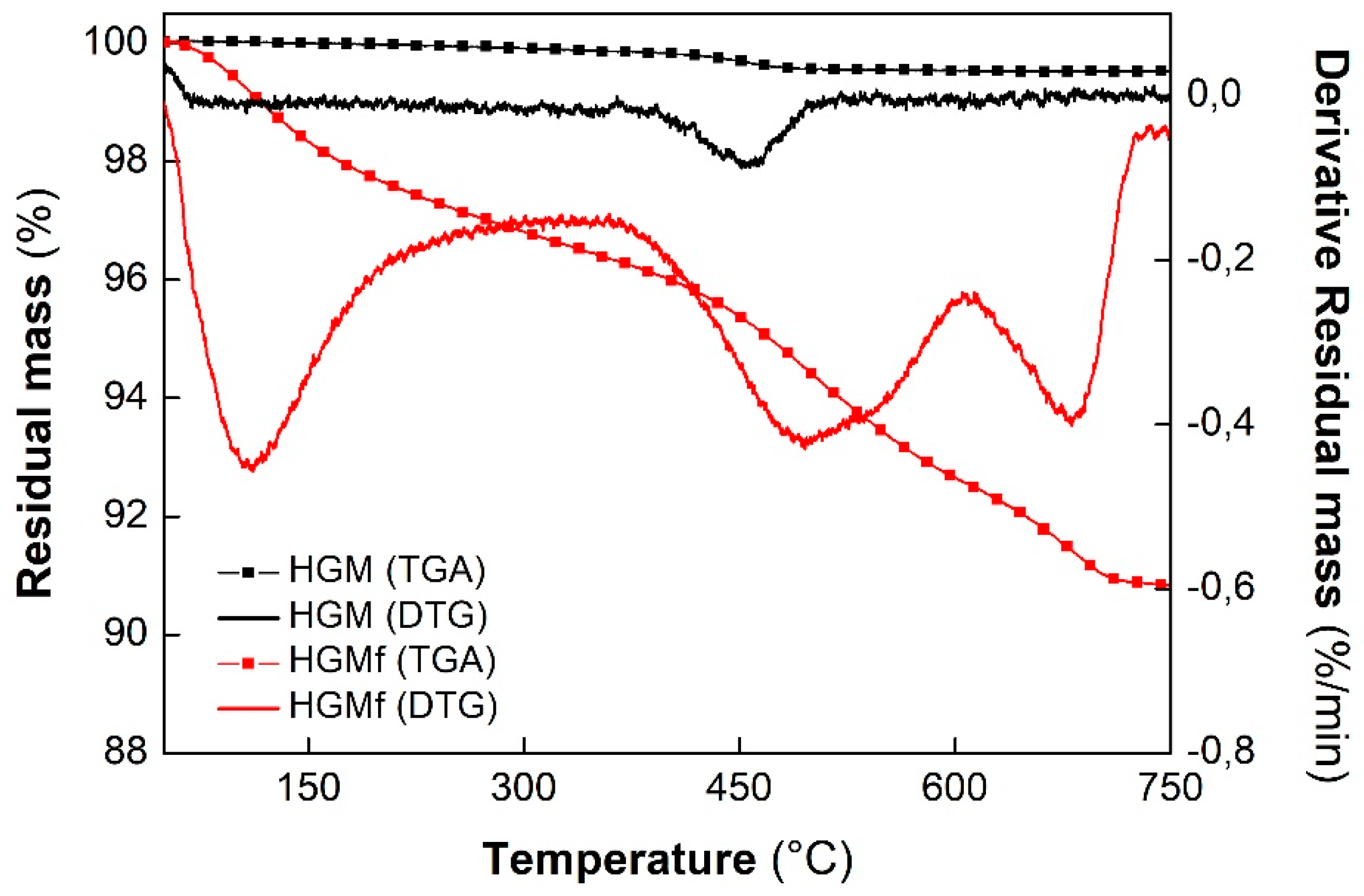
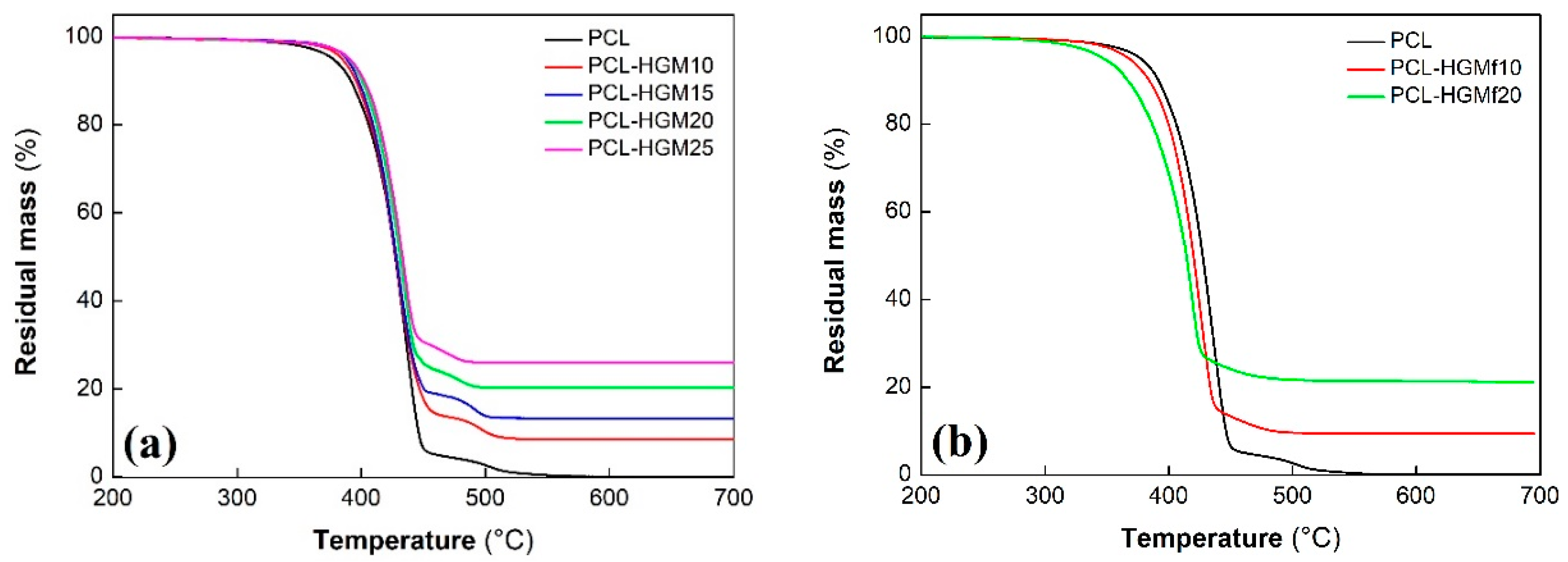
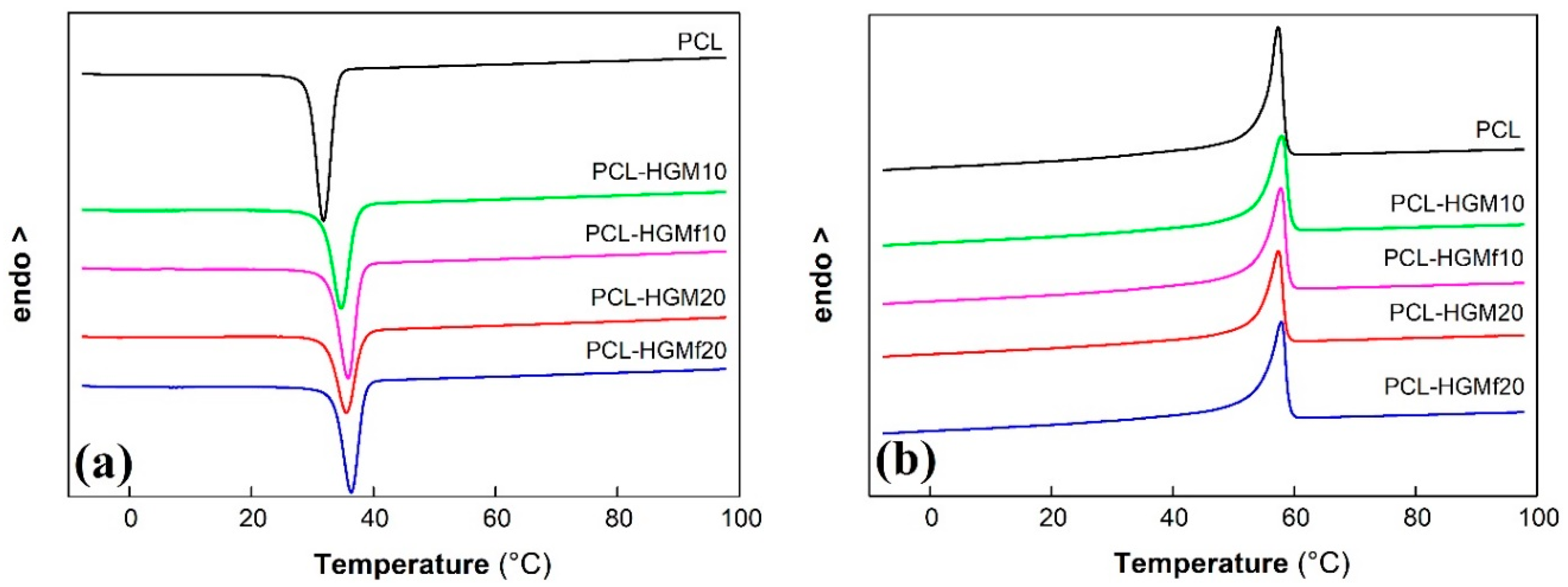
3.2.2. Thermal Behavior
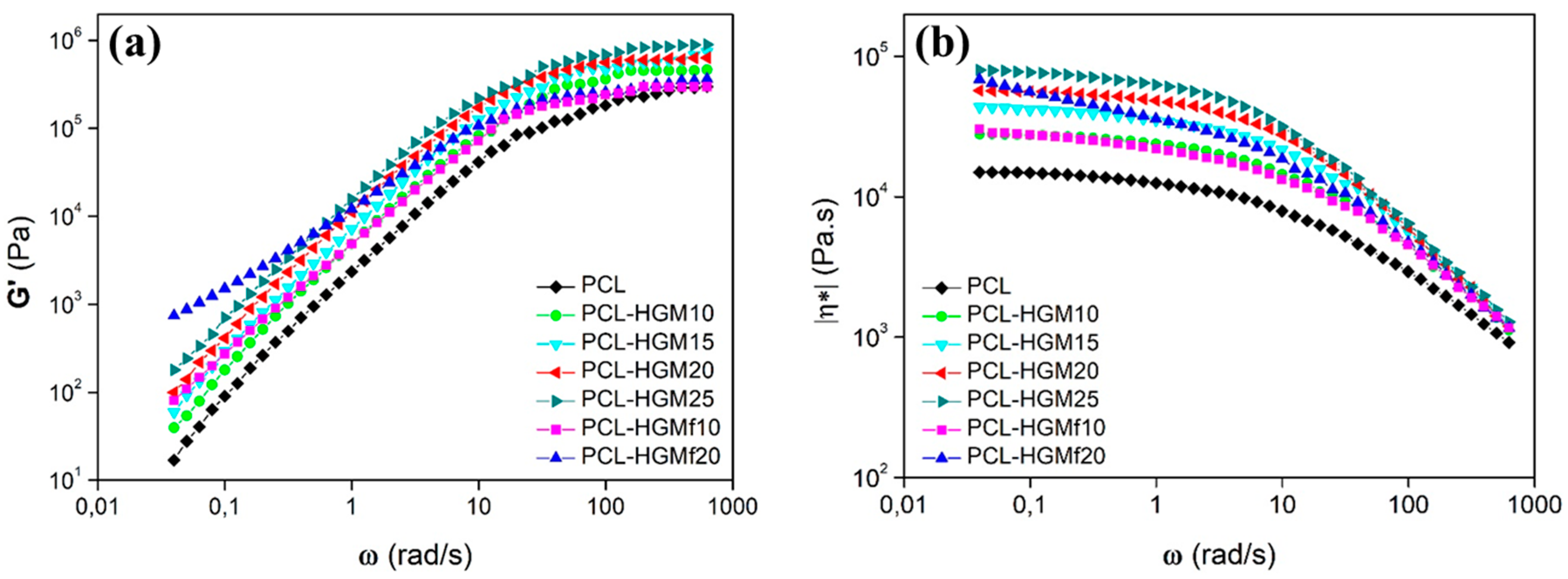
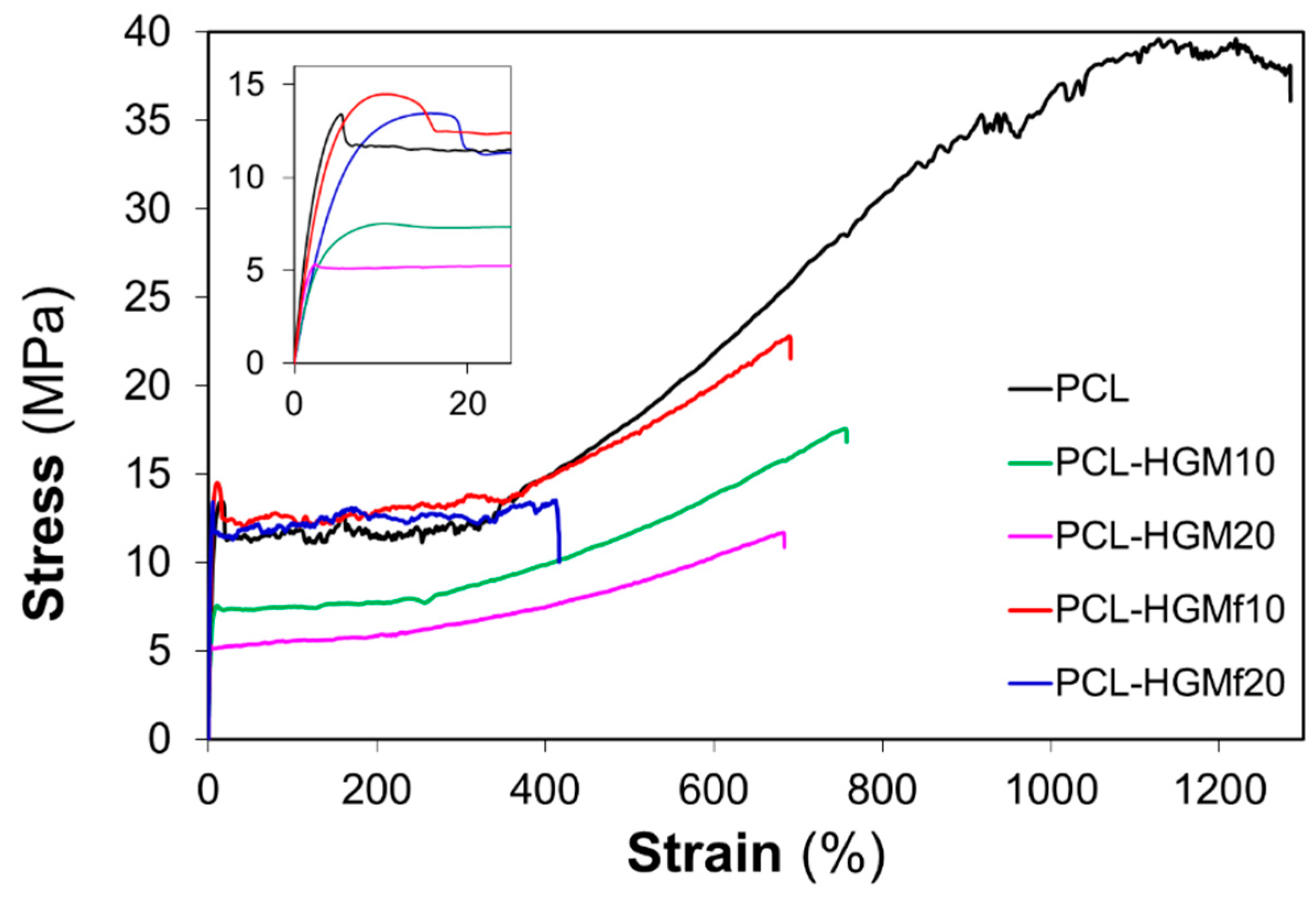
3.2.3. Rheological Properties and Mechanical Behavior
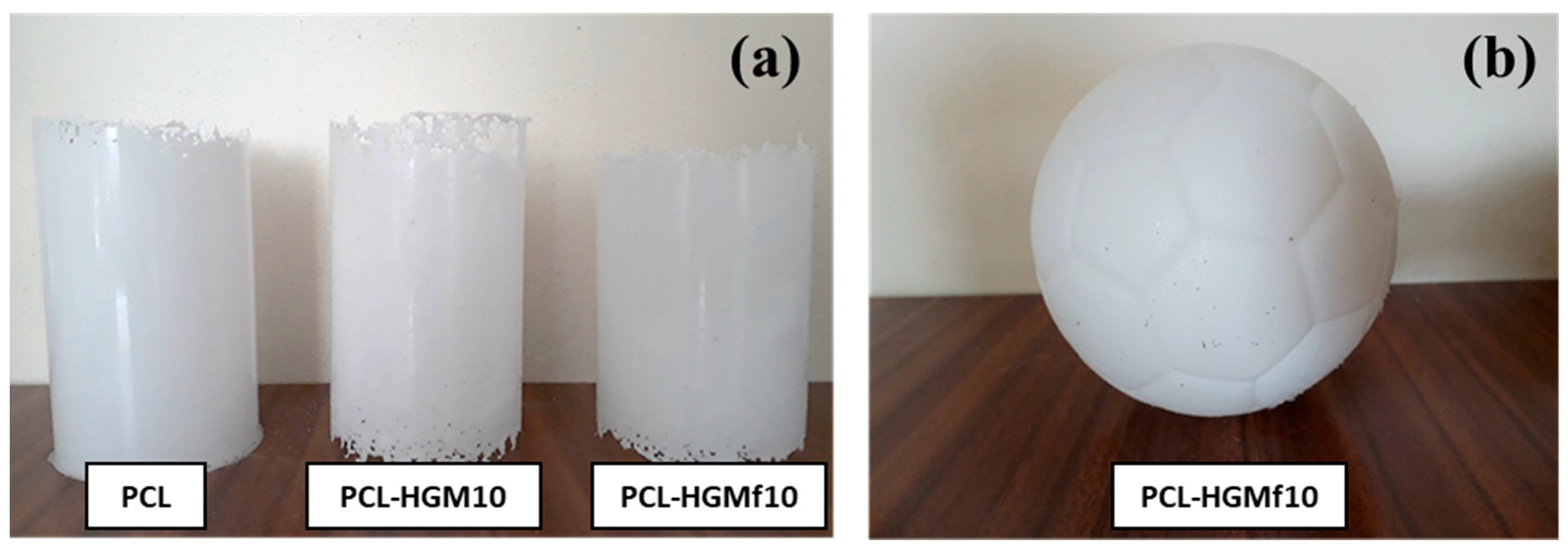
3.3. Rotational Molding Tests
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Vroman, I.; Tighzert, L. Biodegradable polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Luckachan, G.E.; Pillai, C.K.S. Biodegradable polymers—A review on recent trends and emerging perspectives. J. Polym. Environ. 2011, 19, 637–676. [Google Scholar] [CrossRef]
- Reddy, M.M.; Vivekanandhan, S.; Misra, M.; Bhatia, S.K.; Mohanty, A.K. Biobased plastics and bionanocomposites: Current status and future opportunities. Prog. Polym. Sci. 2013, 38, 1653–1689. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer. Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Naira, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Kai, W.; Hirota, Y.; Hua, L.; Inoue, Y. Thermal and mechanical properties of a poly(ε-caprolactone)/graphite oxide composite. J. Appl. Polym. Sci. 2008, 107, 1395–1400. [Google Scholar] [CrossRef]
- Arbelaiz, A.; Fernandez, B.; Valea, A.; Mondragon, I. Mechanical properties of short flax fibre bundle/poly(ε-caprolactone) composites: Influence of matrix modification and fibre content. Carbohydr. Polym. 2006, 64, 224–232. [Google Scholar] [CrossRef]
- Kotek, J.; Kubies, D.; Baldrian, J.; Kovárová, J. Biodegradable polyester nanocomposites: The effect of structure on mechanical and degradation behaviour. Eur. Polym. J. 2011, 47, 2197–2207. [Google Scholar] [CrossRef]
- Williamson, B.R.; Krishnaswamy, R.; Tonelli, A.E. Physical properties of poly(ε-caprolactone) coalesced from its a-cyclodextrin inclusion compound. Polymer 2011, 52, 4517–4527. [Google Scholar] [CrossRef]
- Gurarslan, A.; Caydamli, Y.; Shen, J.; Tse, S.; Yetukuri, M.; Tonelli, A.E. Coalesced poly(ε-caprolactone) fibers are stronger. Biomacromolecules 2015, 16, 890–893. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, B.; Amos, S.E. Hollow Glass Microspheres for Plastics, Elastomers, and Adhesives Compounds; Elsevier: Oxford, UK, 2015. [Google Scholar]
- Gao, J.; Wang, J.; Xu, H.; Wu, C. Preparation and properties of hollow glass bead filled silicone rubber foams with low thermal conductivity. Mater. Des. 2013, 46, 491–496. [Google Scholar] [CrossRef]
- Patankar, S.N.; Kranov, Y.A. Hollow glass microsphere HDPE composites for low energy sustainability. Mater. Sci. Eng. A 2010, 527, 1361–1366. [Google Scholar] [CrossRef]
- Patankar, S.N.; Das, A.; Kranov, Y.A. Interface engineering via compatibilization in HDPE composite reinforced with sodium borosilicate hollow glass microspheres. Compos. Part A Appl. Sci. Manuf. 2009, 40, 897–903. [Google Scholar] [CrossRef]
- Ashton-Patton, M.M.; Hall, M.M.; Shelby, J.E. Formation of low density polyethylene/hollow glass microspheres composites. J. Non Cryst. Solids 2006, 352, 615–619. [Google Scholar] [CrossRef]
- Zhu, B.L.; Zheng, H.; Wang, J.; Ma, J.; Wu, J.; Wu, R. Tailoring of thermal and dielectric properties of LDPE-matrix composites by the volume fraction, density, and surface modification of hollow glass microsphere filler. Compos. Part B Eng. 2014, 58, 91–102. [Google Scholar] [CrossRef]
- Liu, F.; Wu, X.F.; Guo, M.Q.; Yang, Z.Q.; Fan, H.N.; Lu, H.R.; Xu, X.H. Phase structure, thermal, and mechanical properties of polypropylene/hollow glass microsphere composites modified with maleated poly(ethylene-octene). J. Macromol. Sci. B 2012, 51, 1449–1462. [Google Scholar] [CrossRef]
- Doumbia, A.S.; Bourmaud, A.; Jouannet, D.; Falher, T.; Orange, F.; Retoux, R.; Le Pluart, L.; Cauret, L. Hollow microspheres–poly(propylene) blends: Relationship between microspheres degradation and composite properties. Polym. Degrad. Stab. 2015, 114, 146–153. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, Y.; Jiao, C. Synergistic effects between hollow glass microsphere and ammonium polyphosphate on flame-retardant thermoplastic polyurethane. J. Therm. Anal. Calorim. 2014, 117, 857–866. [Google Scholar] [CrossRef]
- Chuanmei, J.; Hongzhi, W.; Shaoxiang, L.; Chen, X. Fire hazard reduction of hollow glass microspheres in thermoplastic polyurethane composites. J. Hazard. Mater. 2017, 332, 176–184. [Google Scholar]
- Yung, K.C.; Zhu, B.L.; Yue, T.M.; Xie, C.S. Preparation and properties of hollow glass microsphere-filled epoxy-matrix composites. Compos. Sci. Technol. 2009, 69, 260–264. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, P.; Zhou, Y.; Li, X.; Yang, E.H.; Yu, T.X.; Yang, J. The effect of strain rate and filler volume fraction on the mechanical properties of hollow glass microspheres modified polymer. Compos. Part B Eng. 2016, 101, 53–63. [Google Scholar] [CrossRef]
- Fei, Y.; Fang, W.; Zhong, M.; Jin, J.; Fan, P.; Yang, J.; Fei, Z.; Chen, F.; Kuang, T. Morphological structure, rheological behavior, mechanical properties and sound insulation performance of thermoplastic rubber composites reinforced by different inorganic fillers. Polymers 2018, 10, 276. [Google Scholar] [CrossRef]
- Kutelova, Z.; Mainka, H.; Mader, K.; Hintz, W.; Tomas, J. Glass spheres: Functionalization, Surface Modification and Mechanical Properties. In Surface Effects in Solid Mechanics; Altenbach, H., Morozov, N.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 95–104. [Google Scholar]
- Crawford, R.J.; Throne, J.L. Rotational Molding Technology; William Andrew Publishing: New York, NY, USA, 2002. [Google Scholar]
- Ogila, K.O.; Shao, M.; Yang, W.; Tan, J. Rotational molding: A review of the models and materials. Express Polym. Lett. 2017, 11, 778–798. [Google Scholar] [CrossRef]
- Yan, W.; Lin, R.J.T.; Bhattacharyya, D. Particulate reinforced rotationally moulded polyethylene composites—Mixing methods and mechanical properties. Compos. Sci. Technol. 2006, 66, 2080–2088. [Google Scholar] [CrossRef]
- Planes, E.; Duchet, J.; Maazouz, A.; Gerard, J.-F. Characterization of new formulations for the rotational molding based on ethylene–propylene copolymer/graphite nanocomposites. Polym. Eng. Sci. 2008, 48, 723–731. [Google Scholar] [CrossRef]
- Lopez-Banuelos, R.H.; Moscoso, F.J.; Ortega-Gudino, P.; Mendizabal, E.; Rodrigue, D.; Gonzalez-Nunez, R. Rotational molding of polyethylene composites based on agave fibers. Polym. Eng. Sci. 2012, 52, 2489–2497. [Google Scholar] [CrossRef]
- Cisneros-López, E.O.; Pérez-Fonseca, A.A.; Fuentes-Talavera, F.J.; Anzaldo, J.; González-Núñez, R.; Rodrigue, D.; Robledo-Ortíz, J.R. Rotomolded polyethylene-agave fiber composites: Effect of fiber surface treatment on the mechanical properties. Polym. Eng. Sci. 2016, 56, 856–865. [Google Scholar] [CrossRef]
- Mutua, F.N.; Lin, P.; Koech, J.K.; Wang, Y. Surface modification of hollow glass microspheres. Mater. Sci. Appl. 2012, 3, 856–860. [Google Scholar] [CrossRef]
- Crescenzi, V.; Manzini, G.; Calzolari, G.; Borri, C. Thermodynamics of fusion of poly-β-propiolactone and poly-ϵ-caprolactone. Comparative analysis of the melting of aliphatic polylactone and polyester chains. Eur. Polym. J. 1972, 8, 449–463. [Google Scholar] [CrossRef]
- Kumar, N.; Mireja, S.; Khandelwal, V.; Arun, B.; Manik, G. Light-weight high-strength hollow glass microspheres and bamboo fiber based hybrid polypropylene composite: A strength analysis and morphological study. Compos. Part B Eng. 2017, 109, 277–285. [Google Scholar] [CrossRef]
- Galeotti, F.; Bertini, F.; Scavia, G.; Bolognesi, A. A controlled approach to iron nanoparticles functionalization for magnetic polymer brushes. J. Colloid Interf. Sci. 2011, 360, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Wang, T.-J.; Gao, H.; Jin, Y. High density silanization of nano-silica particles using γ-aminopropyltriethoxysilane (APTES). Appl. Surf. Sci. 2015, 351, 646–654. [Google Scholar] [CrossRef]
- Li, J.; Luo, X.; Lin, X. Preparation and characterization of hollow glass microsphere reinforced poly(butylene succinate) composites. Mater. Des. 2013, 46, 902–909. [Google Scholar] [CrossRef]
- Bertini, F.; Canetti, M.; Patrucco, A.; Zoccola, M. Wool keratin-polypropylene composites: Properties and thermal degradation. Polym. Degrad. Stab. 2013, 98, 980–987. [Google Scholar] [CrossRef]
- Hu, X.; Xu, H.-S.; Li, Z.-M. Morphology and properties of poly(l-lactide)(PLLA) filled with hollow glass beads. Macromol. Mater. Eng. 2007, 292, 646–654. [Google Scholar] [CrossRef]


| Sample | PCL/HGM/HGMf | Mw (kg/mol) | Mw/Mn | Theoretical Density (g/cm3) |
|---|---|---|---|---|
| PCL unprocessed | 100/0/0 | 177.1 | 2.1 | 1.15 |
| PCL | 100/0/0 | 177.3 | 2.0 | 1.15 |
| PCL–HGM10 | 90/10/0 | 187.8 | 2.0 | 1.08 |
| PCL–HGM15 | 85/15/0 | 183.9 | 2.0 | 1.05 |
| PCL–HGM20 | 80/20/0 | 182.4 | 2.0 | 1.01 |
| PCL–HGM25 | 75/25/0 | 181.4 | 2.0 | 0.98 |
| PCL–HGMf10 | 90/0/10 | 185.0 | 2.1 | 1.08 1 |
| PCL–HGMf20 | 80/0/20 | 180.8 | 2.1 | 1.01 1 |
| Sample | T5% (°C) | T50% (°C) | R (%) |
|---|---|---|---|
| PCL | 378 | 426 | 0 |
| PCL–HGM10 | 384 | 428 | 9 |
| PCL–HGM15 | 388 | 428 | 13 |
| PCL–HGM20 | 390 | 431 | 21 |
| PCL–HGM25 | 391 | 432 | 26 |
| PCL–HGMf10 | 368 | 420 | 9 |
| PCL–HGMf20 | 347 | 415 | 20 |
| Sample | Tc (°C) | ΔHc (J/g) | Tm (°C) | ΔHm (J/g) | χc (%) |
|---|---|---|---|---|---|
| PCL | 31.8 | 52.7 | 57.3 | 52.8 | 38.0 |
| PCL–HGM10 | 34.7 | 47.5 | 57.9 | 48.3 | 38.5 |
| PCL–HGM15 | 35.3 | 44.2 | 57.9 | 46.0 | 38.9 |
| PCL–HGM20 | 35.6 | 40.7 | 57.3 | 42.3 | 38.0 |
| PCL–HGM25 | 35.7 | 38.7 | 57.4 | 39.8 | 38.1 |
| PCL–HGMf10 | 35.9 | 47.2 | 57.8 | 48.0 | 38.3 |
| PCL–HGMf20 | 36.4 | 42.7 | 57.8 | 43.2 | 38.8 |
| Sample | E (MPa) | σ yield (MPa) | ε break (%) |
|---|---|---|---|
| PCL | 241 ± 13 | 12.8 ± 0.9 | 1303 ± 40 |
| PCL–HGM10 | 291 ± 19 | 8.1 ± 0.8 | 724 ± 38 |
| PCL–HGM15 | 343 ± 22 | 6.4 ± 0.8 | 682 ± 34 |
| PCL–HGM20 | 410 ± 20 | 5.6 ± 0.9 | 667 ± 14 |
| PCL–HGM25 | 461 ± 15 | 5.9 ± 0.4 | 567 ± 15 |
| PCL–HGMf10 | 388 ± 21 | 14.1 ± 0.7 | 672 ± 34 |
| PCL–HGMf20 | 513 ± 31 | 13.8 ± 1.2 | 384 ± 53 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vignali, A.; Iannace, S.; Falcone, G.; Utzeri, R.; Stagnaro, P.; Bertini, F. Lightweight Poly(ε-Caprolactone) Composites with Surface Modified Hollow Glass Microspheres for Use in Rotational Molding: Thermal, Rheological and Mechanical Properties. Polymers 2019, 11, 624. https://doi.org/10.3390/polym11040624
Vignali A, Iannace S, Falcone G, Utzeri R, Stagnaro P, Bertini F. Lightweight Poly(ε-Caprolactone) Composites with Surface Modified Hollow Glass Microspheres for Use in Rotational Molding: Thermal, Rheological and Mechanical Properties. Polymers. 2019; 11(4):624. https://doi.org/10.3390/polym11040624
Chicago/Turabian StyleVignali, Adriano, Salvatore Iannace, Giulio Falcone, Roberto Utzeri, Paola Stagnaro, and Fabio Bertini. 2019. "Lightweight Poly(ε-Caprolactone) Composites with Surface Modified Hollow Glass Microspheres for Use in Rotational Molding: Thermal, Rheological and Mechanical Properties" Polymers 11, no. 4: 624. https://doi.org/10.3390/polym11040624
APA StyleVignali, A., Iannace, S., Falcone, G., Utzeri, R., Stagnaro, P., & Bertini, F. (2019). Lightweight Poly(ε-Caprolactone) Composites with Surface Modified Hollow Glass Microspheres for Use in Rotational Molding: Thermal, Rheological and Mechanical Properties. Polymers, 11(4), 624. https://doi.org/10.3390/polym11040624






