Effect of Multivalent Cations on Intermolecular Association of Isotactic and Atactic Poly(Methacrylic Acid) Chains in Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Solutions
2.3. Methods
3. Results
3.1. Calorimetry
3.2. Light Scattering
3.3. pH Measurements
3.4. Fluorimetry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dudev, T.; Lim, C. Principles governing Mg, Ca, and Zn binding and selectivity in proteins. Chem. Rev. 2003, 103, 773–787. [Google Scholar] [CrossRef] [PubMed]
- Dautzenberg, H.; Jaeger, W.; Kötz, J.; Philipp, B.; Seidel, C.; Stscherbina, B. Polyelectrolytes. Formation, Characterization and Application; Hanser: Munich, Germany, 1994. [Google Scholar]
- Cilurzo, F.; Gennari, C.G.M.; Selmin, F.; Vistoli, G. Effects of metal ions on entero-soluble poly(methacrylic acid-methylmethacrylate) coating: A combined analysis by ATR-FTIR spectroscopy and computational approaches. Mol. Pharm. 2010, 7, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Levin, Y. Electrostatic correlations: From plasma to biology. Rep. Prog. Phys. 2002, 65, 924–933. [Google Scholar] [CrossRef]
- Costa, D.; Burrows, H.D.; Miguel, M.G. Changes in hydration of lanthanide ions on binding to DNA in aqueous solution. Langmuir 2005, 21, 10492–10496. [Google Scholar] [CrossRef]
- Burrows, H.D.; Costa, D.; Ramos, M.L.; da Graça Miguel, M.; Teixeira, M.H.; Pais, A.A.C.C.; Valente, A.J.M.; Bastos, M.; Bai, G. Does cation dehydration drive the binding of metal ions to polyelectrolytes in water? What we can learn from the behaviour of aluminium(III) and chromium(III). Phys. Chem. Chem. Phys. 2012, 14, 7950–7953. [Google Scholar] [CrossRef] [PubMed]
- Mattai, J.; Kwak, J.C.T. Binding of La3+ ions by dextran sulfate polyanions in aqueous solutions containing excess sodium chloride. J. Phys. Chem. 1984, 88, 2625–2629. [Google Scholar] [CrossRef]
- Dudev, T.; Lim, C. Effect of carboxylate-binding mode on metal binding/selectivity and function in proteins. Acc. Chem. Res. 2007, 40, 85–93. [Google Scholar] [CrossRef]
- Kaufman Katz, A.; Glusker, J.P.; Beebe, S.A.; Bock, C.W. Calcium ion coordination: A comparison with that of beryllium, magnesium, and zinc. J. Am. Chem. Soc. 1996, 118, 5752–5763. [Google Scholar] [CrossRef]
- Johnen-Dechent, W.; Kettler, M. Magnesium basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef] [PubMed]
- Schauss, J.; Dahms, F.; Fingerhut, B.P.; Elsaesser, T. Phosphate-magnesium ion interactions in water probed by ultrafast 2D-IR spectroscopy. J. Phys. Chem. Lett. 2019, 10, 238–243. [Google Scholar] [CrossRef]
- Maguire, M.E.; Cowan, J.A. Magnesium chemistry and biochemistry. Biometals 2002, 15, 203–210. [Google Scholar] [CrossRef]
- Dudev, T.; Lim, C. Monodentate versus bidentate carboxylate binding in magnesium and calcium proteins: What are the basic principles? J. Phys. Chem. B 2004, 108, 4546–4557. [Google Scholar] [CrossRef]
- Kavitha, S.; Deepa, P.; Karthika, M.; Kanakaraju, R. Hybrid DFT study on non-covalent interactions and their influence on pKa’s of magnesium-carboxylate complexes. J. Mol. Graph. Model. 2018, 85, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Needham, J.V.; Chen, T.Y.; Falke, J.J. Novel ion specificity of a carboxylate cluster Mg(II) binding site: Strong charge selectivity and weak size selectivity. Biochemistry 1993, 32, 3363–3367. [Google Scholar] [CrossRef]
- Collins, K.D. Ion hydration: Implications for cellular function, polyelectrolytes, and protein crystallization. Biophys. Chem. 2006, 119, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Dudev, T.; Cowan, J.A.; Lim, C. Competitive binding in magnesium coordination chemistry: Water versus ligands of biological interest. J. Am. Chem. Soc. 1999, 121, 7665–7673. [Google Scholar] [CrossRef]
- Panichev, A.M. Rare earth elements: Review of medical and biological properties and their abundance in the rock materials and mineralized spring waters in the context of animal and human geophagia reasons evaluation. Achiev. Life Sci. 2015, 9, 95–103. [Google Scholar] [CrossRef]
- Brittain, H.G.; Richardson, F.S.; Martin, R.B. Terbium(III) emission as a probe of calcium(II) binding sites in proteins. J. Am. Chem. Soc. 1976, 98, 8255–8260. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.B.; Richardson, F.S. Lanthanides as probes for calcium in biological systems. Q. Rev. Biophys. 1979, 12, 181–209. [Google Scholar] [CrossRef]
- Dos Remedios, C.G. Lanthanide ion probes of calcium-binding sites on cellular membranes. Cell Calcium 1981, 2, 29–51. [Google Scholar] [CrossRef]
- Persson, I. Hydrated metal ions in aqueous solution: How regular are their structures? Pure Appl. Chem. 2010, 82, 1901–1917. [Google Scholar] [CrossRef]
- Okamoto, S.; Vyprachticky, D.; Furuya, H.; Abe, A.; Okamoto, Y. Ion binding properties of polycarboxylates using terbium(III) as a fluorescent probe: Viscosities and coordinated water molecules in polycarboxylate-terbium(III) complexes in aqueous solutions. Macromolecules 1996, 29, 3511–3514. [Google Scholar] [CrossRef]
- Sitar, S.; Aseyev, V.; Kogej, K. Differences in association behaviour of isotactic and atactic poly(methacrylic acid). Polymer 2014, 55, 848–854. [Google Scholar] [CrossRef]
- Sitar, S.; Aseyev, V.; Kogej, K. Microgel-like aggregates of isotactic and atactic poly(methacrylic acid) chains in aqueous alkali chloride solutions as evidenced by light scattering. Soft Matter 2014, 10, 7712–7722. [Google Scholar] [CrossRef] [PubMed]
- Kogej, K. Thermodynamic analysis of the conformational transition in aqueous solutions of isotactic and atactic poly(methacrylic acid) and the hydrophobic effect. Polymers 2016, 8, 168. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, E.; Keil, Q.; Filipcsei, G.; Berghmans, H.; Reynaers, H. Structure formation in isotactic poly(methacrylic acid). Macromolecules 2004, 37, 9673–9675. [Google Scholar] [CrossRef]
- Hočevar, K.; Sitar, S.; Kogej, K. Aggregates of isotactic poly(methacrylic acid) chains in aqueous CsCl solutions: A static and dynamic light scattering study. Acta Chim. Slov. 2015, 62, 546–554. [Google Scholar] [CrossRef]
- Kogej, K.; Fonseca, S.M.; Rovisco, J.; Azenha, M.E.; Ramos, M.L.; Seixas de Melo, J.S.; Burrows, H.D. Understanding the interaction between trivalent lanthanide ions and stereoregular polymethacrylates through luminescence, binding isotherms, NMR, and interaction with cetylpyridinium chloride. Langmuir 2013, 29, 14429–14437. [Google Scholar] [CrossRef]
- O’Neill, J.J.; Loebl, E.M.; Kandanian, A.Y.; Morawetz, H. Dependence of the association of poly(methacrylic acid) with divalent cations on the stereoregularity of the polymers. J. Polym. Sci. A 1965, 3, 4201–4204. [Google Scholar] [CrossRef]
- Costantino, L.; Crescenzi, V.; Quadrifoglio, F.; Vitagliano, V. Influence of stereoregularity on binding of counterions by poly(methacrylic acid). J. Polym. Sci. A-2 1967, 5, 771–780. [Google Scholar] [CrossRef]
- Kolawole, E.G.; Bello, M.A. Interaction of divalent ions of copper, magnesium and zinc with isotactic polymethacrylic acid. Eur. Polym. J. 1980, 16, 325–332. [Google Scholar] [CrossRef]
- Morcellet, M. Effect of tacticity on the association of poly(methacrylic acid) with divalent metal ions. J. Polym. Sci. C Polym. Lett. 1985, 23, 99–102. [Google Scholar] [CrossRef]
- Jacobson, A.L. Configurational effects on binding of magnesium to polyacrylic acid. J. Polym. Sci. 1962, 57, 321–336. [Google Scholar] [CrossRef]
- Konradi, R.; Rühe, J. Interaction of poly(methacrylic acid) brushes with metal ions: An infrared investigation. Macromolecules 2004, 37, 6954–6961. [Google Scholar] [CrossRef]
- Burrows, H.D.; Chimamkpam, T.O.; Encarnação, T.; Fonseca, S.M.; Pereira, R.F.P.; Ramos, M.L.; Valente, A.J.M. Trivalent metal ion binding to surfactants and polyelectrolytes—A review. J. Surf. Sci. Technol. 2010, 26, 197–212. [Google Scholar]
- Nagata, I.; Okamoto, Y. Investigation on ion binding in synthetic polyelectrolyte solutions using rare earth metal fluorescence probes. Macromolecules 1983, 16, 749–753. [Google Scholar] [CrossRef]
- Choppin, G.R.; Graffeo, A.J. Complexes of trivalent lanthanide and actinide ions. II. Inner-sphere complexes. Inorg. Chem. 1965, 4, 1254–1257. [Google Scholar] [CrossRef]
- Horváth, J.; Nagy, M. Thermodynamic characterization of rare earth salts of strong polyacid copolymers. J. Phys. Chem. B 2007, 111, 5140–5148. [Google Scholar] [CrossRef]
- Goode, W.E.; Owens, F.H.; Fellmann, R.F.; Snyder, W.H.; Moore, J.E. Crystalline acrylic polymers. I. Stereospecific anionic polymerization of methyl methacrylate. J. Polym. Sci. 1960, 46, 317–331. [Google Scholar] [CrossRef]
- Jerman, B.; Breznik, M.; Kogej, K.; Paoletti, S. Osmotic and Volume Properties of Stereoregular Poly(methacrylic acids) in Aqueous Solution: Role of Intermolecular Association. J. Phys. Chem. B 2007, 111, 8435–8443. [Google Scholar] [CrossRef]
- Kogej, K.; Škerjanc, J. Fluorescence and conductivity studies of polyelectrolyte-induced aggregation of alkyltrimethylammonium bromides. Langmuir 1999, 15, 4251–4258. [Google Scholar] [CrossRef]
- Chu, D.Y.; Thomas, J.K. Effect of cationic surfactants on the conformation transition of poly(methacrylic acid). J. Am. Chem. Soc. 1986, 108, 6270–6276. [Google Scholar] [CrossRef]
- Eliassaf, J.; Silberberg, A.; Katchalsky, A. Negative thixotropy of aqueous solutions of polymethacrylic acid. Nature 1955, 176, 1119. [Google Scholar] [CrossRef]
- Ohoya, S.; Hashiya, S.; Tsubakiyama, K.; Matsuo, T. Shear-induced viscosity change of aqueous polymethacrylic acid solution. Polym. J. 2000, 32, 133–139. [Google Scholar] [CrossRef]
- Urban, C.; Schurtenberger, P. Characterization of turbid colloidal suspensions using light scattering techniques combined with cross-correlation methods. J. Colloid Interface Sci. 1998, 207, 150–158. [Google Scholar] [CrossRef]
- Crescenzi, V.; Quadrifoglio, F.; Delben, F. Calorimetric investigation of poly(methacrylic acid) and poly(acrylic acid) in aqueous solution. J. Polym. Sci. A-2 1972, 10, 357–368. [Google Scholar] [CrossRef]
- Vlachy, N.; Dolenc, J.; Jerman, B.; Kogej, K. Influence of stereoregularity of the polymer chain on interactions with surfactants: Binding of cetylpyridinium chloride by isotactic and atactic poly(methacrylic acid). J. Phys. Chem. B 2006, 110, 9061–9071. [Google Scholar] [CrossRef] [PubMed]
- Kratochvil, P. Particle scattering functions. In Particle Scattering Functions in Light Scattering from Polymer Solutions; Huglin, M.B., Ed.; Academic Press Inc.: London, UK; New York, NY, USA, 1972; pp. 333–384. [Google Scholar]
- Savin, G.; Burchard, W. Uncommon solution behavior of poly(N-vinylimidazole). Angular dependence of scattered light from aggregates in ethanol. Macromolecules 2004, 37, 3005–3017. [Google Scholar] [CrossRef]
- Boyko, V.; Richter, S.; Burchard, W.; Arndt, K.-F. Chain dynamics in microgels: Poly(N-vinylcaprolactam-co-N-vinylpyrrolidone) microgels as examples. Langmuir 2007, 23, 776–784. [Google Scholar] [CrossRef]
- Tarassova, E.; Aseyev, V.; Filippov, A.; Tenhu, H. Structure of poly(vinyl pyrrolidone) – C70 complexes in aqueous solutions. Polymer 2007, 48, 4503–4510. [Google Scholar] [CrossRef]
- Kalyanasundaram, K.; Thomas, J.K. Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J. Am. Chem. Soc. 1977, 99, 2039–2044. [Google Scholar] [CrossRef]
- Karpovich, D.S.; Blanchard, G.J. Relating the polarity-dependent fluorescence response of pyrene to vibronic coupling. Achieving a fundamental understanding of the py polarity scale. J. Phys. Chem. 1995, 99, 3951–3958. [Google Scholar] [CrossRef]
- Jerman, B.; Podlipnik, Č.; Kogej, K. Molecular dynamics simulation of poly(methacrylic acid) chains in water. Acta Chim. Slov. 2007, 54, 509–516. [Google Scholar]
- Eigen, M. Fast elementary steps in chemical reaction mechanisms. Pure Appl. Chem. 1963, 6, 97–115. [Google Scholar] [CrossRef]
- Diebler, H.; Eigen, M.; Ilgenfritz, G.; Maass, G.; Winkler, R. Kinetics and mechanism of reactions of main group metal ions with biological carriers. Pure Appl. Chem. 1969, 20, 93–116. [Google Scholar] [CrossRef]


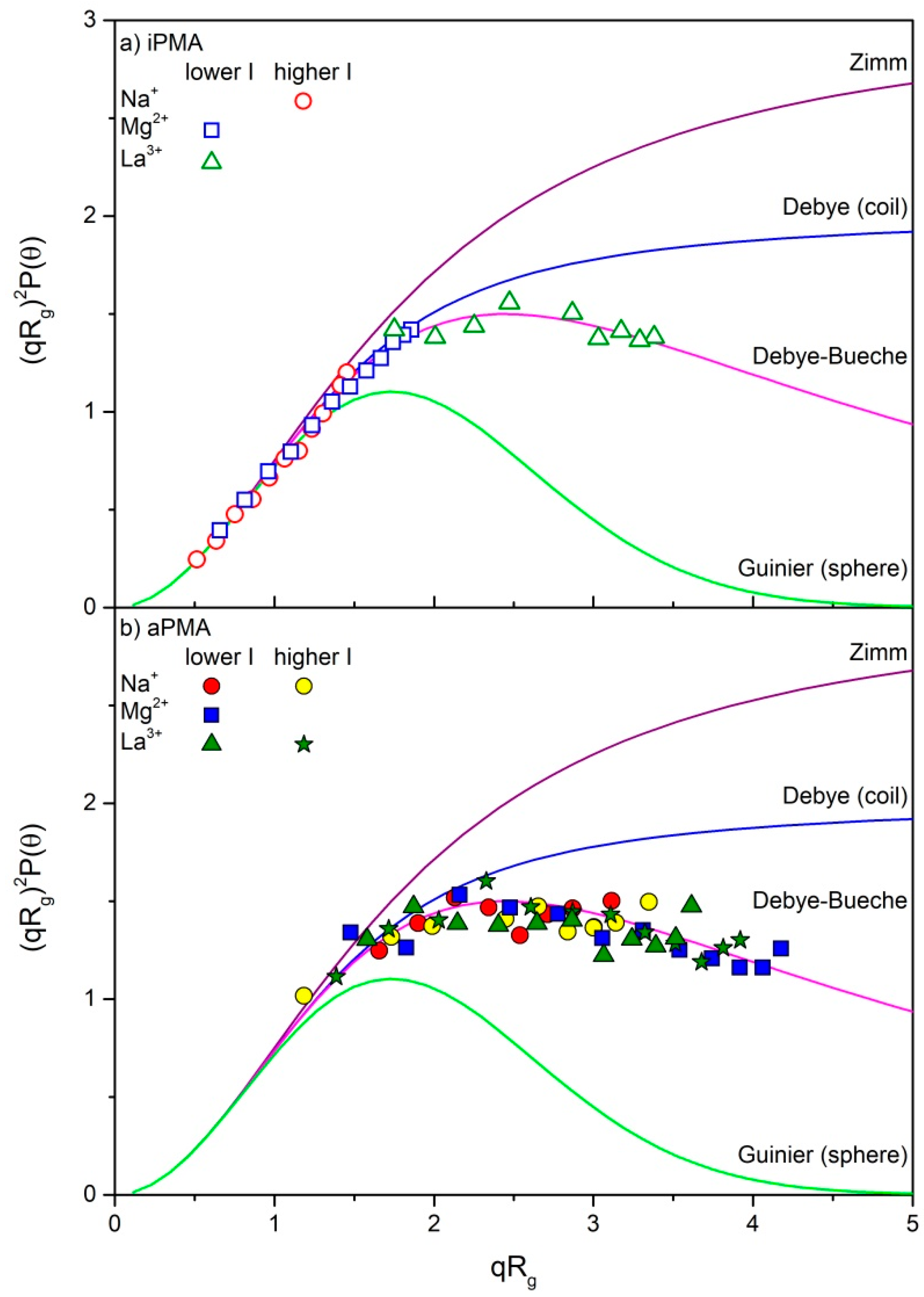
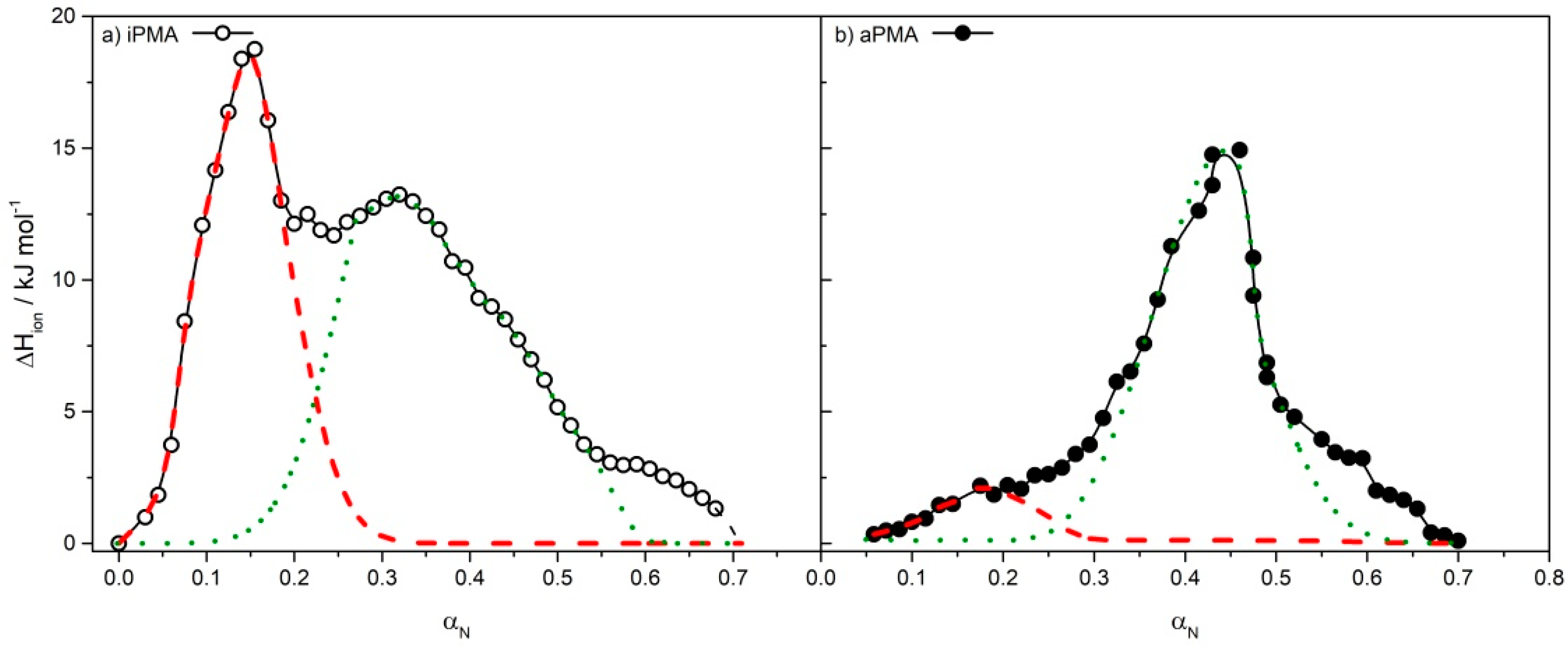
| iPMA | aPMA | ||||
|---|---|---|---|---|---|
| Added Salt | I/mol·L−1 | cs/mol·L−1 | αN | I/mol·L−1 | cs/mol·L−1 |
| NaCl | 0.01 | 0.01 | 0.19 | 0.1 | 0.1 |
| 0.02 | 0.02 | 0.19 | 0.2 | 0.2 | |
| MgCl2 | 0.01 | 0.0033 | 0.19 | 0.1 | 0.033 |
| LaCl3 | 0.005 | 0.00083 | 0.22 | 0.05 | 0.0083 |
| 0.1 | 0.0167 | ||||
| iPMA | aPMA | |
|---|---|---|
| Added Salt | ΔHtr/kJ·mol−1 | ΔHtr/kJ·mol−1 |
| 0.01 M NaCl | 4.83 | 1.12 |
| 0.0033 M MgCl2 | 5.52 | 0.83 |
| 0.00167 M LaCl3 | 5.77 | 2.97 |
| iPMA | aPMA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Added Salt | I/mol·L−1 | αN | Rh,ass/nm | Rg,ass/nm | ρ | I/mol·L−1 | Rh,ass/nm | Rg,ass/nm | ρ |
| NaCl | 0.01 | 0.19 | 59 | / | / | 0.1 | 172 | 126 | 0.73 |
| 0.02 | 0.19 | 80 | 57 | 0.71 | 0.2 | 177 | 131 | 0.74 | |
| MgCl2 | 0.01 | 0.19 | 75 | 73 | 0.97 | 0.1 | 178 | 164 | 0.92 |
| LaCl3 | 0.005 | 0.22 | 191 | 132 | 0.69 | 0.05 | 183 | 142 | 0.77 |
| 0.1 | 200 | 152 | 0.76 | ||||||
| iPMA | aPMA | |||
|---|---|---|---|---|
| Added Salt | I/mol·L−1 | I1/I3 | I/mol·L−1 | I1/I3 |
| / | / | 1.51 | / | 0.90 |
| NaCl | 0.01 | 1.50 | 0.1 | 0.89 |
| 0.02 | 1.48 | 0.2 | 0.90 | |
| MgCl2 | 0.01 | 1.49 | 0.1 | 0.89 |
| LaCl3 | 0.005 (a) | 1.09 | 0.05 | 0.98 |
| 0.1 | 0.94 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hriberšek, P.; Kogej, K. Effect of Multivalent Cations on Intermolecular Association of Isotactic and Atactic Poly(Methacrylic Acid) Chains in Aqueous Solutions. Polymers 2019, 11, 605. https://doi.org/10.3390/polym11040605
Hriberšek P, Kogej K. Effect of Multivalent Cations on Intermolecular Association of Isotactic and Atactic Poly(Methacrylic Acid) Chains in Aqueous Solutions. Polymers. 2019; 11(4):605. https://doi.org/10.3390/polym11040605
Chicago/Turabian StyleHriberšek, Patricija, and Ksenija Kogej. 2019. "Effect of Multivalent Cations on Intermolecular Association of Isotactic and Atactic Poly(Methacrylic Acid) Chains in Aqueous Solutions" Polymers 11, no. 4: 605. https://doi.org/10.3390/polym11040605
APA StyleHriberšek, P., & Kogej, K. (2019). Effect of Multivalent Cations on Intermolecular Association of Isotactic and Atactic Poly(Methacrylic Acid) Chains in Aqueous Solutions. Polymers, 11(4), 605. https://doi.org/10.3390/polym11040605






