pH-Responsive Micelles Assembled by Three-Armed Degradable Block Copolymers with a Cholic Acid Core for Drug Controlled-Release
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Synthesis of Polymers
2.3.1. Synthesis of CA-(PCL28)3
2.3.2. Synthesis of CA-(PCL28-Br)3
2.3.3. Synthesis of CA-(PCL28-b-PDEAEMA5)3
2.3.4. Synthesis of CA-(PCL28-b-PDEAEMA5-b-PPEGMA5)3 (CA-CDEP)
2.3.5. Synthesis of CA-(PCL28-b-PPEGMA7)3 (CA-CP)
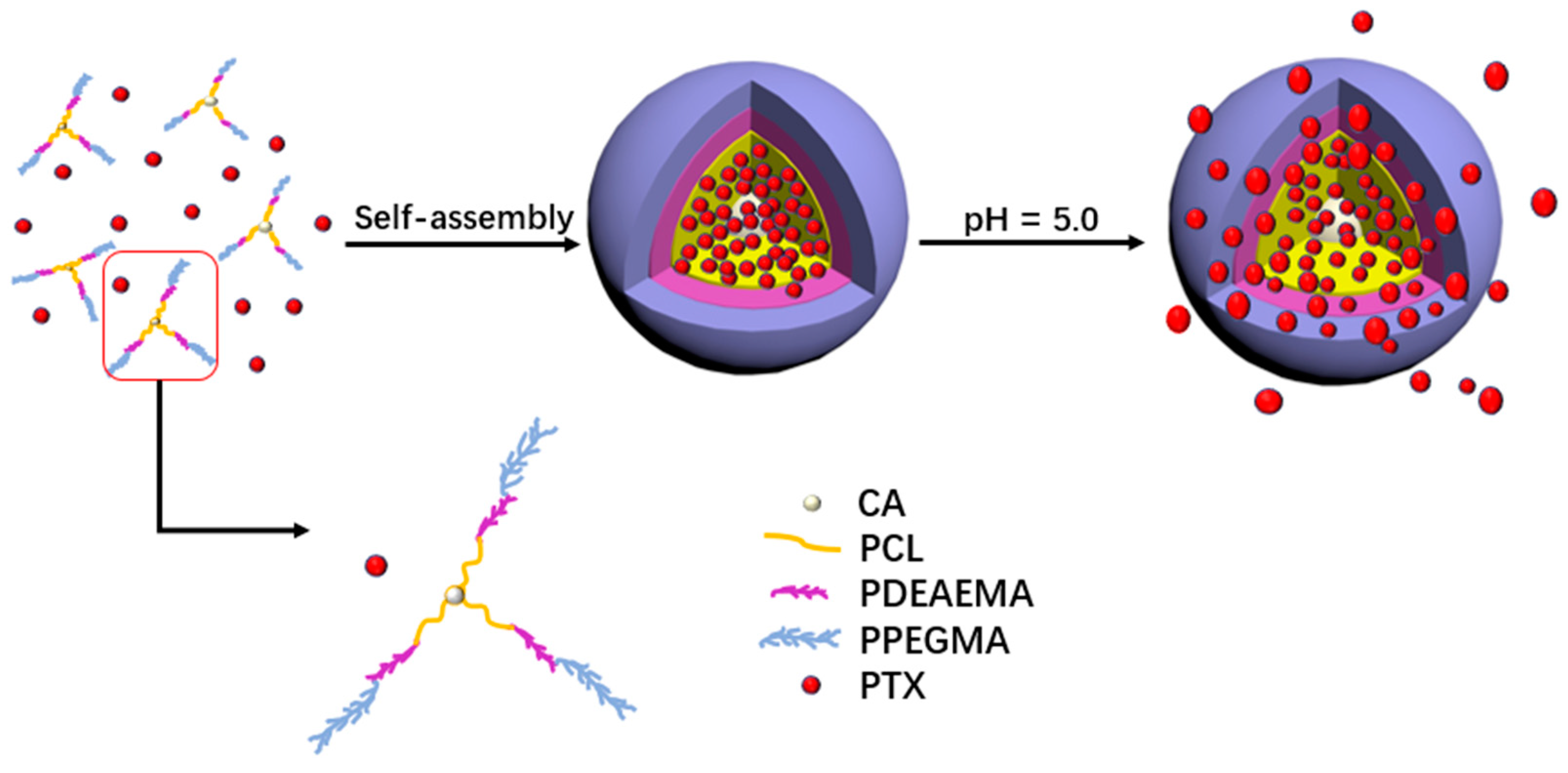
2.4. Self-Assembly of the Micelles
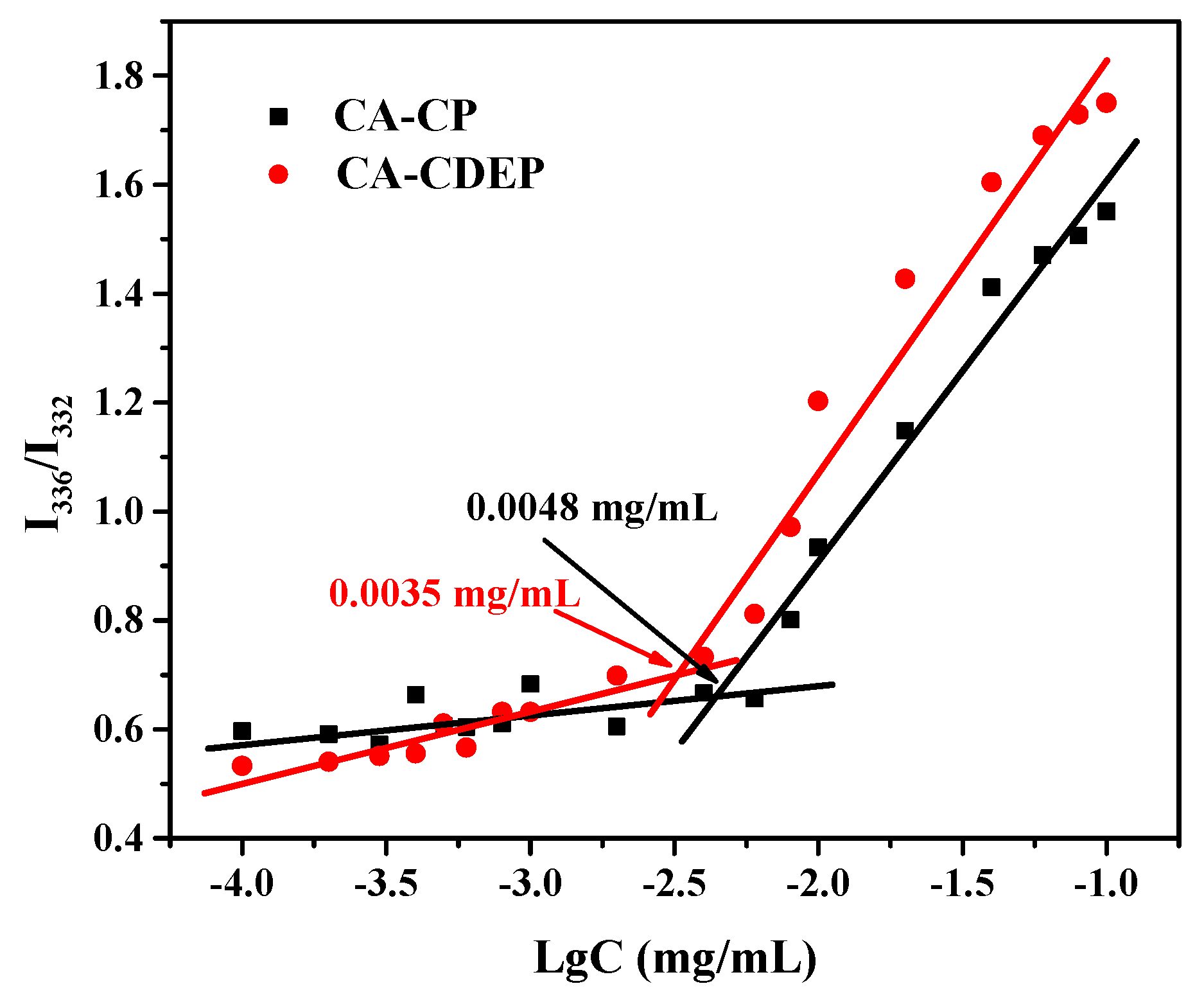
2.5. CMC of the Micelles
2.6. Drug Loading and In Vitro Release
2.7. In Vitro Cytotoxicity Assay
3. Results and Discussion
3.1. Synthesis of Block Polymers
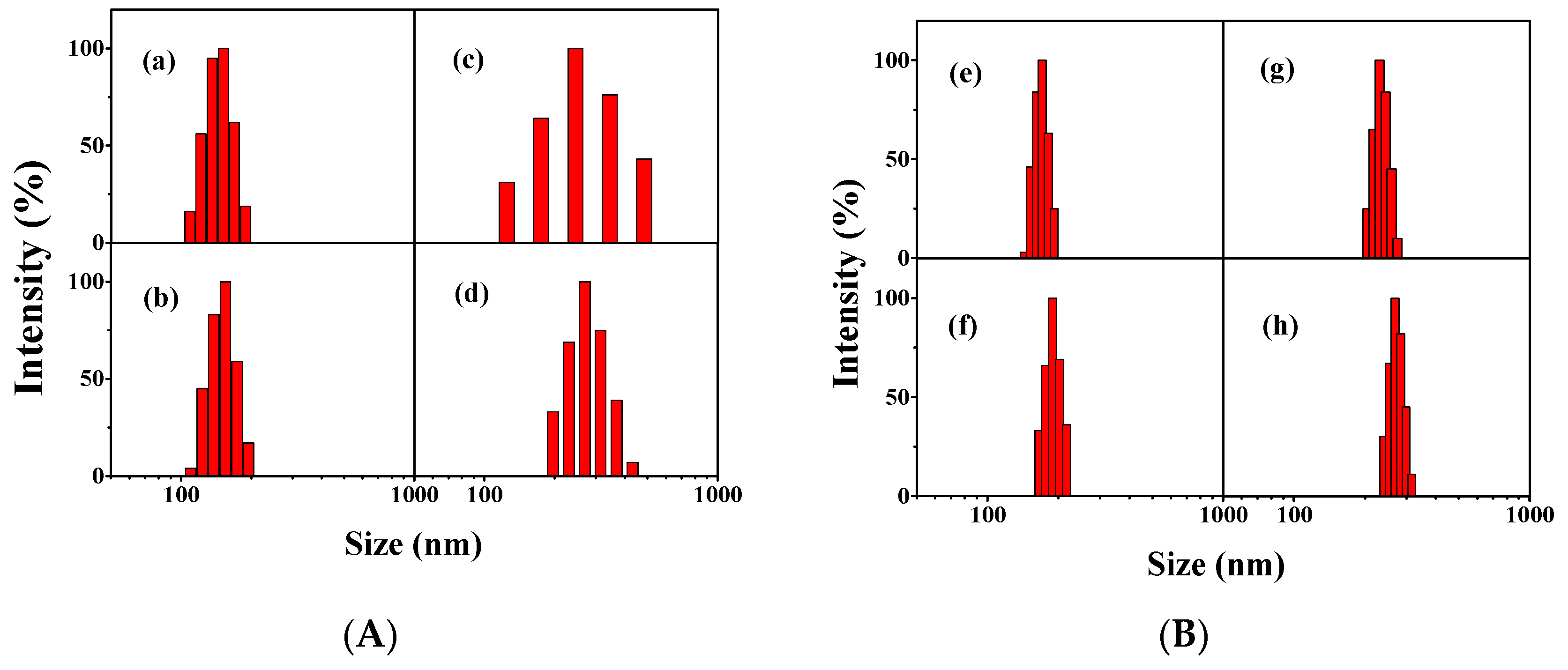
3.2. Formation and Characterization of the Micelles
3.3. Drug Release Assay
3.4. In Vitro Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Tew, W.P.; Muss, H.B.; Kimmick, G.G.; Von Gruenigen, V.E.; Lichtman, S.M. Breast and ovarian cancer in the older woman. J. Clin. Oncol. 2014, 32, 2553–2561. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2017. CA J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Schorzman, A.N.; Ma, P.; Madden, A.J.; Zamboni, W.C.; Benhabbour SRMumper, U.J. 2′-(2-bromohexadecanoyl)-paclitaxel conjugate nanoparticles for the treatment of non-small cell lung cancer in an orthotopic xenograft mouse model. Int. J. Nanomed. 2014, 9, 3601–3610. [Google Scholar] [CrossRef]
- Pawar, V.K.; Panchal, S.B.; Singh, Y.; Meher, J.G.; Sharma, K.; Singh, P.; Bora, H.K.; Singh, A.; Datta DChourasia, M.K. Immunotherapeutic vitamin E nanoemulsion synergies the antiproliferative activity of paclitaxel in breast cancer cells via modulating Th1 and Th2 immune response. J. Control. Release 2014, 196, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Gawde, K.A.; Sau, S.; Tatiparti, K.; Kashaw, S.K.; Mehrmohammadi, M.; Azmi Asiyer, A.K. Paclitaxel and di-fluorinated curcumin loaded in albumin nanoparticles for targeted synergistic combination therapy of ovarian and cervical cancers. Colloids Surf. B Biointerfaces 2018, 167, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, L.; Saunders, D.N.; Ranson, M.; Thurecht, K.J.; Storm, G.; Vine, K.L. Towards clinical translation of ligand-functionalized liposomes in targeted cancer therapy: Challenges and opportunities. J. Control. Release 2018, 277, 1–13. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J.H.; Park, H.; Kim, Y.S.; Park, K.; Nam, H.; Lee, S.; Park, J.H.; Park, R.W.; Kim, I.S.; et al. Tumor-homing multifunctional nanoparticles for cancer theragnosis: Simultaneous diagnosis, drug delivery, and therapeutic monitoring. J. Control. Release 2010, 146, 219–227. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, Y.A.; Chen, Y.Z.; Ye, J.A.; Sha, X.Y.; Fang, X.L. Multifunctional Pluronic P123/F127 mixed polymeric micelles loaded with paclitaxel for the treatment of multidrug resistant tumors. Biomaterials 2011, 32, 2894–2906. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.W.; Wu, S.J.; Hu, C.Y.; Chen, Z.; Wang, H.; Fan, F.; Qin, Y.; Wang, C.; Sun, H.F.; Leng, X.G.; et al. Folate-targeted polymersomes loaded with both paclitaxel and doxorubicin for the combination chemotherapy of hepatocellular carcinoma. Acta Biomater. 2017, 58, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Maiti, C.; Banerjee, R.; Maiti, S.; Dhara, D. pH-Induced vesicle-to-micelle transition in amphiphilic diblock copolymer: Investigation by energy transfer between in situ formed polymer embedded gold nanoparticles and fluorescent dye. Langmuir 2015, 31, 32–41. [Google Scholar] [CrossRef]
- Meng, H.; Wang, M.Y.; Liu, H.Y.; Liu, X.S.; Situ, A.; Wu, B.; Ji, Z.X.; Chang, C.H.; Nel, A.E. Use of a lipid-coated mesoporous silica nanoparticle platform for synergistic gemcitabine and paclitaxel delivery to human pancreatic cancer in mice. ACS Nano 2015, 9, 3540–3557. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.H.; He, C.L.; Tian, H.Y.; Ding, J.X.; Hsiao, B.S.; Chu, B.; Chen, X.S. Polymeric nanostructured materials for biomedical applications. Prog. Polym. Sci. 2016, 60, 86–128. [Google Scholar] [CrossRef]
- Su, J.H.; Sun, H.P.; Meng, Q.S.; Yin, Q.; Zhang, P.C.; Zhang, Z.W.; Yu, H.J.; Li, Y.P. Bioinspired nanoparticles with NIR-controlled drug release for synergetic chemophotothermal therapy of metastatic breast cancer. Adv. Funct. Mater. 2016, 26, 7495–7506. [Google Scholar] [CrossRef]
- Jaskula-Sztul, R.; Chen, G.J.; Dammalapati, A.; Harrison, A.; Tang, W.P.; Gong, S.Q.; Chen, H. AB3-loaded and tumor-targeted unimolecular micelles for medullary thyroid cancer treatment. J. Mater. Chem. B 2017, 5, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.J.; Nie, S.Y.; Zhong, Q.; Yang, Y.Q.; Cai, C.Z.; Wang, J.F.; Zhang, L.J. Amphiphilic miktoarm star copolymer (PCL)(3)-(PDEAEMA-b-PPEGMA)(3) as pH-sensitive micelles in the delivery of anticancer drug. J. Mater. Chem. B 2014, 2, 4008–4020. [Google Scholar] [CrossRef]
- Guo, J.W.; Gao, X.L.; Su, L.N.; Xia, H.M.; Gu, G.Z.; Pang, Z.Q.; Jiang, X.G.; Yao, L.; Chen, J.; Chen, H.Z. Aptamer-functionalized PEG-PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials 2011, 32, 8010–8020. [Google Scholar] [CrossRef] [PubMed]
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in biomaterials for drug delivery. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.M.; Fay, F.; Hak, S.; Perez-Aguilar, J.M.; Sanchez-Gaytan, B.L.; Goode, B.; Duivenvoorden, R.; Davies, C.D.; Bjorkoy, A.; Weinstein, H.; et al. Augmenting drug-carrier compatibility improves tumour nanotherapy efficacy. Nat. Commun. 2016, 7, 11221. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Huang, L.X.; Moingeon, F.; Gauthier, M.; Yang, G. pH-responsive poly(ethylene glycol)-block-polylactide micelles for tumor-targeted drug delivery. Biomacromolecules 2017, 18, 2711–2722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Song, C.C.; Ji, R.; Qiao, Z.Y.; Yang, C.; Qiu, F.Y.; Liang, D.H.; Du, F.S.; Li, Z.C. Oxidation and temperature dual responsive polymers based on phenylboronic acid and N-isopropylacrylamide motifs. Polym. Chem. 2016, 7, 1494–1504. [Google Scholar] [CrossRef]
- Gao, F.; Xing, Y.H.; Yao, Y.; Sun, L.Y.; Sun, Y.; He, X.H.; Lin, S.L. Self-assembly and multi-stimuli responsive behavior of PAA-b-PAzoMA-b-PNIPAM triblock copolymers. Polym. Chem. 2017, 8, 7529–7536. [Google Scholar] [CrossRef]
- Thambi, T.; Park, J.H.; Lee, D.S. Stimuli-responsive polymersomes for cancer therapy. Biomater. Sci. 2016, 4, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.Q.; Wu, H.; Dong, J.; Wang, G.J. Quadruple-stimuli-sensitive polymeric nanocarriers for controlled release under combined stimulation. Macromolecules 2014, 47, 8777–8783. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, L.; Torchilin, V.P. pH-sensitive poly(histidine)-PEG/DSPE-PEG co-polymer micelles for cytosolic drug delivery. Biomaterials 2013, 34, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.X.; Ma, X.Q.; Hou, M.L.; Gao, Y.E.; Bai, S.; Xiao, B.; Xue, P.; Kang, Y.J.; Xu, Z.G.; Li, C.M. pH-Responsive unimolecular micelles based on amphiphilic star-like copolymers with high drug loading for effective drug delivery and cellular imaging. J. Mater. Chem. B 2017, 5, 6847–6859. [Google Scholar] [CrossRef]
- Yildirim, T.; Traeger, A.; Sungur, P.; Hoeppener, S.; Kellner, C.; Yildirim, I.; Pretzel, D.; Schubert, S.; Schubert, U.S. Polymersomes with endosomal pH-induced vesicle-to-micelle morphology transition and a potential application for controlled doxorubicin delivery. Biomacromolecules 2017, 18, 3280–3290. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.Q.; Chen, Y.Z.; Chen, Y.; Liu, H. Lipid-polymer hybrid nanoparticles for synergistic drug delivery to overcome cancer drug resistance. New J. Chem. 2017, 41, 1518–1525. [Google Scholar] [CrossRef]
- Strandman, S.; Le Devedec, F.; Zhu, X.X. Thermosensitivity of bile acid-based oligo(ethylene glycol) stars in aqueous solutions. Macromol. Rapid Commun. 2011, 32, 1185–1189. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Deng, J.R.; Deng, J.P. Optically active helical polyacetylene self-assembled into chiral micelles used as nanoreactor for helix-sense-selective polymerization. ACS Macro Lett. 2017, 6, 6–10. [Google Scholar] [CrossRef]
- Posa, M.; Popovic, K. Structure-property relationships in sodium muricholate derivative (bile salts) micellization: The effect of conformation of steroid skeleton on hydrophobicity and micelle formation-pattern recognition and potential membranoprotective properties. Mol. Pharm. 2017, 14, 3343–3355. [Google Scholar] [CrossRef] [PubMed]
- Le Devedec, F.; Fuentealba, D.; Strandman, S.; Bohne, C.; Zhu, X.X. Aggregation behavior of pegylated bile acid derivatives. Langmuir 2012, 28, 13431–13440. [Google Scholar] [CrossRef] [PubMed]
- Le Devedec, F.; Strandman, S.; Baille, W.E.; Zhu, X.X. Functional star block copolymers with a cholane core: Thermo-responsiveness and aggregation behavior. Polymer 2013, 54, 3898–3903. [Google Scholar] [CrossRef]
- Shao, Y.; Jia, Y.G.; Shi, C.Y.; Luo, J.T.; Zhu, X.X. Block and random copolymers bearing cholic acid and oligo(ethylene glycol) pendant groups: Aggregation, thermosensitivity, and drug loading. Biomacromolecules 2014, 15, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Despa, F.; Luo, J.T.; Li, J.; Duan, Y.; Lam, K.S. Cholic acid micelles-controlling the size of the aqueous cavity by PEGylation. Phys. Chem. Chem. Phys. 2010, 12, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.J.; Robinson, M.; Banquy, X.; Leblond, J.; Zhu, X.X. Bile acid-based drug delivery systems for enhanced doxorubicin encapsulation: Comparing hydrophobic and ionic interactions in drug loading and release. Mol. Pharm. 2018, 15, 1266–1276. [Google Scholar] [CrossRef]
- Topel, O.; Cakir, B.A.; Budama, L.; Hoda, N. Determination of critical micelle concentration of polybutadiene-block-poly(ethyleneoxide) diblock copolymer by fluorescence spectroscopy and dynamic light scattering. J. Mol. Liq. 2013, 177, 40–43. [Google Scholar] [CrossRef]
- Xiong, X.B.; Binkhathlan, Z.; Molavi, O.; Lavasanifar, A. Amphiphilic block co-polymers: Preparation and application in nanodrug and gene delivery. Acta Biomater. 2012, 8, 2017–2033. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.C.; Chan, D.P.Y.; Shoichet, M.S. Polymeric micelle stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Pramanik, P.; Ray, D.; Aswal, V.K.; Ghosh, S. Supramolecularly engineered amphiphilic macromolecules: Molecular interaction overrules packing parameters. Angew. Chem. Int. Ed. 2017, 56, 3516–3520. [Google Scholar] [CrossRef]
- Mai, Y.Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Guo, J.W.; Tong, R.; Yang, C.F.; Chen, J.K. pH-sensitive micelles based on star copolymer ad-(pcl-b-pdeaema-b-ppegma)4 for controlled drug delivery. Polymers 2018, 10, 443. [Google Scholar] [CrossRef]
- Ojha, T.; Pathak, V.; Shi, Y.; Hennink, W.E.; Moonen, C.T.W.; Storm, G.; Kiessling, F.; Lammers, T. Pharmacological and physical vessel modulation strategies to improve EPR-mediated drug targeting to tumors. Adv. Drug Deliv. Rev. 2017, 119, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Wei, M.; Lu, J.; Jin, L. pH-Responsive composite based on prednisone-block copolymer micelle intercalated inorganic layered matrix: Structure and in vitro drug release. Chem. Eng. J. 2009, 151, 359–366. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.Z.; Li, Z.B. A review of drug release mechanisms from nanocarrier systems. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 76, 1440–1453. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Y.H.; Sun, J.; Zhao, N.N.; Sun, M.Y.; He, Z.G. Preparation and in vitro/in vivo evaluation of sustained-release venlafaxine hydrochloride pellets. Int. J. Pharm. 2012, 426, 21–28. [Google Scholar] [CrossRef] [PubMed]





| Polymer | Mn,th 1 (g/mol) | Mn,GPC (g/mol) | PDI 2 |
|---|---|---|---|
| CA-(PCL28)3 | 9500 | 9400 | 1.46 |
| CA-(PCL28-b-PDEAEMA5)3 | 16,900 | 12,800 | 1.55 |
| CA-CDEP | 31,780 | 19,500 | 1.44 |
| CA-CP | 32,900 | 19,400 | 1.31 |
| Sample | PTX/Polymer | Diameter (nm) | PDI 1 (DLS) | Potential (Zeta, mV) | DLC (wt %) | EE (%) | |
|---|---|---|---|---|---|---|---|
| DLS | TEM | ||||||
| CA-CP | -- | 151.0 | 100 ± 20 | 0.093 | −14.96 | -- | -- |
| CA-CDEP | -- | 170.4 | 120 ± 20 | 0.205 | −16.04 | -- | -- |
| CA-CP + PTX | 30/100 | 245.2 | -- | 0.147 | -- | 6.96 | 22.17 |
| 50/100 | 269.2 | 220 ± 20 | 0.105 | -- | 11.4 | 23.14 | |
| CA-CDEP + PTX | 30/100 | 230.6 | -- | 0.097 | -- | 20.48 | 41.77 |
| 50/100 | 268.2 | 210 ± 20 | 0.171 | -- | 29.92 | 48.24 | |
| Sample | pH | n | k | R2 |
|---|---|---|---|---|
| CA-CP | 7.4 | 0.45 | 0.11 | 0.991 |
| 6.5 | 0.50 | 0.10 | 0.982 | |
| 5.0 | 0.52 | 0.10 | 0.993 | |
| CA-CDEP | 7.4 | 0.47 | 0.11 | 0.986 |
| 6.5 | 0.28 | 0.23 | 0.973 | |
| 5.0 | 0.29 | 0.22 | 0.967 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Wen, W.; Jia, Y.-G.; Liu, S.; Guo, J. pH-Responsive Micelles Assembled by Three-Armed Degradable Block Copolymers with a Cholic Acid Core for Drug Controlled-Release. Polymers 2019, 11, 511. https://doi.org/10.3390/polym11030511
Feng J, Wen W, Jia Y-G, Liu S, Guo J. pH-Responsive Micelles Assembled by Three-Armed Degradable Block Copolymers with a Cholic Acid Core for Drug Controlled-Release. Polymers. 2019; 11(3):511. https://doi.org/10.3390/polym11030511
Chicago/Turabian StyleFeng, Jingjie, Weiqiu Wen, Yong-Guang Jia, Sa Liu, and Jianwei Guo. 2019. "pH-Responsive Micelles Assembled by Three-Armed Degradable Block Copolymers with a Cholic Acid Core for Drug Controlled-Release" Polymers 11, no. 3: 511. https://doi.org/10.3390/polym11030511
APA StyleFeng, J., Wen, W., Jia, Y.-G., Liu, S., & Guo, J. (2019). pH-Responsive Micelles Assembled by Three-Armed Degradable Block Copolymers with a Cholic Acid Core for Drug Controlled-Release. Polymers, 11(3), 511. https://doi.org/10.3390/polym11030511





