Deuteration-Induced Volume Phase Transition Temperature Shift of PNIPMAM Microgels
Abstract
:1. Introduction
2. Materials and Methods
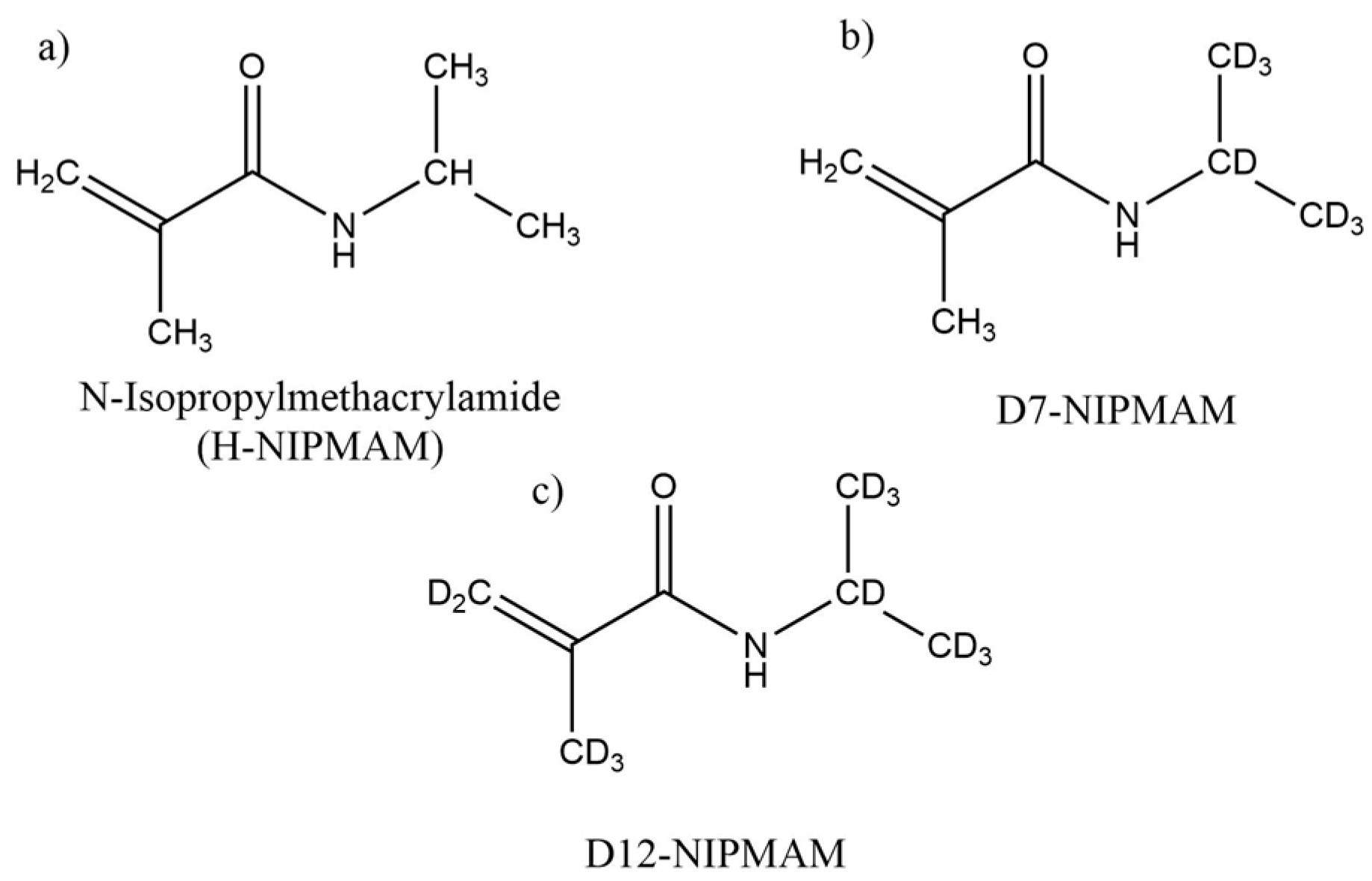
2.1. Synthesis
2.2. Photon Correlation Spectroscopy
2.3. Infrared Spectroscopy
2.4. Atomic Force Microscopy
3. Results
3.1. Particle Size and Shape
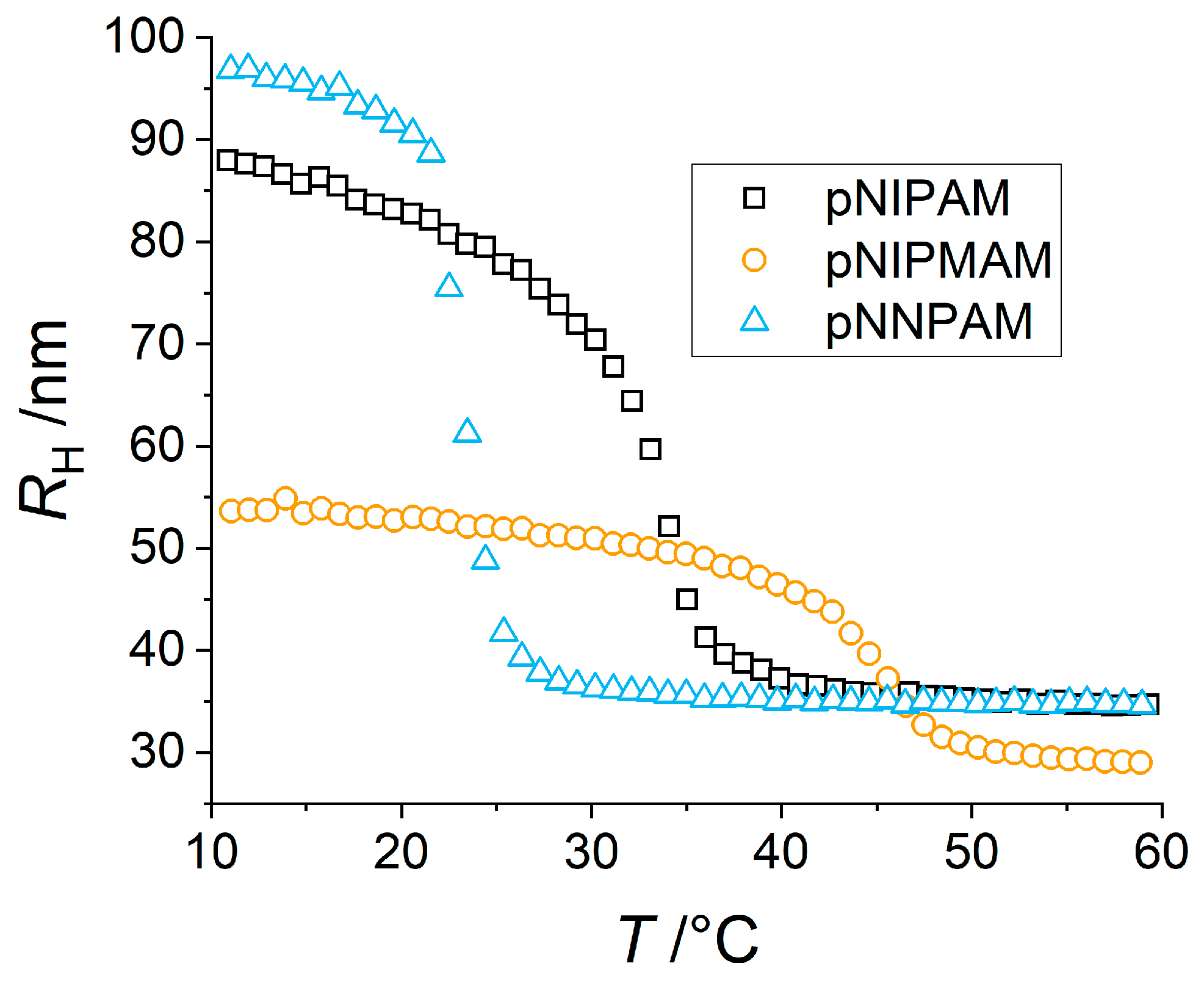
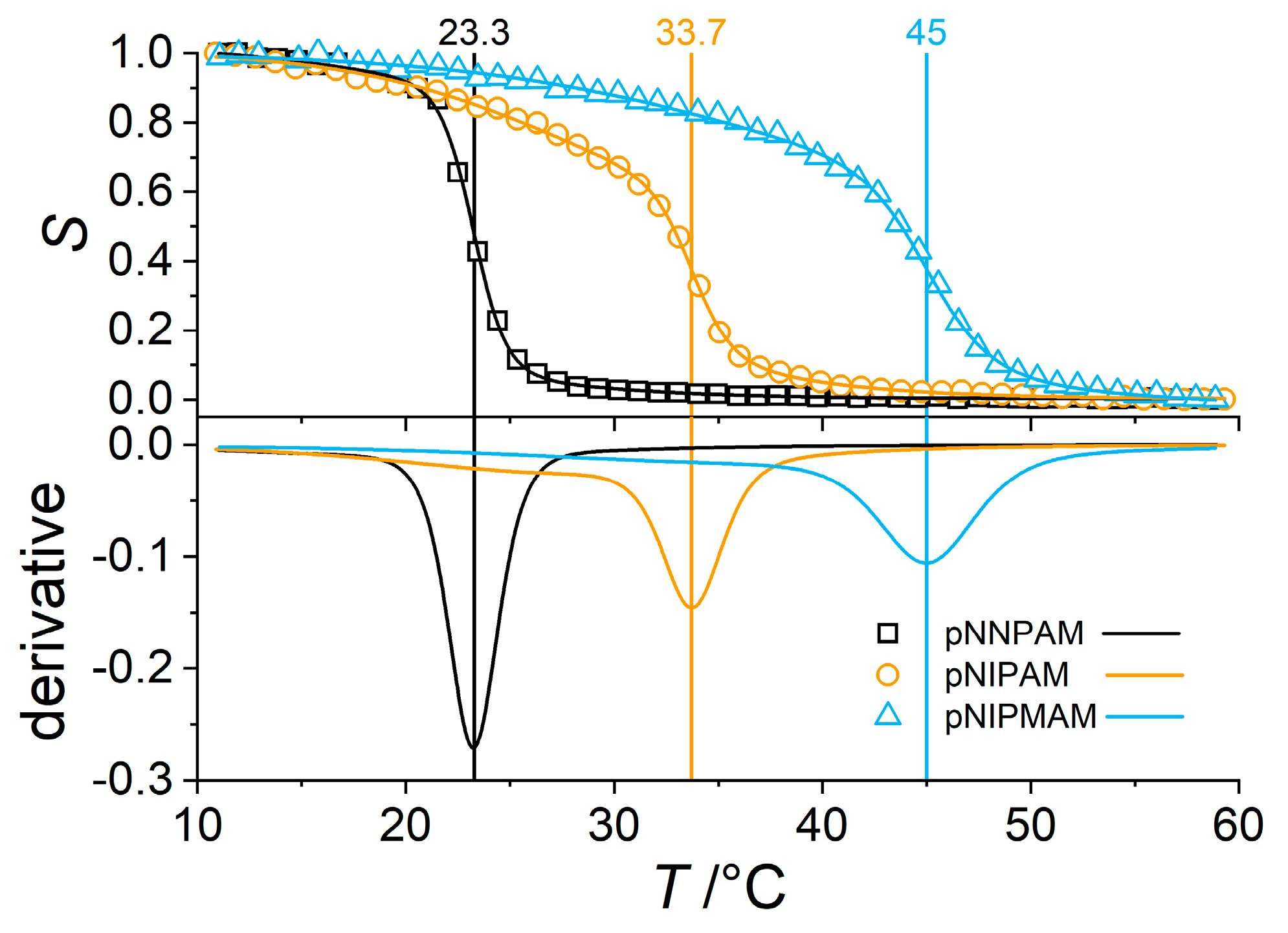
3.2. Swelling Behavior of Microgels
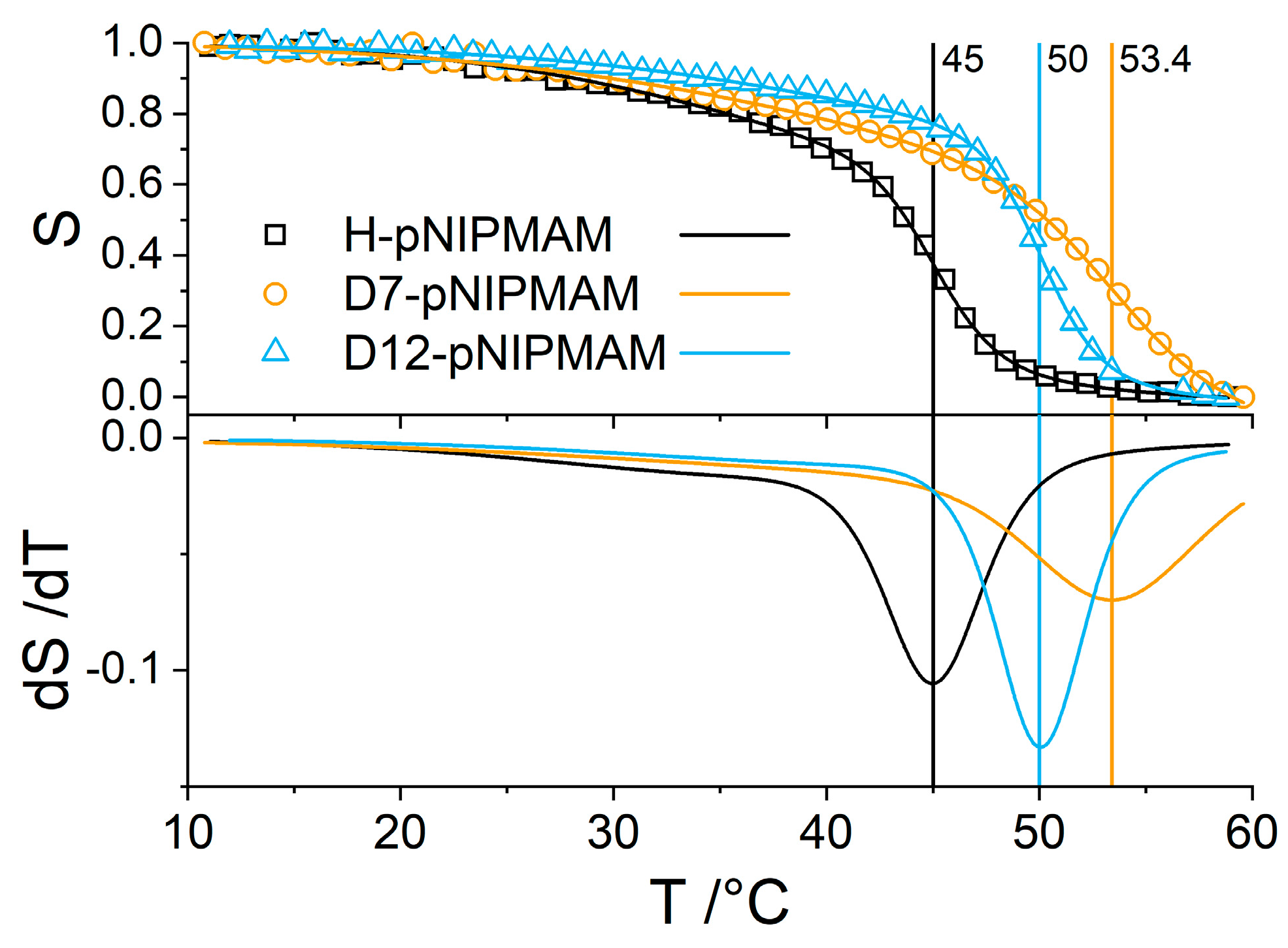
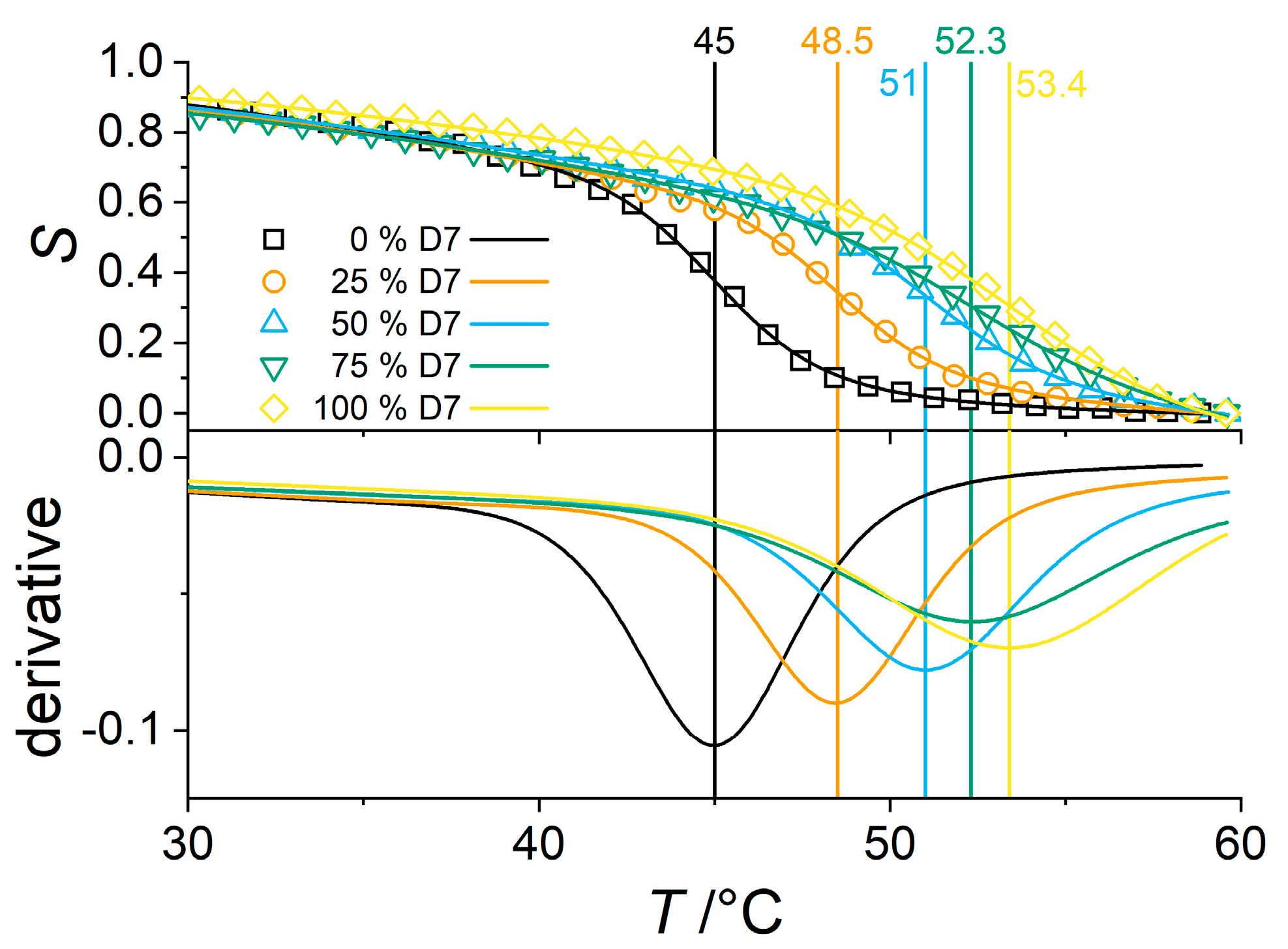
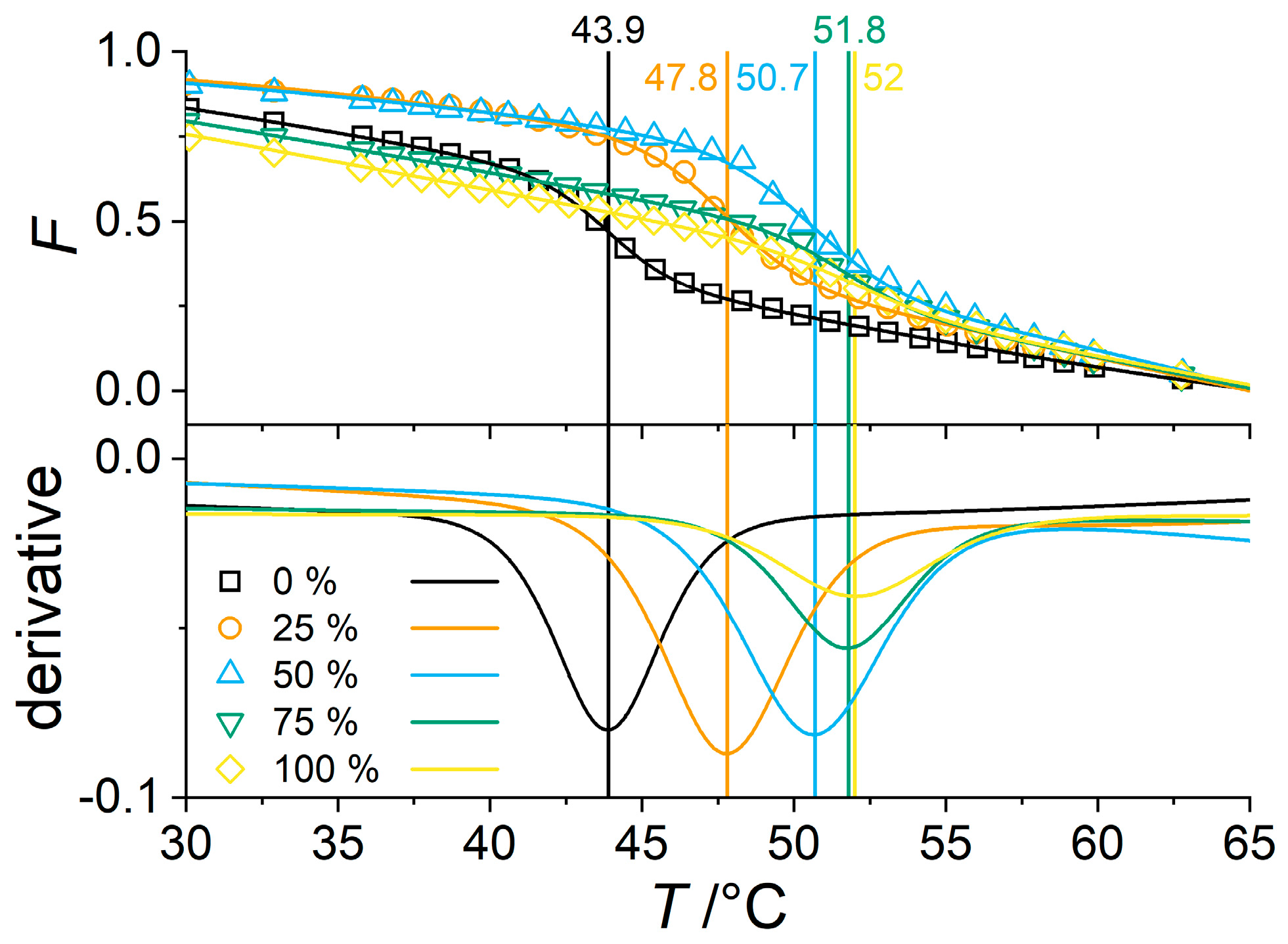
3.3. Effect of PNIPMAM Monomer Deuteration
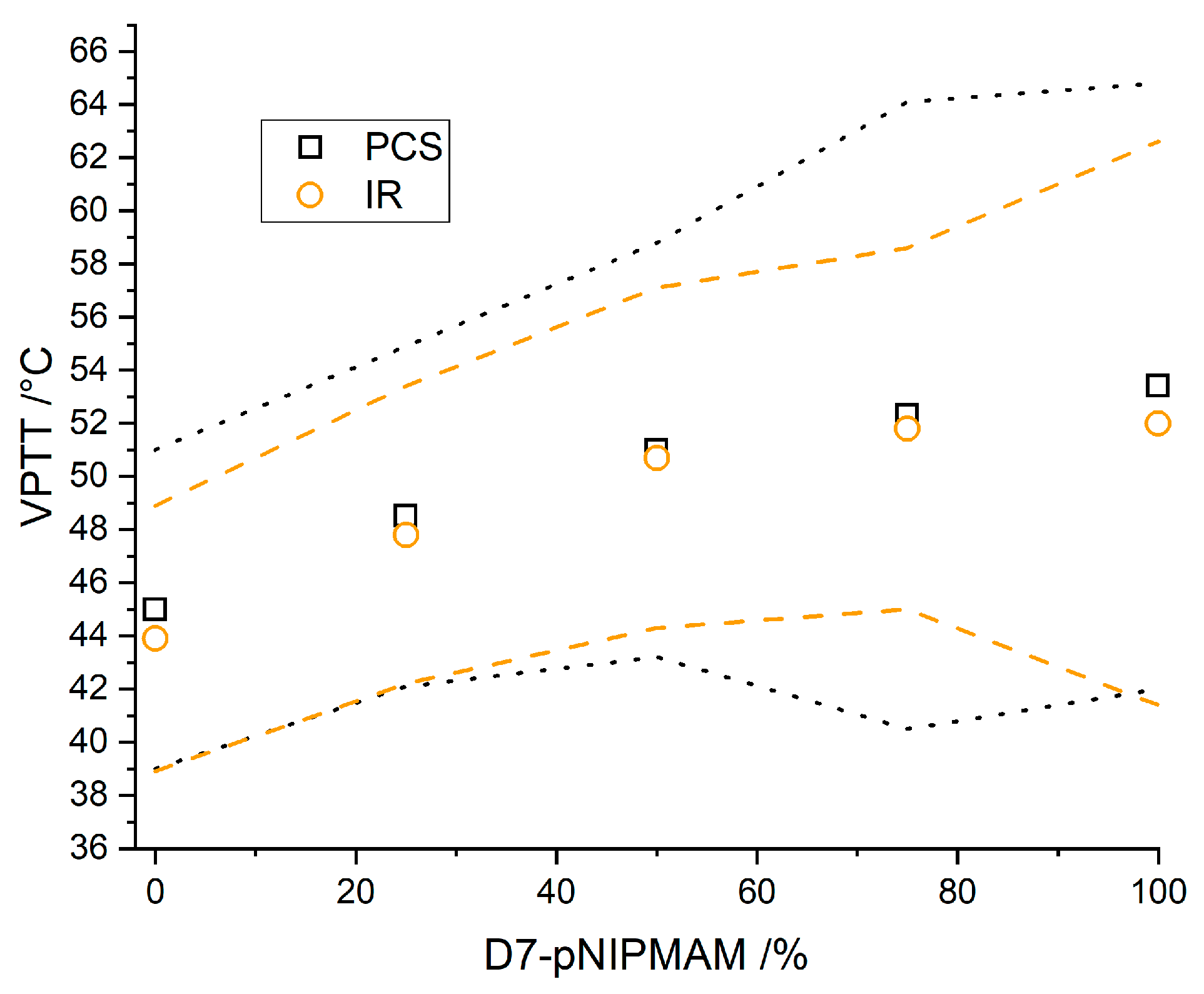
3.4. Partially Deuterated Co-Polymers
3.5. Influence of the Solvent on the VPTT of PNIPMAM Microgels
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pelton, R. Temperature-sensitive aqueous microgels. Adv. Colloid Interface Sci. 2000, 85, 1–33. [Google Scholar] [CrossRef]
- Richtering, W.; Saunders, B.R. Gel architectures and their complexity. Soft Matter 2014, 10, 3695–3702. [Google Scholar] [CrossRef]
- Nolan, C.M.; Serpe, M.J.; Lyon, L.A. Thermally modulated insulin release from microgel thin films. Biomacromolecules 2004, 5, 1940–1946. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Lyon, L.A. Soft nanotechnology with soft nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 7686–7708. [Google Scholar] [CrossRef]
- Serpe, M.J.; Yarmey, K.A.; Nolan, C.M.; Lyon, L.A. Doxorubicin uptake and release from microgel thin films. Biomacromolecules 2005, 6, 408–413. [Google Scholar] [CrossRef]
- Nolan, C.M.; Serpe, M.J.; Lyon, L.A. Pulsatile release of insulin from layer-by-layer assembled microgel thin films. Macromol. Symp. 2005, 227, 285–294. [Google Scholar] [CrossRef]
- Saunders, B.R.; Laajam, N.; Daly, E.; Teow, S.; Hu, X.; Stepto, R. Microgels: From responsive polymer colloids to biomaterials. Adv. Colloid Interface Sci. 2009, 147–148, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Hellweg, T. Towards large-scale photonic crystals with tuneable bandgaps. Angew. Chem. Int. Ed. 2009, 48, 6777–6778. [Google Scholar] [CrossRef]
- Clara-Rahola, J.; Contreras-Caceres, R.; Sierra-Martin, B.; Maldonado-Valdivia, A.; Hund, M.; Fery, A.; Hellweg, T.; Fernandez-Barbero, A. Structure and plasmon coupling of gold-poly(N-isopropylacrylamide) coreshell microgel arrays with thermally controlled interparticle gap. Colloids Surf. A Physicochem. Eng. Aspects 2014, 463, 18–27. [Google Scholar] [CrossRef]
- Ballauff, M.; Lu, Y. smart nanoparticles: Preparation, characterization and applications. Polymer 2007, 48, 1815–1823. [Google Scholar] [CrossRef]
- Lu, Y.; Proch, S.; Schrinner, M.; Drechsler, M.; Kempe, R.; Ballauff, M. Thermosensitive core-shell microgel as a nanoreactor for catalytic active metal nanoparticles. J. Mater. Chem. 2009, 19, 3955. [Google Scholar] [CrossRef]
- Lu, Y.; Ballauff, M. Thermosensitive coreshell microgels: From colloidal model systems to nanoreactors. Prog. Polym. Sci. 2011, 36, 767–792. [Google Scholar] [CrossRef]
- Welsch, N.; Becker, A.L.; Dzubiella, J.; Ballauff, M. Coreshell microgels as smart carriers for enzymes. Soft Matter 2012, 8, 1428–1436. [Google Scholar] [CrossRef]
- Wellert, S.; Richter, M.; Hellweg, T.; von Klitzing, R.; Hertle, Y. Responsive microgels at surfaces and interfaces. Z. Phys. Chem. 2015, 229, 1225–1250. [Google Scholar] [CrossRef]
- Cors, M.; Wrede, O.; Genix, An.; Anselmetti, D.; Oberdisse, J.; Hellweg, T. Coreshell microgel-based surface coatings with linear thermoresponse. Langmuir 2017, 33, 6804–6811. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Serpe, M.J. Understanding and controlling the self-folding behavior of poly (N-isopropylacrylamide) microgel-based devices. Adv. Funct. Mater. 2014, 24, 4119–4126. [Google Scholar] [CrossRef]
- Zhang, Q.M.; Berg, D.; Mugo, S.M.; Serpe, M.J. Lipase-modified pH-responsive microgel-based optical device for triglyceride sensing. Chem. Commun. 2015, 51, 9726–9728. [Google Scholar] [CrossRef] [PubMed]
- Yoshitsugu Hirokawa and Toyoichi Tanaka. Volume phase transition in a nonionic gel. J. Chem. Phys. 1984, 81, 6379–6380. [Google Scholar] [CrossRef]
- Saunders, B.R.; Vincent, B. Microgel particles as model colloids: Theory, properties and applications. Adv. Colloid Interface Sci. 1999, 80, 1–25. [Google Scholar] [CrossRef]
- Balaceanu, A.; Demco, D.E.; Möller, M.; Pich, A. Heterogeneous morphology of random copolymer microgels as reflected in temperature-induced volume transition and 1H high-resolution transverse relaxation NMR. Macromol. Chem. Phys. 2011, 212, 2467–2477. [Google Scholar] [CrossRef]
- Wu, Y.; Wiese, S.; Balaceanu, A.; Richtering, W.; Pich, A. Behavior of temperature-responsive copolymer microgels at the oil/water interface. Langmuir 2014, 30, 7660–7669. [Google Scholar] [CrossRef]
- Berndt, I.; Pedersen, J.; Richtering, W. Temperature-sensitive coreshell microgel particles with dense shell. Angew. Chem. Int. Ed. 2006, 45, 1737–1741. [Google Scholar] [CrossRef]
- Wedel, B.; Zeiser, M.; Hellweg, T. Non NIPAM based smart microgels: Systematic variation of the volume phase transition temperature by copolymerization. Z. Phys. Chem. 2012, 226, 737–748. [Google Scholar] [CrossRef]
- Crassous, J.J.; Mihut, A.M.; Månsson, L.K.; Schurtenberger, P. Anisotropic responsive microgels with tuneable shape and interactions. Nanoscale 2015, 7, 15971–15982. [Google Scholar] [CrossRef]
- Zeiser, M.; Freudensprung, I.; Hellweg, T. Linearly thermoresponsive coreshell microgels: Towards a new class of nanoactuators. Polymer 2012, 53, 6096–6101. [Google Scholar] [CrossRef]
- Iwai, K.; Matsumura, Y.; Uchiyama, S.; de Silva, A.P. Development of fluorescent microgel thermometers based on thermo-responsive polymers and their modulation of sensitivity range. J. Mater. Chem. 2005, 15, 2796. [Google Scholar] [CrossRef]
- Pusey, P.N. Introduction to Scattering Experiments. In Neutron, X-Rays and Light. Scattering Methods Applied to Soft Condensed Matter; Lindner, T.Z.P., Ed.; Chapter Introduction to Scattering Experiments; Elsevier Science B. V.: Amsterdam, The Netherlands, 2002; pp. 3–22. [Google Scholar]
- Stieger, M.; Richtering, W.; Pedersen, J.; Lindner, P. Small-angle neutron scattering study of structural changes in temperature sensitive microgel colloids. J. Chem. Phys. 2004, 120, 6197–6206. [Google Scholar] [CrossRef]
- Boon, N.; Schurtenberger, P. Swelling of micro-hydrogels with a crosslinker gradient. Phys. Chem. Chem. Phys. 2017, 19, 23740–23746. [Google Scholar] [CrossRef] [PubMed]
- Cors, M.; Wiehemeier, L.; Hertle, Y.; Feoktystov, A.; Cousin, F.; Hellweg, T.; Oberdisse, J. Determination of internal density profiles of smart acrylamide-based microgels by small-angle neutron scattering: A multishell reverse monte carlo approach. Langmuir 2018, 34, 15403–15415. [Google Scholar] [CrossRef]
- Keerl, M.; Pedersen, J.; Richtering, W. Temperature sensitive copolymer microgels with nanophase separated structure. J. Am. Chem. Soc. 2009, 131, 3093–3097. [Google Scholar] [CrossRef]
- Balaceanu, A.; Mayorga, V.; Lin, W.; Schürings, Ma.; Demco, D.E.; Böker, A.; Winnik, M.A.; Pich, A. Copolymer microgels by precipitation polymerisation of N-vinylcaprolactam and N-isopropylacrylamides in aqueous medium. Colloid Polym. Sci. 2012, 291, 21–31. [Google Scholar] [CrossRef]
- Wellert, S.; Hertle, Y.; Richter, M.; Medebach, M.; Magerl, D.; Wang, W.; Demé, B.; Radulescu, A.; Müller-Buschbaum, P.; Hellweg, T.; von Klitzing, R. Inner structure of adsorbed ionic microgel particles. Langmuir 2014, 30, 7168–7176. [Google Scholar] [CrossRef]
- Berndt, I.; Richtering, W. Doubly temperature sensitive core-shell microgels. Macromolecules 2003, 36, 8780–8785. [Google Scholar] [CrossRef]
- Berndt, I.; Pedersen, J.; Richtering, W. Structure of multiresponsive intelligent core-shell microgels. J. Am. Chem. Soc. 2005, 127, 9372–9373. [Google Scholar] [CrossRef]
- Berndt, I.; Pedersen, J.; Lindner, P.; Richtering, W. Influence of shell thickness and cross-link density on the structure of temperature-sensitive poly-N-isopropylacrylamide-poly-N-isopropylmethacrylamide core-shell microgels investigated by small-angle neutron scattering. Langmuir 2006, 22, 459–468. [Google Scholar] [CrossRef]
- Hellweg, T. Responsive core-shell microgels: Synthesis, characterization, and possible applications. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1073–1083. [Google Scholar] [CrossRef]
- Malmgren, J.; Santoro, S.; Jalalian, N.; Himo, F.; Olofsson, B. Arylation with unsymmetrical diaryliodonium salts: A chemoselectivity study. Chem. Eur. J. 2013, 19, 10334–10342. [Google Scholar] [CrossRef]
- Dubbert, J.; Nothdurft, K.; Karg, M.; Richtering, W. Core-shell-shell and hollow double-shell microgels with advanced temperature responsiveness. Macromol. Rapid Commun. 2014, 36, 159–164. [Google Scholar] [CrossRef]
- Brugnoni, M.; Scotti, A.; Rudov, A.A.; Gelissen, A.P.H.; Caumanns, T.; Radulescu, A.; Eckert, T.; Pich, A.; Potemkin, I.I.; Richtering, W. Swelling of a responsive network within different constraints in multi-thermosensitive microgels. Macromolecules 2018, 51, 2662–2671. [Google Scholar] [CrossRef]
- Dubbert, J.; Honold, T.; Pedersen, J.; Radulescu, A.; Drechsler, M.; Karg, M.; Richtering, W. How hollow are thermoresponsive hollow nanogels? Macromolecules 2014, 47, 8700–8708. [Google Scholar] [CrossRef]
- Schmid, A.J.; Dubbert, J.; Rudov, A.A.; Pedersen, J.; Lindner, P.; Karg, M.; Potemkin, I.I.; Richtering, W. Multi-shell hollow nanogels with responsive shell permeability. Sci. Rep. 2016, 6, 22736. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, P.S.; Nöjd, S.; van Gruijthuijsen, K.; Crassous, J.J.; Obiols-Rabasa, M.; Schweins, R.; Stradner, A.; Schurtenberger, P. Interpenetration of polymeric microgels at ultrahigh densities. Sci. Rep. 2017, 7, 1487. [Google Scholar] [CrossRef] [PubMed]
- Scotti, A.; Brugnoni, M.; Rudov, A.A.; Houston, J.E.; Potemkin, I.I.; Richtering, W. Hollow microgels squeezed in overcrowded environments. J. Chem. Phys. 2018, 148, 174903. [Google Scholar] [CrossRef]
- Nöjd, S.; Holmqvist, P.; Boon, N.; Obiols-Rabasa, M.; Mohanty, P.S.; Schweins, R.; Schurtenberger, P. Deswelling behaviour of ionic microgel particles from low to ultra-high densities. Soft Matter 2018, 14, 4150–4159. [Google Scholar] [CrossRef] [PubMed]
- Holstein, P.; Barmatov, E.B.; Geschke, D.; Bender, M.; Shibaev, V.P. Ordering in a nematic side-chain polymer. A proton and deuterium nuclear magnetic resonance study. Colloid Polym. Sci. 2000, 278, 711–718. [Google Scholar] [CrossRef]
- Russell, R.A.; Garvey, C.J.; Darwish, T.A.; Foster, L.J.R.; Holden, P.J. Biopolymer deuteration for neutron scattering and other isotope-sensitive techniques. In Isotope Labeling of Biomolecules—Labeling Methods; Elsevier: Amsterdam, The Netherlands, 2015; pp. 97–121. [Google Scholar] [CrossRef]
- Hu, R.; Kan, W.; Xiong, X.; Wei, H. Preparation of a deuterated polymer: Simulating to produce a solid tritium radioactive source. J. Nucl. Mater. 2017, 492, 171–177. [Google Scholar] [CrossRef]
- Saunders, B.R.; Crowther, H.M.; Morris, G.E.; Mears, S.J.; Cosgrove, T.; Vincent, B. Factors affecting the swelling of poly(n-isopropylacrylamide) microgel particles: Fundamental and commercial implications. Colloids Surf. A Physicochem. Eng. Aspects 1999, 149, 57–64. [Google Scholar] [CrossRef]
- Crowther, H.M.; Saunders, B.R.; Mears, S.J.; Cosgrove, T.; Vincent, B.; King, S.M.; Yu, G.-F. Poly(NIPAM) microgel particle de-swelling: A light scattering and small-angle neutron scattering study. Colloids Surf. A Physicochem. Eng. Aspects 1999, 152, 327–333. [Google Scholar] [CrossRef]
- Koppel, D.E. Analysis of macromolecular polydispersity in intensity correlation spectroscopy: The method of cumulants. J. Chem. Phys. 1972, 57, 4814–4820. [Google Scholar] [CrossRef]
- Hassan, P.A.; Kulshreshtha, S.K. Modification to the cumulant analysis of polydispersity in quasielastic light scattering data. J. Colloid Interface Sci. 2006, 300, 744–748. [Google Scholar] [CrossRef]
- Wiehemeier, L.; Cors, M.; Wrede, O.; Oberdisse, J.; Hellweg, T.; Kottke, T. Swelling behaviour of coreshell microgels in H2O, analysed by temperature-dependent FTIR spectroscopy. Phys. Chem. Chem. Phys. 2019, 21, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Burmistrova, A.; von Klitzing, R. Control of number density and swelling/shrinking behavior of p(NIPAMAAc) particles at solid surfaces. J. Mater. Chem. 2010, 20, 3502. [Google Scholar] [CrossRef]
- Vikulina, A.S.; Aleed, S.T.; Paulraj, T.; Vladimirov, Y.A.; Duschl, C.; von Klitzing, R.; Volodkin, D. Temperature-induced molecular transport through polymer multilayers coated with PNIPAM microgels. Phys. Chem. Chem. Phys. 2015, 17, 12771–12777. [Google Scholar] [CrossRef]
- Bergmann, S.; Wrede, O.; Huser, T.; Hellweg, T. Super-resolution optical microscopy resolves network morphology of smart colloidal microgels. Phys. Chem. Chem. Phys. 2018, 20, 5074–5083. [Google Scholar] [CrossRef]
- Kratz, K.; Hellweg, T.; Eimer, W. Structural changes in PNIPAM microgel particles as seen by SANS, DLS, and EM techniques. Polymer 2001, 42, 6631–6639. [Google Scholar] [CrossRef]
- Wu, C. A comparison between the coil-to-globule transition of linear chains and the volume phase transition of spherical microgels1dedicated to the 80th birthday of professor renyuan qian.1. Polymer 1998, 39, 4609–4619. [Google Scholar] [CrossRef]
- von Nessen, K.; Karg, M.; Hellweg, T. Thermoresponsive poly-(N-isopropylmethacrylamide) microgels: Tailoring particle size by interfacial tension control. Polymer 2013, 54, 5499–5510. [Google Scholar] [CrossRef]
- Perrin, C.L.; Karri, P. Position-specific secondary deuterium isotope effects on basicity of pyridine. J. Am. Chem. Soc. 2010, 132, 12145–12149. [Google Scholar] [CrossRef]
- Keerl, M.; Smirnovas, V.; Winter, R.; Richtering, W. Interplay between hydrogen bonding and macromolecular architecture leading to unusual phase behavior in thermosensitive microgels. Angew. Chem. Int. Ed. 2008, 47, 338–341. [Google Scholar] [CrossRef]
- Turowski, M.; Yamakawa, N.; Meller, J.; Kimata, K.; Ikegami, T.; Hosoya, K.; Tanaka, N.; Thornton, E.R. Deuterium isotope effects on hydrophobic interactions: The importance of dispersion interactions in the hydrophobic phase. J. Am. Chem. Soc. 2003, 125, 13836–13849. [Google Scholar] [CrossRef]
- Shibayama, M.; Tanaka, T.; Han, C.C. Small angle neutron scattering study on poly(n-isopropyl acrylamide) gels near their volume-phase transition temperature. J. Chem. Phys. 1992, 97, 6829–6841. [Google Scholar] [CrossRef]
- Djokpé, E.; Vogt, W. N-isopropylacrylamide andN-isopropylmethacryl-amide: Cloud points of mixtures and copolymers. Macromol. Chem. Phys. 2001, 202, 750–757. [Google Scholar] [CrossRef]
- Udagawa, T.; Ishimoto, T.; Tokiwa, H.; Tachikawa, M.; Nagashima, U. Geometric isotope effect of various intermolecular and intramolecular c-ho hydrogen bonds, using the multicomponent molecular orbital method. J. Phys. Chem. A 2006, 110, 7279–7285. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Ma, K.; An, Z.; Wu, P. Exploring the volume phase transition behavior of POEGA- and PNIPAM-based coreshell nanogels from infrared-spectral insights. Macromolecules 2014, 47, 1144–1154. [Google Scholar] [CrossRef]
- Keerl, M.; Smirnovas, V.; Winter, R.; Richtering, W. Copolymer microgels from mono- and disubstituted acrylamides: Phase behavior and hydrogen bonds. Macromolecules 2008, 41, 6830–6836. [Google Scholar] [CrossRef]
- Maeda, Y.; Higuchi, T.; Ikeda, I. Change in hydration state during the coil-globule transition of aqueous solutions of poly(n-isopropylacrylamide) as evidenced by FTIR spectroscopy. Langmuir 2000, 16, 7503–7509. [Google Scholar] [CrossRef]
- Dahlgren, G.; Long, F.A. Relative hydrogen bonding of deuterium. I. ionization constants of maleic and fumaric acids and of their monoethyl esters in H2O and D2O1. J. Am. Chem. Soc. 1960, 82, 1303–1308. [Google Scholar] [CrossRef]
- Bellamy, L.J.; Rogasch, P.E. Proton transfer in hydrogen bonded systems. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1960, 257, 98–108. [Google Scholar] [CrossRef]
- Grimison, A. The deuterium isotope effect in the hydrogen bonding of imidazole in naphthalene solutions. J. Phys. Chem. 1963, 67, 962–964. [Google Scholar] [CrossRef]
- Singh, S.; Rao, C.N.R. Deuterium isotope effects on hydrogen bonding. Can. J. Chem. 1966, 44, 2611–2615. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cors, M.; Wiehemeier, L.; Oberdisse, J.; Hellweg, T. Deuteration-Induced Volume Phase Transition Temperature Shift of PNIPMAM Microgels. Polymers 2019, 11, 620. https://doi.org/10.3390/polym11040620
Cors M, Wiehemeier L, Oberdisse J, Hellweg T. Deuteration-Induced Volume Phase Transition Temperature Shift of PNIPMAM Microgels. Polymers. 2019; 11(4):620. https://doi.org/10.3390/polym11040620
Chicago/Turabian StyleCors, Marian, Lars Wiehemeier, Julian Oberdisse, and Thomas Hellweg. 2019. "Deuteration-Induced Volume Phase Transition Temperature Shift of PNIPMAM Microgels" Polymers 11, no. 4: 620. https://doi.org/10.3390/polym11040620
APA StyleCors, M., Wiehemeier, L., Oberdisse, J., & Hellweg, T. (2019). Deuteration-Induced Volume Phase Transition Temperature Shift of PNIPMAM Microgels. Polymers, 11(4), 620. https://doi.org/10.3390/polym11040620






