MgAl-Layered-Double-Hydroxide/Sepiolite Composite Membrane for High-Performance Water Treatment Based on Layer-by-Layer Hierarchical Architectures
Abstract
:1. Introduction
2. Experimental Section
2.1. Material
2.2. Synthesis of MgAl-Layered-Double-Hydroxide (MgAl-LDH)
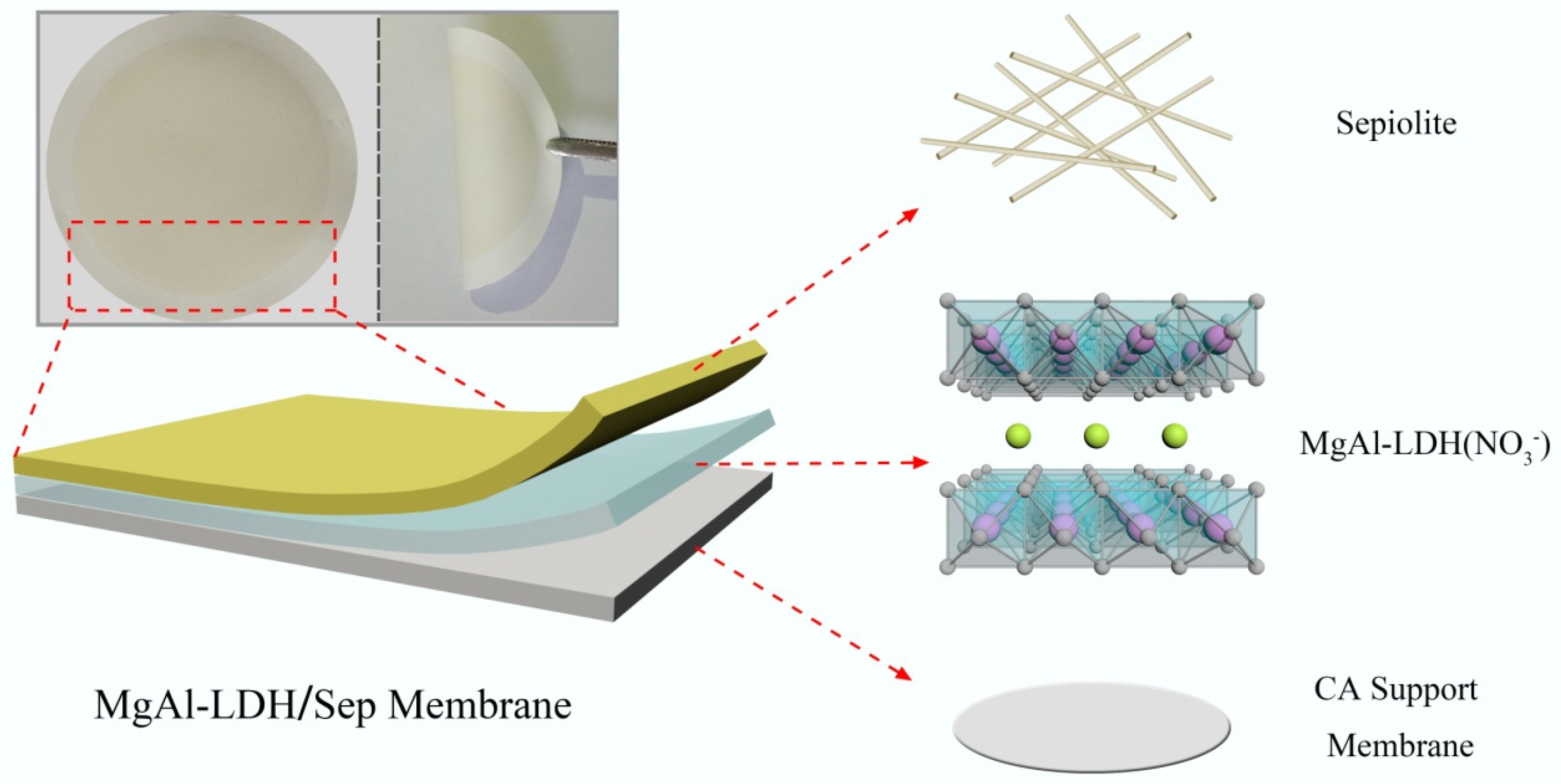
2.3. Fabrication of the MgAl-Layered-Double-Hydroxide/Sepiolite (MgAl-LDH/Sep) Membranes
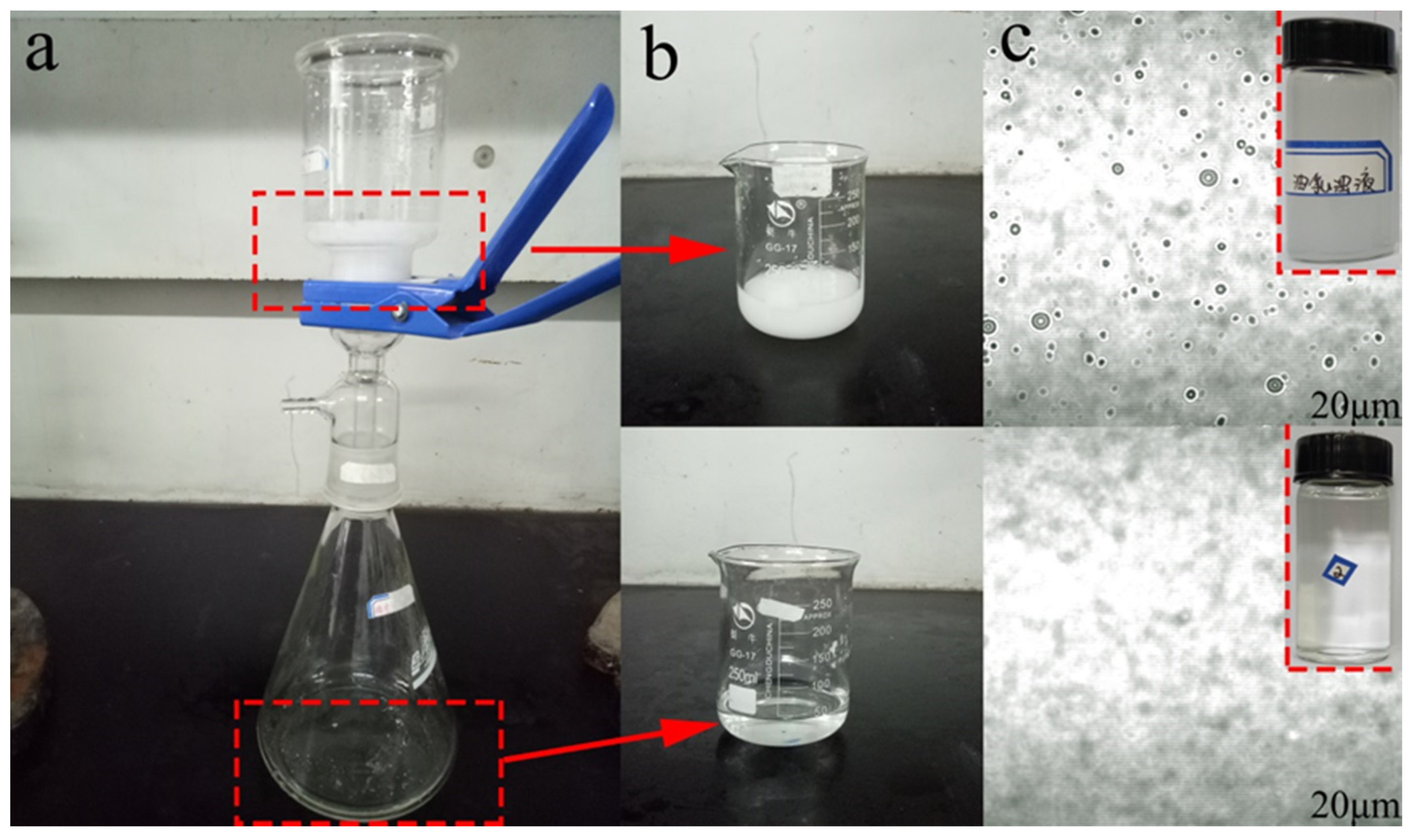
2.4. Preparation of Oil-in-Water (O/W) Emulsions
2.5. Characterization
2.6. Permeation and Separation Performances
3. Results and Discussion
3.1. Characterization
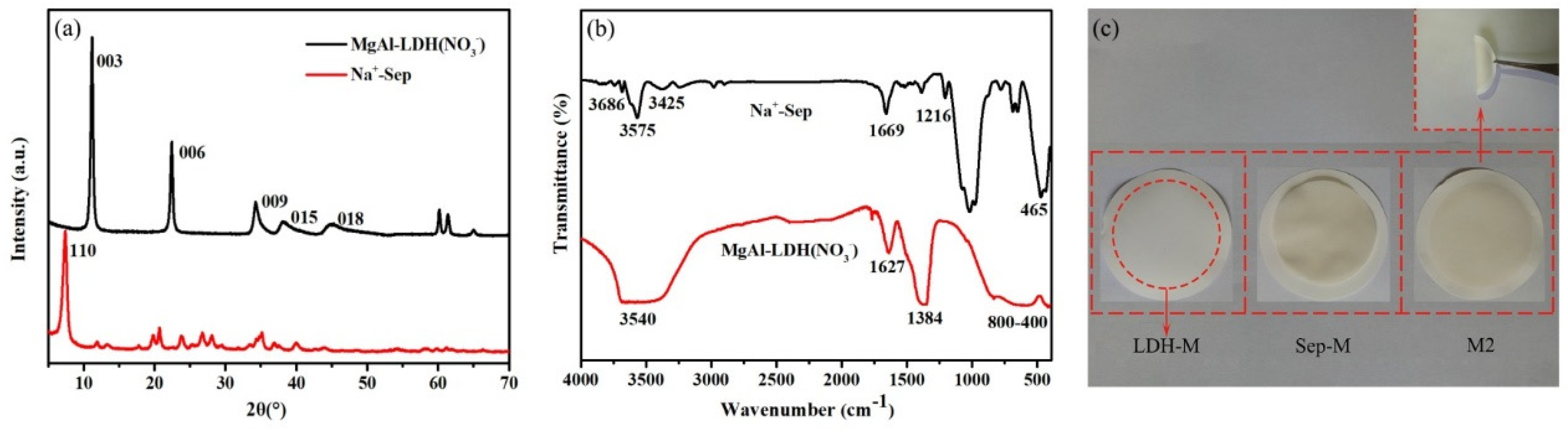
3.1.1. X-ray Diffraction (XRD)
3.1.2. Fourier Transform Infrared Spectroscopy (FT-IR)
3.1.3. Scanning Electron Microscopy (SEM)
3.1.4. Atomic Force Microscopy (AFM)
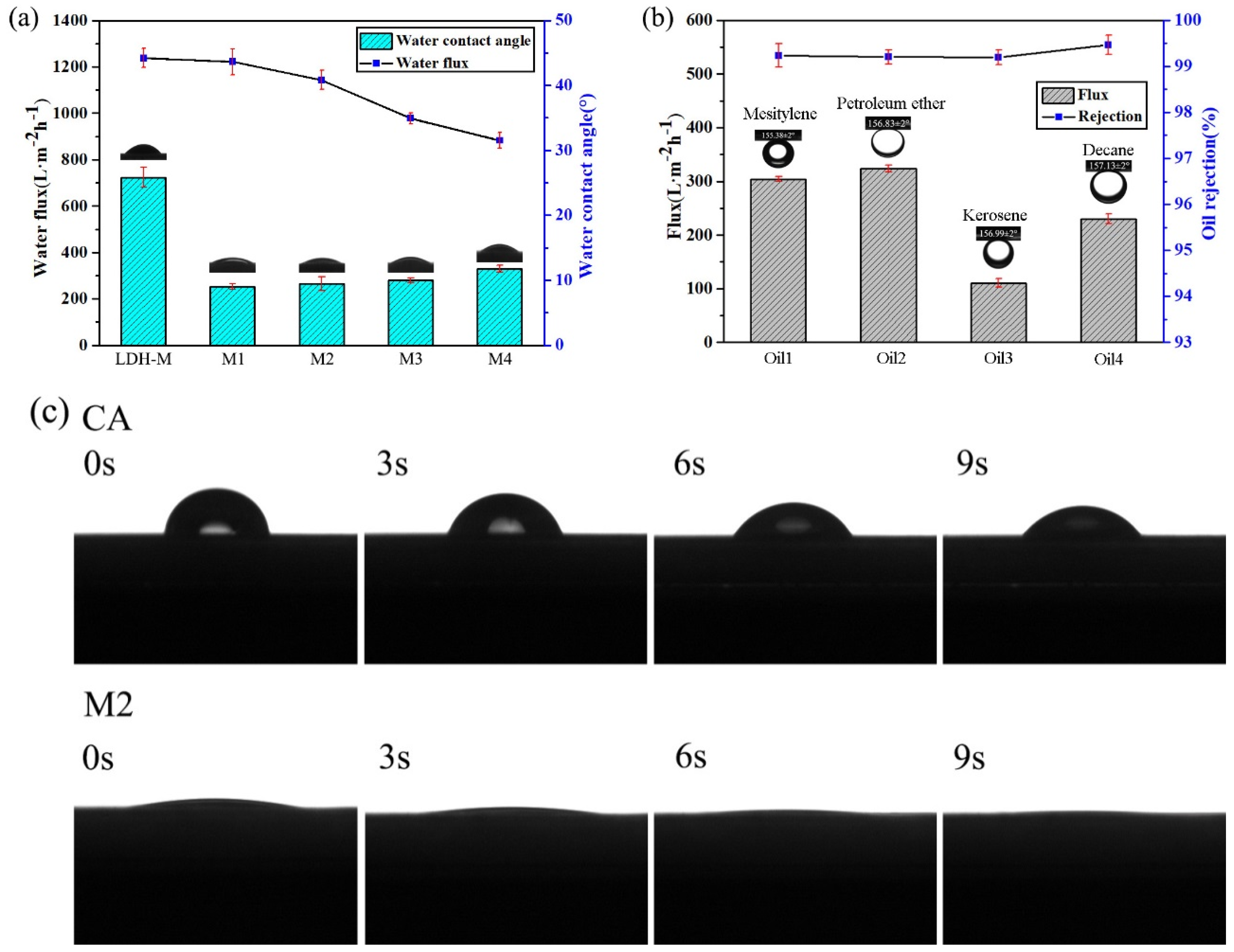
3.3. Wettability of MgAl-LDH/Sep Membrane
3.4. Dye Removal Separation Performance
3.5. Oil/Water Separation Performance
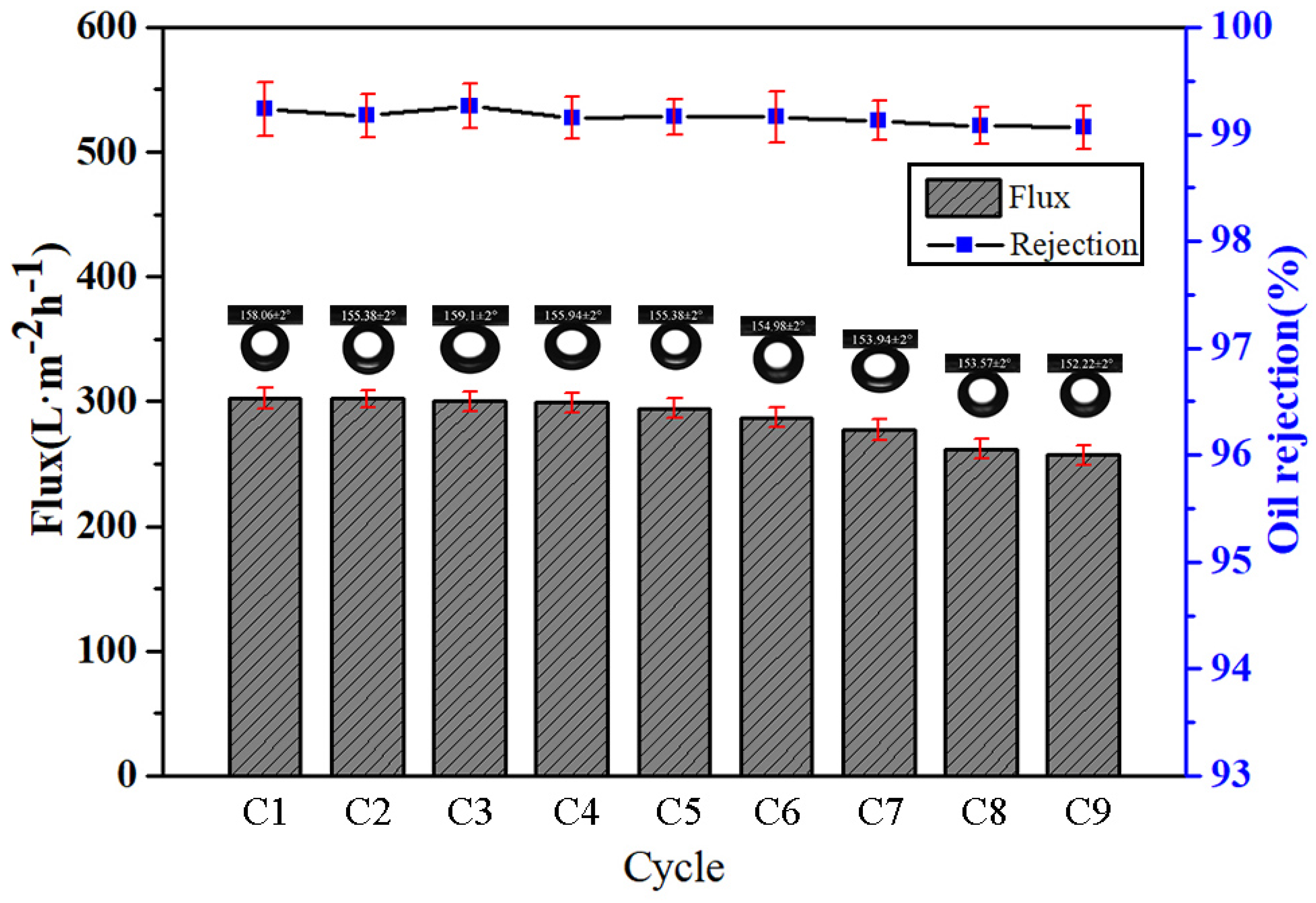
3.6. Durability and Stability of MgAl-LDH/Sep Membranes
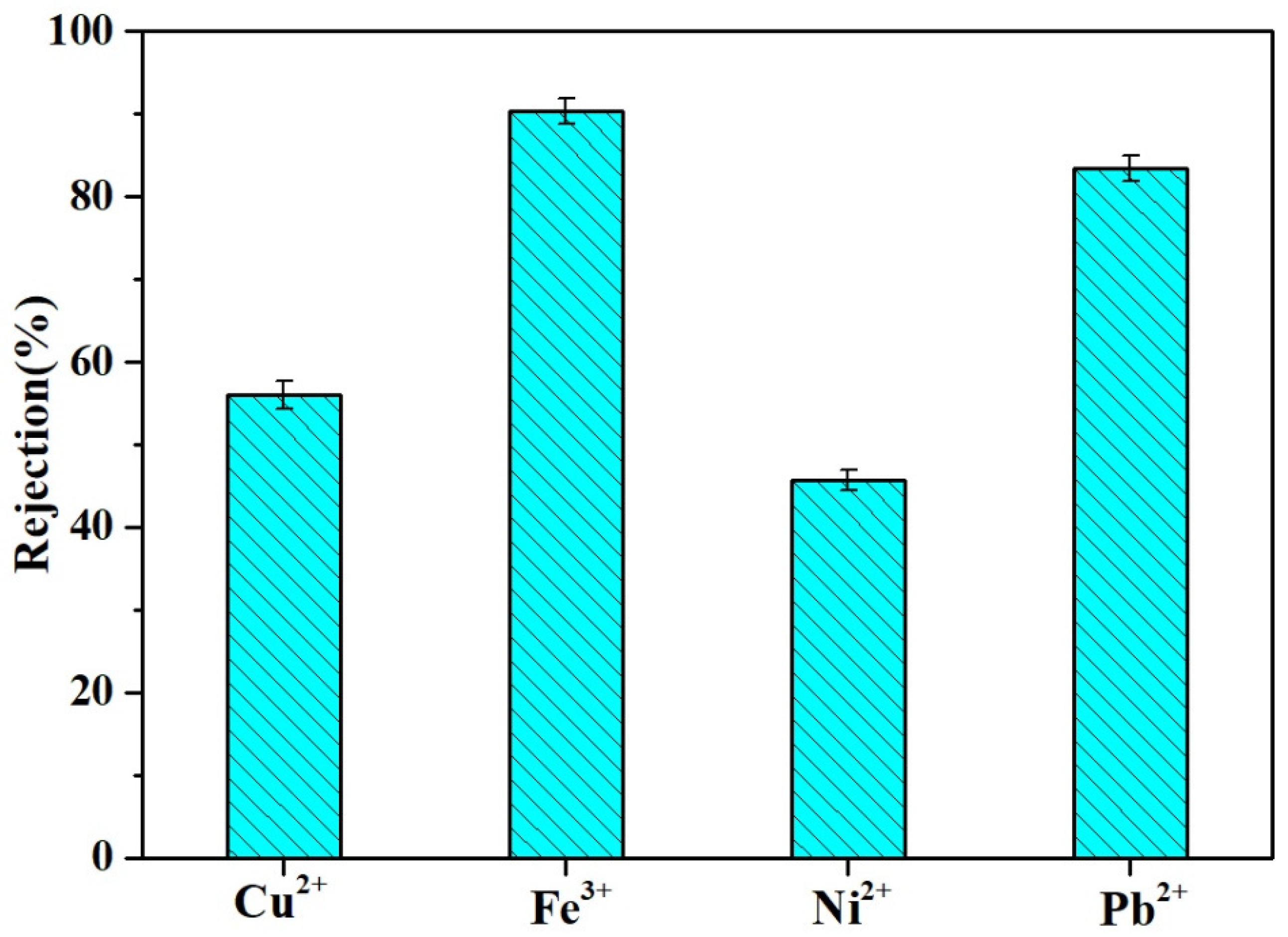
3.7. Other Water Treatment Applications
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sen, S.; Demirer, G.N. Anaerobic treatment of real textile wastewater with a fluidized bed reactor. Water Res. 2003, 37, 1868–1878. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, H.; Liu, Y.; Craig, V.S.J.; Wang, C.; Li, L.H.; Chen, Y. Superhydrophobic and Superoleophilic Porous Boron Nitride Nanosheet/Polyvinylidene Fluoride Composite Material for Oil-Polluted Water Cleanup. Adv. Mater. Interfaces 2015, 2. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Gossen, L.P.; Velichkina, L.M. Environmental problems of the oil-and-gas industry (Review). Pet. Chem. 2006, 46, 67–72. [Google Scholar] [CrossRef]
- Calcagnile, P.; Fragouli, D.; Bayer, I.S.; Anyfantis, G.C.; Martiradonna, L.; Cozzoli, P.D.; Cingolani, R.; Athanassiou, A. Magnetically Driven Floating Foams for the Removal of Oil Contaminants from Water. ACS Nano 2012, 6, 5413–5419. [Google Scholar] [CrossRef] [PubMed]
- Geise, G.M.; Freeman, B.D.; Paul, D.R. Characterization of a sulfonated pentablock copolymer for desalination applications. Polymer 2010, 51, 5815–5822. [Google Scholar] [CrossRef]
- Reichle, W.T. Synthesis of anionic clay minerals (mixed metal hydroxides, hydrotalcite). Solid State Ion. 1986, 22, 135–141. [Google Scholar] [CrossRef]
- Ovarlez, S.; Giulieri, F.; Chaze, A.M.; Delamare, F.; Raya, J.; Hirschinger, J. The incorporation of indigo molecules in sepiolite tunnels. Chem. Eur. J. 2009, 15, 11326–11332. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Hitzky, E. Molecular access to intracrystalline tunnels of sepiolite. J. Mater. Chem. 2000, 11, 86–91. [Google Scholar] [CrossRef]
- Hui, Z.; Tao, Z.; Rongjun, T.; Chunyu, C. Robust tunicate cellulose nanocrystal/palygorskite nanorod membranes for multifunctional oil/water emulsion separation. ACS Sustain. Chem. Eng. 2018, 6, 10833–10840. [Google Scholar]
- Lafi, R.; Charradi, K.; Djebbi, M.A.; Amara, A.B.H.; Hafiane, A. Adsorption study of Congo red dye from aqueous solution to Mg–Al–layered double hydroxide. Adv. Powder Technol. 2016, 27, 232–237. [Google Scholar] [CrossRef]
- Jiang, L.; Zhao, Y.; Zhai, J. A Lotus-Leaf-like Superhydrophobic Surface: A Porous Microsphere/Nanofiber Composite Film Prepared by Electrohydrodynamics. Angew. Chem. Int. Ed. Engl. 2004, 43, 4338–4341. [Google Scholar] [CrossRef]
- Parker, A.R.; Lawrence, C.R. Water capture by a desert beetle. Nature 2001, 414, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Her, E.K. Fabrication of Superhydrophobic Surfaces with High and Low Adhesion Inspired from Rose Petal. Langmuir ACS J. Surf. Colloids 2010, 26, 8207–8217. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Gao, R.; Wu, T.; Li, Y.J. Role of layered materials in emulsified oil/water separation and anti-fouling performance of modified cellulose acetate membranes with hierarchical structure. J. Membr. Sci. 2017, 543, 163–171. [Google Scholar] [CrossRef]
- Bui, N.N.; Mccutcheon, J.R. Hydrophilic nanofibers as new supports for thin film composite membranes for engineered osmosis. Environ. Sci. Technol. 2013, 47, 1761. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, Q.; Hua, D.; Xiong, R.; Zhao, J.; Rao, W.; Huang, S.; Zhan, X.; Chen, F.; Huang, C. Electrospun fibers for oil–water separation. RSC Adv. 2016, 6, 12868–12884. [Google Scholar] [CrossRef]
- Wagner, A.; Poursorkhabi, V.; Mohanty, A.K.; Misra, M. Analysis of Porous Electrospun Fibers from Poly(l-lactic acid)/Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Blends. ACS Sustain. Chem. Eng. 2014, 2, 1976–1982. [Google Scholar] [CrossRef]
- Lee, M.W.; An, S.; Latthe, S.S.; Lee, C.; Hong, S.; Yoon, S.S. Electrospun polystyrene nanofiber membrane with superhydrophobicity and superoleophilicity for selective separation of water and low viscous oil. Appl. Mater. Interfaces 2013, 5, 10597–10604. [Google Scholar] [CrossRef]
- Li, J.J.; Zhou, Y.N.; Luo, Z.H. Smart Fiber Membrane for pH-Induced Oil/Water Separation. ACS Appl. Mater. Interfaces 2015, 7, 19643–19650. [Google Scholar] [CrossRef]
- Xiong, L.; Yu, X.; Cheng, C.; Deng, L.; Wang, M.; Wang, X.F. Electrospun Superhydrophobic Organic/Inorganic Composite Nanofibrous Membranes for Membrane Distillation. ACS Appl. Mater. Interfaces 2015, 7, 21919–21930. [Google Scholar]
- Peng, S.; Jin, G.; Li, L.; Li, K.; Srinivasan, M.; Ramakrishna, S.; Chen, J. Multi-functional electrospun nanofibres for advances in tissue regeneration, energy conversion & storage, and water treatment. Chem. Soc. Rev. 2016, 45, 1225. [Google Scholar] [PubMed]
- Shami, Z.; Amininasab, S.M.; Shakeri, P. Structure-Property Relationships of Nanosheeted 3D Hierarchical Roughness MgAl-Layered Double Hydroxide Branched to an Electrospun Porous Nanomembrane: A Superior Oil-Removing Nanofabric. ACS Appl. Mater. Interfaces 2016, 8, 28964–28973. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Tan, L.; Chen, Y.; Hayat, T.; Hu, J.; Alsaedi, A.; Ahmad, B.; Guo, W.; Wang, X. Performances and mechanisms of Mg/Al and Ca/Al layered double hydroxides for graphene oxide removal from aqueous solution. Chem. Eng. J. 2016, 297, 106–115. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Cheminform 1992, 23, 173–301. [Google Scholar] [CrossRef]
- Yu, S.; Zhai, L.; Zhong, S.; Qiu, Y.; Cheng, L.; Ren, X. Synthesis and structural characterization of magnetite/sepiolite composite and its sorptive properties for Co(II) and Cd(II). J. Taiwan Inst. Chem. Eng. 2016, 59, 221–228. [Google Scholar] [CrossRef]
- Kim, D.Y.; Joo, E.H.; Mishra, A.K.; Kim, N.H.; Na, Y.J.; Lee, J.H. Synthesis and Characterization of SPEEK/Layered Double Hydroxide Polymer Nanocomposite for Fuel Cell Applications. Adv. Mater. Res. 2013, 747, 234–237. [Google Scholar] [CrossRef]
- Li, F.; Yu, Z.; Shi, H.; Yang, Q.; Chen, Q.; Pan, Y.; Zeng, G.; Yan, L. A Mussel-inspired method to fabricate reduced graphene oxide/g-C3N4, composites membranes for catalytic decomposition and oil-in-water emulsion separation. Chem. Eng. J. 2017, 322, 33–45. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, S.L.; Chai, B.X.; Shun, X.D. Effect of nano-sized Al2O3-particle addition on PVDF ultrafiltration membrane performance. J. Membr. Sci. 2006, 276, 162–167. [Google Scholar]
- Zhao, X.; Su, Y.; Liu, Y.; Lip, Y.; Jiang, Z. Free-Standing Graphene Oxide-Palygorskite Nanohybrid Membrane for Oil/Water Separation. Acs Appl Mater Interfaces 2016, 8, 8247–8256. [Google Scholar] [CrossRef]
- Lin, J.; Ye, W.; Baltaru, M.C.; Tang, Y.P.; Bernstein, N.J.; Gao, P.; Balta, S.; Vald, M.; Volodin, A.; Sotto, A.; et al. Tight ultrafiltration membranes for enhanced separation of dyes and Na2SO4, during textile wastewater treatment. J. Membr. Sci. 2016, 514, 217–228. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, Y.; Wang, Y.; Zhang, H.; Liu, J. High flux, positively charged loose nanofiltration membrane by blending with poly (ionic liquid) brushes grafted silica spheres. J. Hazard. Mater. 2015, 287, 373–383. [Google Scholar] [CrossRef]
- Zhu, J.; Tian, M.; Hou, J.; Wang, J.; Lin, J.; Zhang, Y.; Liu, J.; Van der Bruggen, B. Surface zwitterionic functionalized graphene oxide for a novel loose nanofiltration membrane. J. Mater. Chem. A 2015, 4, 1980–1990. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Zhu, J.; Hou, J.; Liu, J.; Van der Bruggen, B. Zwitterionic functionalized layered double hydroxides nanosheets for a novel charged mosaic membrane with high salt permeability. J. Membr. Sci. 2016, 510, 27–37. [Google Scholar] [CrossRef]
- Liu, C.; Mao, H.; Zheng, J.; Zhang, S. Tight ultrafiltration membrane: Preparation and characterization of thermally resistant carboxylated cardo poly (arylene ether ketone)s (PAEKCOOH) tight ultrafiltration membrane for dye removal. J. Membr. Sci. 2017, 530, 1–10. [Google Scholar] [CrossRef]
- Hebbar, R.S.; Isloor, A.M.; Inamuddin; Abdullah, M.S.; Ismail, A.F.; Asiri, A.M. Fabrication of polyetherimide nanocomposite membrane with amine functionalised halloysite nanotubes for effective removal of cationic dye effluents. J. Taiwan Inst. Chem. Eng. 2018, 93, 42–53. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, G.; Zhang, L.; Zhang, W.; Gu, J.; Huang, Y.; Zhang, J.; Chen, T. Robust Construction of Underwater Superoleophobic CNTs/Nanoparticles Multifunctional Hybrid Membranes via Interception Effect for Oily Wastewater Purification. J. Membr. Sci. 2018, 59. [Google Scholar] [CrossRef]






| Membrane | Pure Water Flux (L·m−2·h−1·bar−1) | Dye Permeate Flux (L·m−2·h−1·bar−1) | Dye Rejection (%) | Operating Conditions | Ref. | |
|---|---|---|---|---|---|---|
| pH limitation | Max. Temp. (°C) | |||||
| UH004 (hydrophilic PES) | 27.5 | 27 | 98.9 | 0–14.0 | 95 | [31] |
| SIO2-PIL/PES blending NF | 21.2 | null | 90-93 | – | – | [32] |
| GO-PSBMA/PES blending NF | 12.5 | 8.8 | 95 | – | – | [33] |
| Zwitterion-hydrotalcite incorporated PES | 20.1 | null | 86.7 | – | – | [34] |
| PAEK-COOH | 29.5 | 25 | 95 | 1.0–10.0 | 95 | [35] |
| MgAl-LDH/Sep | 1200 | 900 | 99.8 | – | – | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Li, X.; Peng, Y.; Min, X.; Yin, D.; Shao, L. MgAl-Layered-Double-Hydroxide/Sepiolite Composite Membrane for High-Performance Water Treatment Based on Layer-by-Layer Hierarchical Architectures. Polymers 2019, 11, 525. https://doi.org/10.3390/polym11030525
Yu Z, Li X, Peng Y, Min X, Yin D, Shao L. MgAl-Layered-Double-Hydroxide/Sepiolite Composite Membrane for High-Performance Water Treatment Based on Layer-by-Layer Hierarchical Architectures. Polymers. 2019; 11(3):525. https://doi.org/10.3390/polym11030525
Chicago/Turabian StyleYu, Zongxue, Xiuhui Li, Yixin Peng, Xia Min, Di Yin, and Liangyan Shao. 2019. "MgAl-Layered-Double-Hydroxide/Sepiolite Composite Membrane for High-Performance Water Treatment Based on Layer-by-Layer Hierarchical Architectures" Polymers 11, no. 3: 525. https://doi.org/10.3390/polym11030525
APA StyleYu, Z., Li, X., Peng, Y., Min, X., Yin, D., & Shao, L. (2019). MgAl-Layered-Double-Hydroxide/Sepiolite Composite Membrane for High-Performance Water Treatment Based on Layer-by-Layer Hierarchical Architectures. Polymers, 11(3), 525. https://doi.org/10.3390/polym11030525





