The Influence of DMDBS on Crystallization Behavior and Crystalline Morphology of Weakly-Phase-Separated Olefin Block Copolymer
Abstract
:1. Introduction
2. Experimental
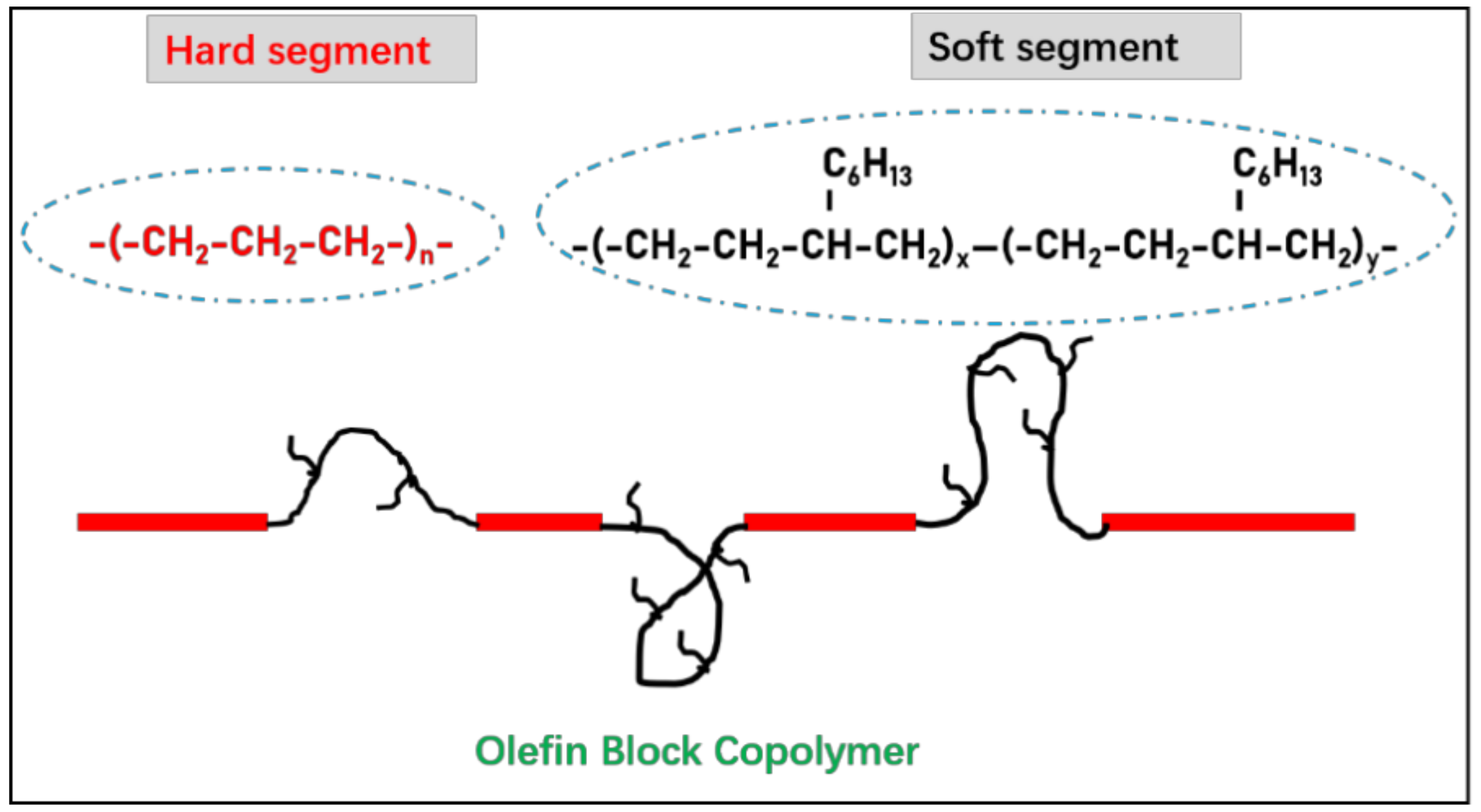
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
3. Results and Discussion
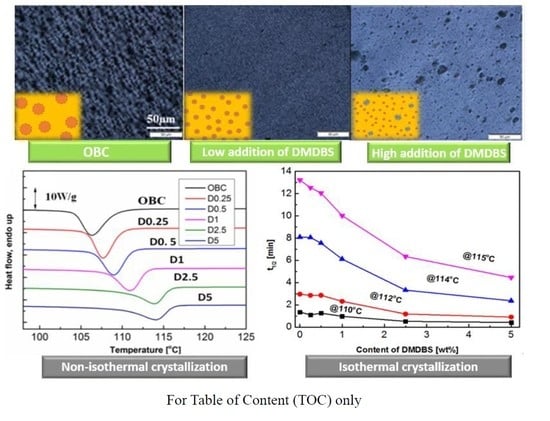
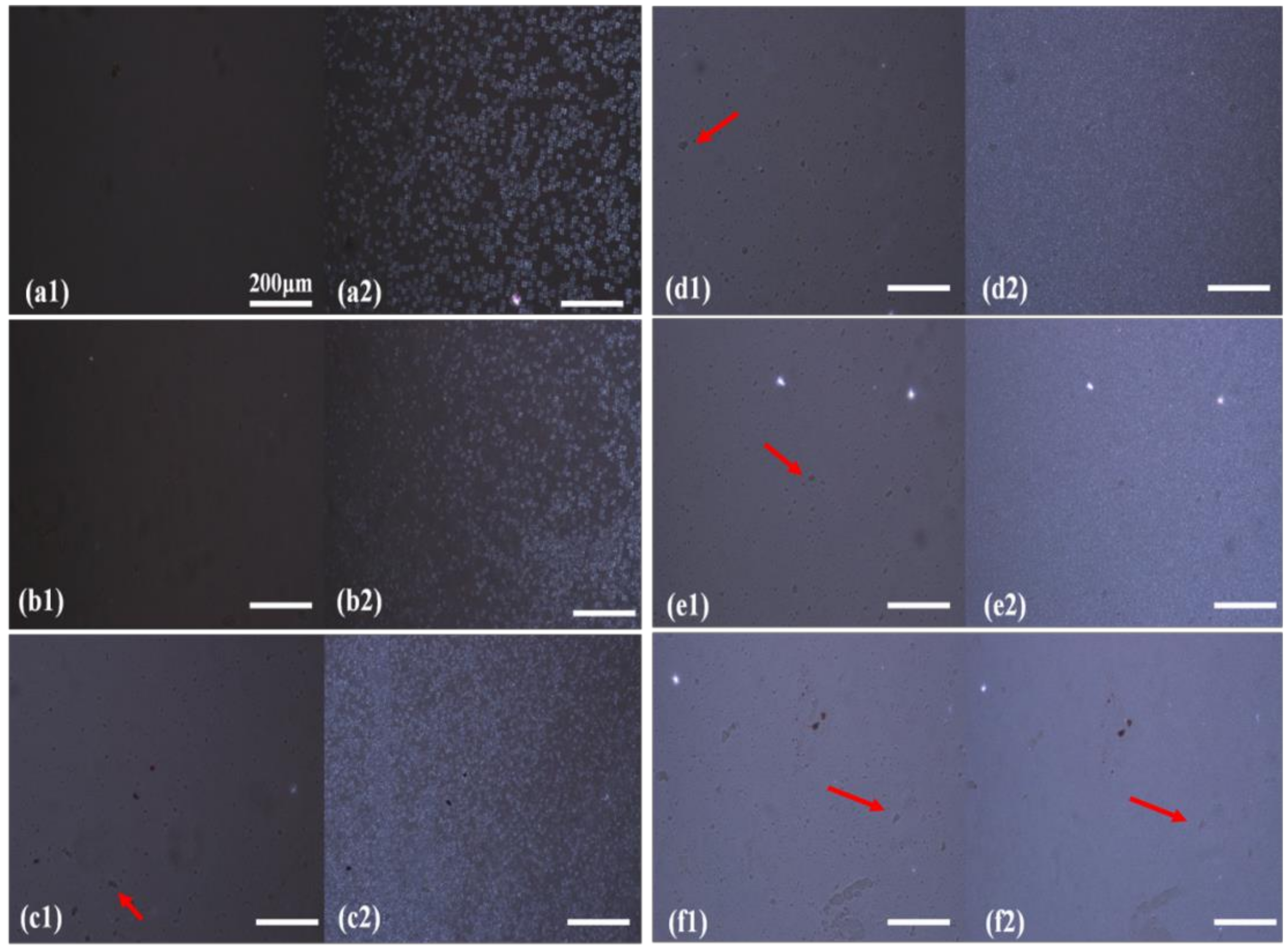
3.1. Phase Separation of DMDBS from OBC Melt
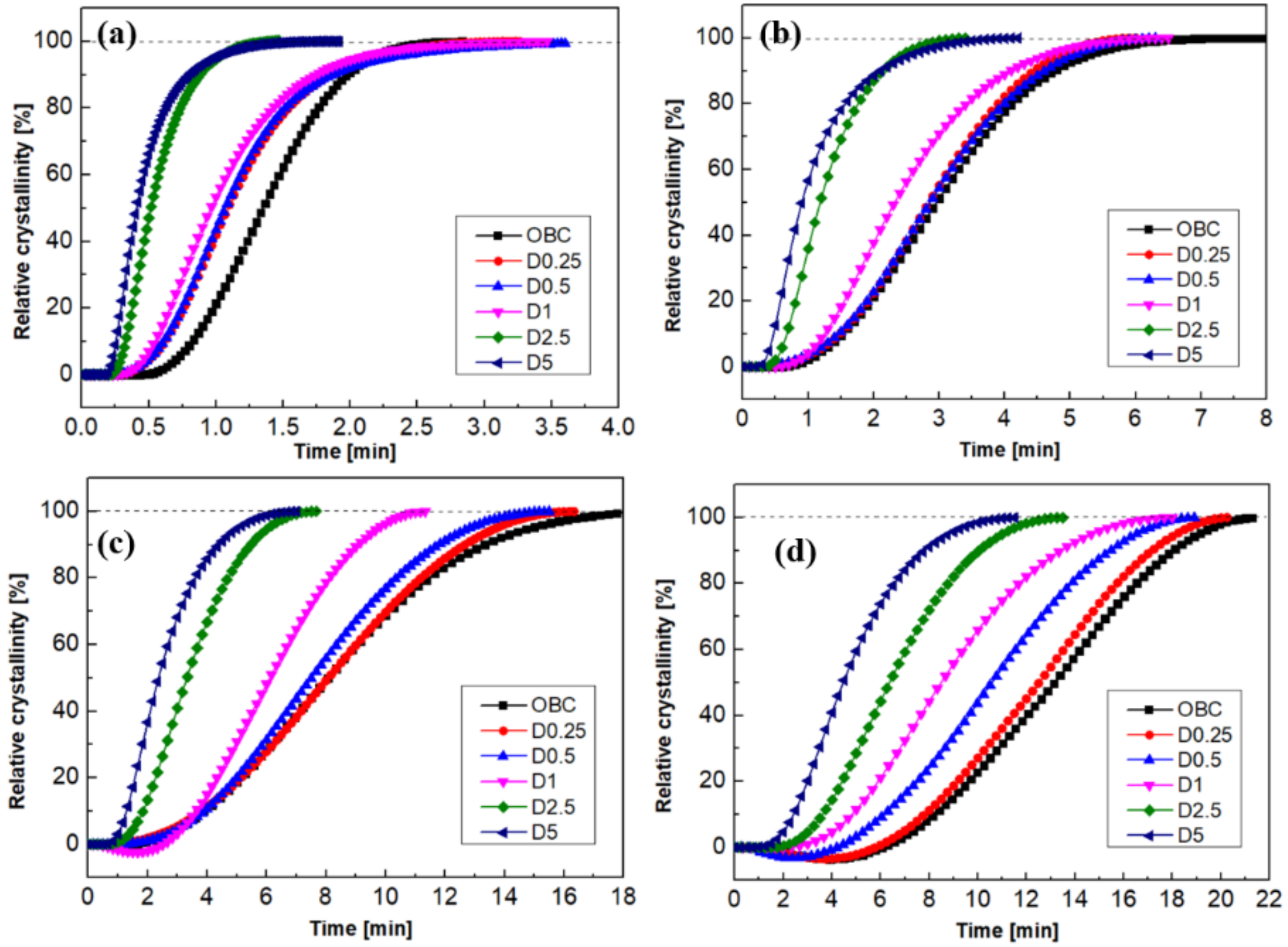
3.2. Isothermal and Non-Isothermal Crystallization Behavior of OBC/DMDBS Samples
3.3. The Crystalline Morphology of OBC/DMDBS Samples
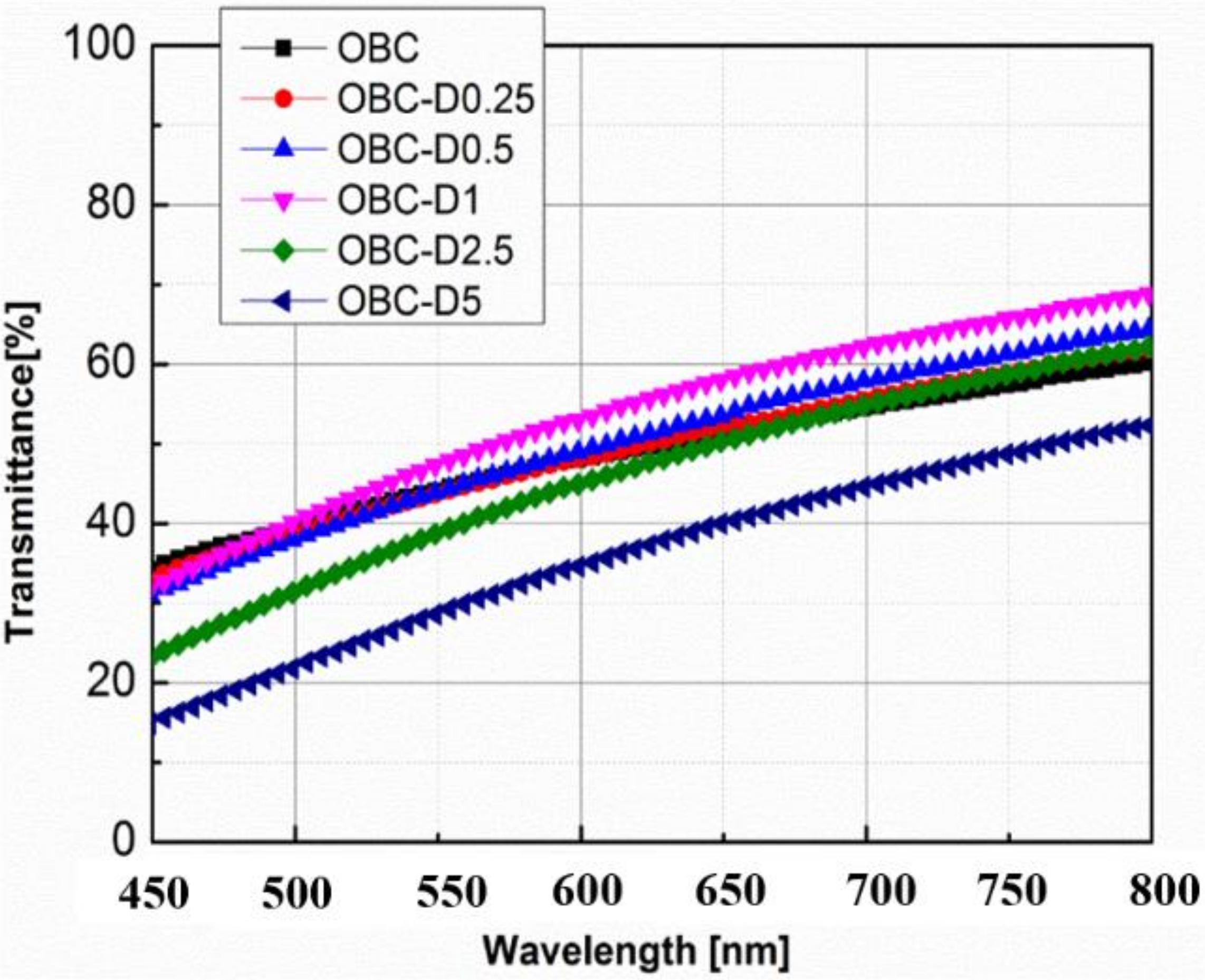
3.4. The Optical Properties of OBC/DMDBS Samples
3.5. The Mechanical Properties of OBC/DMDBS Samples
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zuo, F.; Mao, Y.; Li, X.; Burger, C.; Hsiao, B.S.; Chen, H.; Marchand, G.R. Effects of Block Architecture on Structure and Mechanical Properties of Olefin Block Copolymers under Uniaxial Deformation. Macromolecules 2011, 44, 3670–3673. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Y.; Sui, G.; Chen, F.; Zhang, Q.; Fu, Q. The Effect of Hard Block Content on the Orientation and Mechanical Properties of Olefin Block Copolymer Films as Obtained via Melt Stretching. RSC Adv. 2015, 5, 82535–82543. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Y.; Sui, G.; Chen, F.; Fu, Q. Tailoring the Crystalline Morphology and Mechanical Property of Olefin Block Copolymer via Blending with a Small Amount of UHMWPE. Polymer 2017, 109, 137–145. [Google Scholar] [CrossRef]
- Khariwala, D.U.; Taha, A.; Chum, S.P.; Hiltner, A.; Baer, E. Crystallization Kinetics of Some New Olefinic Block Copolymers. Polymer 2008, 49, 1365–1375. [Google Scholar] [CrossRef]
- Carnahan, E.M.; Hustad, P.D.; Kuhlman, R.L.; Wenzel, T.T. Catalytic Production of Olefin Block Copolymers via Chain Shuttling Polymerization. Science 2006, 312, 714–719. [Google Scholar]
- Vittoria, A.; Busico, V.; Cannavacciuolo, F.D.; Cipullo, R. Molecular Kinetic Study of “Chain Shuttling” Olefin Copolymerization. ACS Catal. 2018, 8, 5051–5061. [Google Scholar] [CrossRef]
- Kuhlman, R.L.; Klosin, J. Tuning Block Compositions of Polyethylene Multi-Block Copolymers by Catalyst Selection. Macromolecules 2010, 43, 7903–7904. [Google Scholar] [CrossRef]
- Lin, Y.; Yakovleva, V.; Chen, H.; Hiltner, A.; Baer, E. Comparison of Olefin Copolymers as Compatibilizers for Polypropylene and High-Density Polyethylene. J. Appl. Polym. Sci. 2009, 113, 1945–1952. [Google Scholar] [CrossRef]
- Dobrynin, A.V.; Hill, C.; Carolina, N. Theory of Polydisperse Multiblock Copolymers. Macromol. Rapid Commun. 1997, 9297, 4756–4765. [Google Scholar] [CrossRef]
- Liu, G.; Guan, Y.; Wen, T.; Wang, X.; Zhang, X.; Wang, D.; Li, X.; Loos, J.; Chen, H.; Walton, K.; et al. Effect of Mesophase Separation and Crystallization on the Elastomeric Behavior of Olefin Multi-Block Copolymers. Polymer 2011, 52, 5221–5230. [Google Scholar] [CrossRef]
- Park, H.E.; Dealy, J.M.; Marchand, G.R.; Wang, J.; Li, S.; Register, R.A. Rheology and Structure of Molten, Olefin Multiblock Copolymers. Macromolecules 2010, 43, 6789–6799. [Google Scholar] [CrossRef]
- Hustad, P.D.; Marchand, G.R.; Garcia-Meitin, E.I.; Roberts, P.L.; Weinhold, J.D. Photonic Polyethylene from Self-Assembled Mesophases of Polydisperse Olefin Block Copolymers. Macromolecules 2009, 42, 3788–3794. [Google Scholar] [CrossRef]
- Wang, H.P.; Khariwala, D.U.; Cheung, W.; Chum, S.P.; Hiltner, A.; Baer, E. Characterization of Some New Olefinic Block Copolymers. Macromolecules 2007, 40, 2852–2862. [Google Scholar] [CrossRef]
- Nie, Z.; He, P.; Yu, W.; Zhou, C. In Situ Raman Spectroscopic Investigation of Olefin Multiblock Copolymers during Melting and Crystallization. Polym. Test. 2018, 67, 31–36. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, X.; Liu, C.; Chen, H.; Walton, K.; Wang, D. Morphology and Mechanical Properties of Binary Blends of Polypropylene with Statistical and Block Ethylene-Octene Copolymers. J. Appl. Polym. Sci. 2011, 119, 3591–3597. [Google Scholar] [CrossRef]
- Zhao, Y.; Si, L.; Wang, L.; Dang, W.; Bao, J.; Lu, Z.; Zhang, M. Tuning the Mechanical Properties of Weakly Phase-Separated Olefin Block Copolymer by Establishing Co-Crystallization Structure with the Aid of Linear Polyethylene: The Dependence on Molecular Chain Length. CrystEngComm 2017, 19, 2884–2893. [Google Scholar] [CrossRef]
- Wu, M.; Wu, Z.; Wang, K.; Zhang, Q.; Fu, Q. Simultaneous the Thermodynamics Favorable Compatibility and Morphology to Achieve Excellent Comprehensive Mechanics in PLA/OBC Blend. Polymer 2014, 55, 6409–6417. [Google Scholar] [CrossRef]
- Fu, S.; Yu, B.; Duan, L.; Bai, H.; Chen, F.; Wang, K.; Deng, H.; Zhang, Q.; Fu, Q. Combined Effect of Interfacial Strength and Fiber Orientation on Mechanical Performance of Short Kevlar Fiber Reinforced Olefin Block Copolymer. Compos. Sci. Technol. 2015, 108, 23–31. [Google Scholar] [CrossRef]
- Lu, Z.; Si, L.; Dang, W.; Zhao, Y. Transparent and Mechanically Robust Poly (Para-Phenylene Terephthamide) PPTA Nanopaper toward Electrical Insulation Based on Nanoscale Fibrillated Aramid-Fibers. Compos. Part A Appl. Sci. Manuf. 2018, 115, 321–330. [Google Scholar] [CrossRef]
- Sui, G.; Zhao, Y.; Zhang, Q.; Fu, Q. Enhanced Mechanical Properties of Olefin Block Copolymer by Adding a Quaternary Ammonium Salt Functionalized Graphene Oxide. RSC Adv. 2016, 6, 54785–54792. [Google Scholar] [CrossRef]
- Zhao, Y.; Dang, W.; Lu, Z.; Deng, J.; Hao, Y.; Su, Z.; Zhang, M. Fabrication of Mechanically Robust and UV-Resistant Aramid Fiber-Based Composite Paper by Adding Nano-TiO2 and Nanofibrillated Cellulose. Cellulose 2018, 25, 3913–3925. [Google Scholar] [CrossRef]
- Li, T.; Pu, J.-H.; Ma, L.-F.; Bao, R.-Y.; Qi, G.-Q.; Yang, W.; Xie, B.-H.; Yang, M.-B. An Extremely Uniform Dispersion of MWCNTs in Olefin Block Copolymers Significantly Enhances Electrical and Mechanical Performances. Polym. Chem. 2015, 6, 7160–7170. [Google Scholar] [CrossRef]
- Wu, S.; Li, H.; Huang, G.; Wu, J. Nucleating Effect of Multi-Walled Carbon Nanotubes and Graphene on the Crystallization Kinetics and Melting Behavior of Olefin Block Copolymers. RSC Adv. 2014, 4, 19024–19033. [Google Scholar] [CrossRef]
- Tong, Z.-Z.; Zhou, B.; Huang, J.; Xu, J.-T.; Fan, Z.-Q. Olefinic Blocky Copolymer/Montmorillonite Nanocomposites with Collapsed Clay Layers. Compos. Sci. Technol. 2013, 85, 111–117. [Google Scholar] [CrossRef]
- Tong, Z.-Z.; Huang, J.; Zhou, B.; Xu, J.-T.; Fan, Z.-Q. Self-Nucleation Behaviors of Olefinic Blocky Copolymer/Montmorillonite Nanocomposites with Collapsed and Intercalated Clay Layers. J. Appl. Polym. Sci. 2015, 132, 41771–41779. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Wei, X. Non-Isothermal Crystallization Kinetics of Isotactic Polypropylene Nucleated with 1,3:2,4-Bis(3, 4-Dimethylbenzylidene) Sorbitol. J. Therm. Anal. Calorim. 2010, 100, 661–665. [Google Scholar] [CrossRef]
- Vaughan, A.S.; Hosier, I.L. The Effect of Dibenzylidene Sorbitol on the Crystallization Behaviour of Polyethylene. J. Mater. Sci. 2008, 43, 2922–2928. [Google Scholar]
- Liu, S.; Yu, W.; Zhou, C. Molecular Self-Assembly Assisted Liquid-Liquid Phase Separation in Ultrahigh Molecular Weight Polyethylene/Liquid Paraffin/Dibenzylidene Sorbitol Ternary Blends. Macromolecules 2013, 46, 6309–6318. [Google Scholar] [CrossRef]
- Sreenivas, K.; Pol, H.V.; Kumaraswamy, G. The Influence of DMDBS on the Morphology and Mechanical Properties of Polypropylene Cast Films. Polym. Eng. Sci. 2011. [Google Scholar] [CrossRef]
- Supaphol, P.; Charoenphol, P.; Junkasem, J. Effect of Nucleating Agents on Crystallization and Melting Behavior and Mechanical Properties of Nucleated Syndiotactic Poly (Propylene). Macromol. Rapid Commun. 2004, 289, 818–827. [Google Scholar] [CrossRef]
- Balzano, L.; Portale, G.; Peters, G.W.M.; Rastogi, S. Thermoreversible DMDBS Phase Separation in IPP: The Effects of Flow on the Morphology. Macromolecules 2008, 41, 5350–5355. [Google Scholar] [CrossRef]
- Lai, W.; Cheng, L. Preparation and Characterization of Novel Poly (Vinylidene fluoride) Membranes Using Self-Assembled Dibenzylidene Sorbitol for Membrane Distillation. Desalination 2014, 332, 7–17. [Google Scholar] [CrossRef]
- Sreenivas, K.; Basargekar, R.; Kumaraswamy, G. Phase Separation of DMDBS from PP: Effect of Polymer Molecular Weight and Tacticity. Macromolecules 2011, 44, 2358–2364. [Google Scholar] [CrossRef]
- Lipp, J.; Shuster, M.; Terry, A.E.; Cohen, Y.; Engineering, C.; Eindho, V.; Uni, V.; Ox, O. Fibril Formation of 1,3:2,4-Di(3,4-Dimethylbenzylidene) Sorbitol in a Polypropylene Melt. Langmuir 2006, 65, 6398–6402. [Google Scholar] [CrossRef]
- He, P.; Shen, W.; Yu, W.; Zhou, C. Mesophase Separation and Rheology of Ole Fi n Multiblock Copolymers. Macromolecules 2014, 47, 807–820. [Google Scholar] [CrossRef]
- Fu, F.; Chen, Z.; Zhao, Z.; Wang, H.; Shang, L.; Gu, Z.; Zhao, Y. Bio-Inspired Self-Healing Structural Color Hydrogel. Proc. Natl. Acad. Sci. USA 2017, 114, 5900–5905. [Google Scholar] [CrossRef]
- Balzano, L.; Rastogi, S.; Peters, G.W.M. Flow Induced Crystallization in Isotactic Polypropylene-1,3:2,4-Bis(3,4-Dimethylbenzylidene)Sorbitol Blends: Implications on Morphology of Shear and Phase Separation. Macromolecules 2008, 41, 399–408. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Y.; Qin, Y.; Voigt, M.; Liu, X.; Zheng, G.; Chen, Q.; Schubert, D.W.; Liu, C.; Shen, C. Creep and Recovery Behavior of Injection-Molded Isotactic Polypropylene with Controllable Skin-Core Structure. Polym. Test. 2018, 69, 478–484. [Google Scholar] [CrossRef]
- Coburn, N.; Douglas, P.; Kaya, D.; Gupta, J.; Mcnally, T. Advanced Industrial and Engineering Polymer Research Isothermal and Non-Isothermal Crystallization Kinetics of Composites of Poly (Propylene) and MWCNTs. Adv. Ind. Eng. Polym. Res. 2018, 1, 99–110. [Google Scholar] [CrossRef]





| Sample | Tm [°C] | Tc,p [°C] | ΔHm [J/g] | Xc [%] |
|---|---|---|---|---|
| OBC | 121.4 | 106.3 | 45.362 | 15.6% |
| D0.25 | 121.8 | 107.7 | 45.914 | 15.8% |
| D0.5 | 122.0 | 108.9 | 47.119 | 16.1% |
| D1 | 122.1 | 110.9 | 45.954 | 15.8% |
| D2.5 | 122.6 | 113.9 | 44.441 | 15.3% |
| D5 | 122.8 | 114.0 | 42.862 | 14.7% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Yao, C.; Chang, T.; Zhu, Y. The Influence of DMDBS on Crystallization Behavior and Crystalline Morphology of Weakly-Phase-Separated Olefin Block Copolymer. Polymers 2019, 11, 552. https://doi.org/10.3390/polym11030552
Zhao Y, Yao C, Chang T, Zhu Y. The Influence of DMDBS on Crystallization Behavior and Crystalline Morphology of Weakly-Phase-Separated Olefin Block Copolymer. Polymers. 2019; 11(3):552. https://doi.org/10.3390/polym11030552
Chicago/Turabian StyleZhao, Yongsheng, Cheng Yao, Tao Chang, and Yanling Zhu. 2019. "The Influence of DMDBS on Crystallization Behavior and Crystalline Morphology of Weakly-Phase-Separated Olefin Block Copolymer" Polymers 11, no. 3: 552. https://doi.org/10.3390/polym11030552
APA StyleZhao, Y., Yao, C., Chang, T., & Zhu, Y. (2019). The Influence of DMDBS on Crystallization Behavior and Crystalline Morphology of Weakly-Phase-Separated Olefin Block Copolymer. Polymers, 11(3), 552. https://doi.org/10.3390/polym11030552