Synthesis and Properties of Plasma-Polymerized Methyl Methacrylate via the Atmospheric Pressure Plasma Polymerization Technique
Abstract
1. Introduction
2. Experimental and Characterizations
2.1. Atmospheric Pressure Plasma Synthesis and Materials
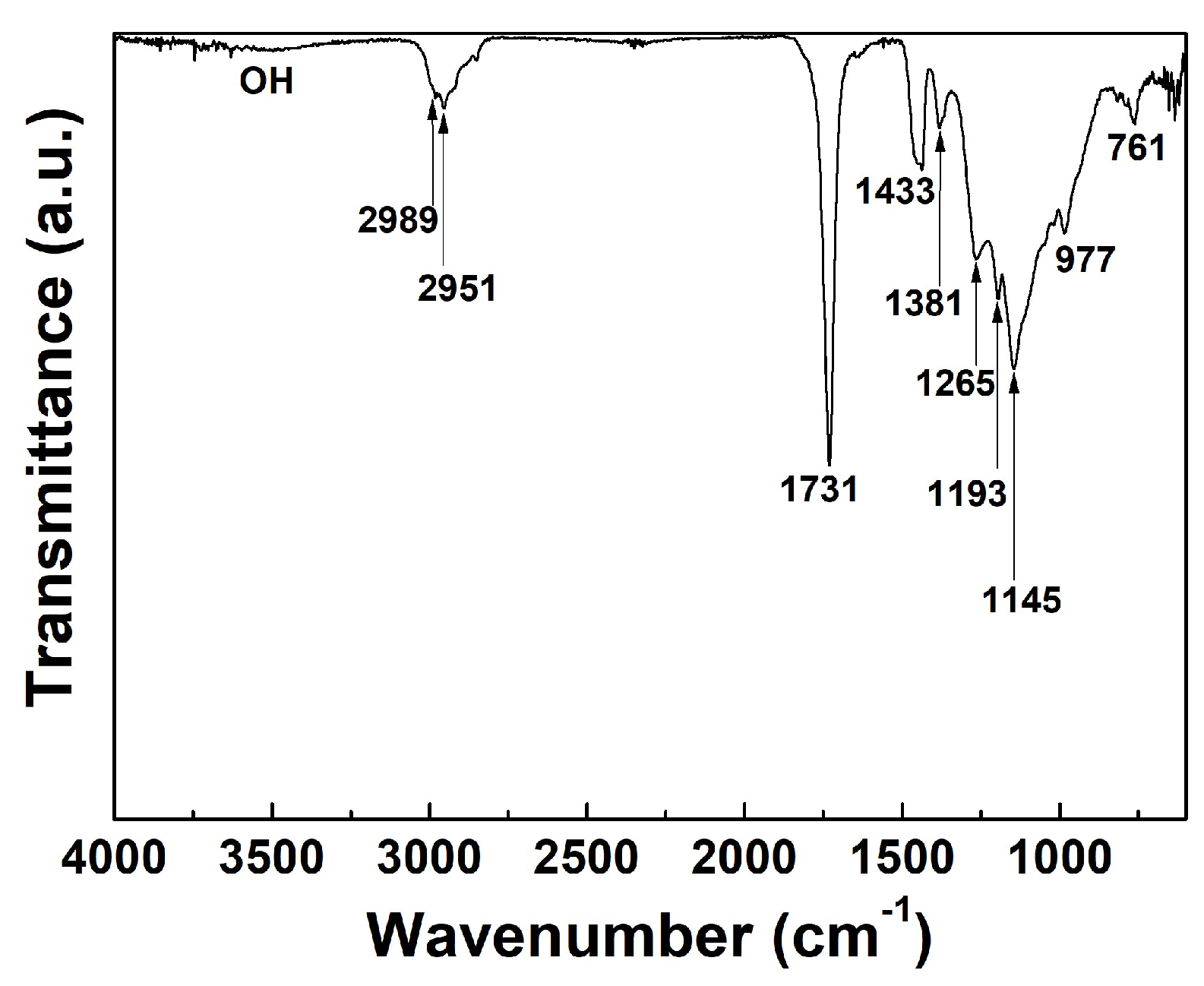
2.2. Fourier Transform-Infrared Spectroscopy Analysis
2.3. X-ray Photoelectron Spectroscopy Analysis
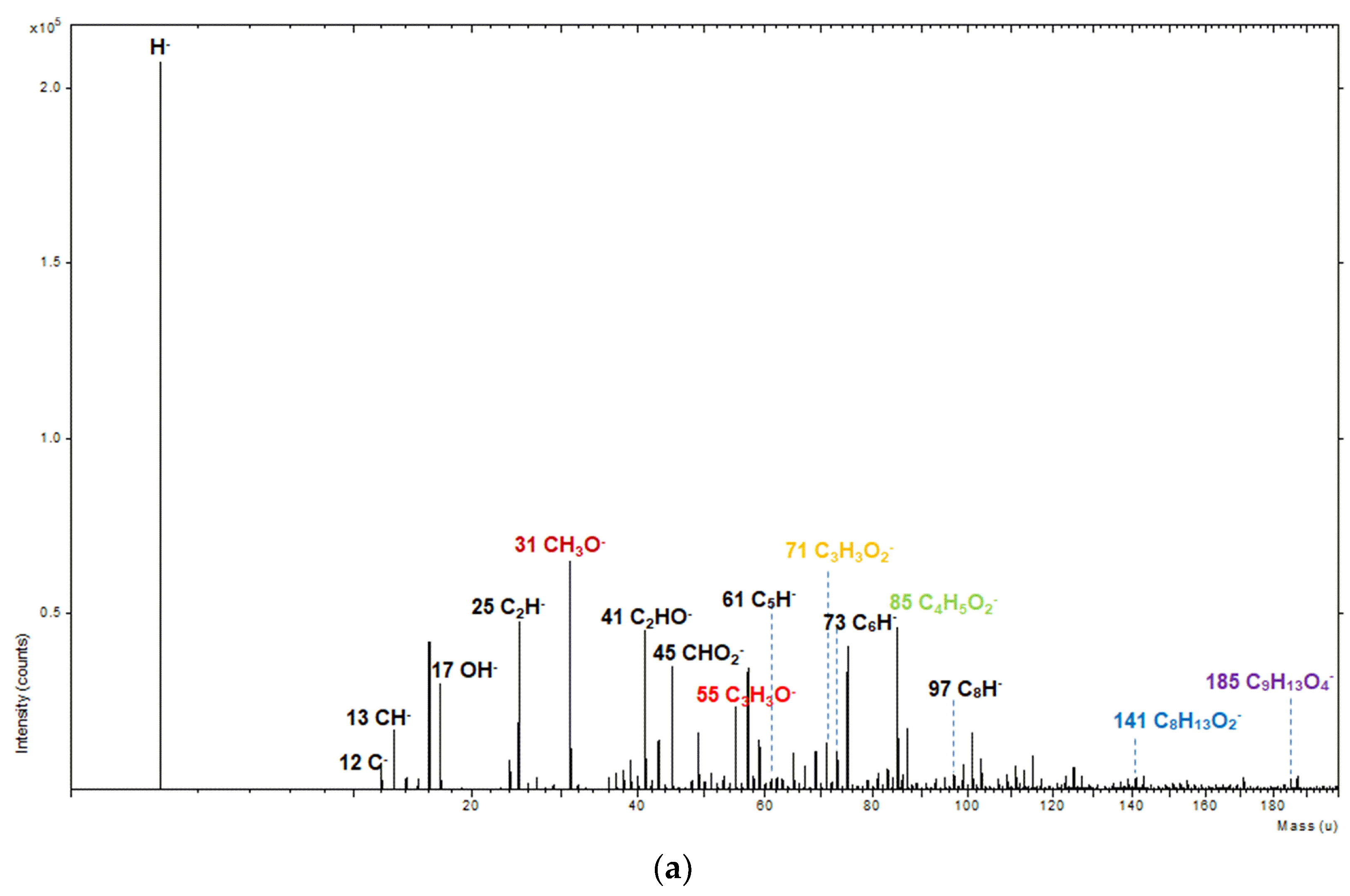
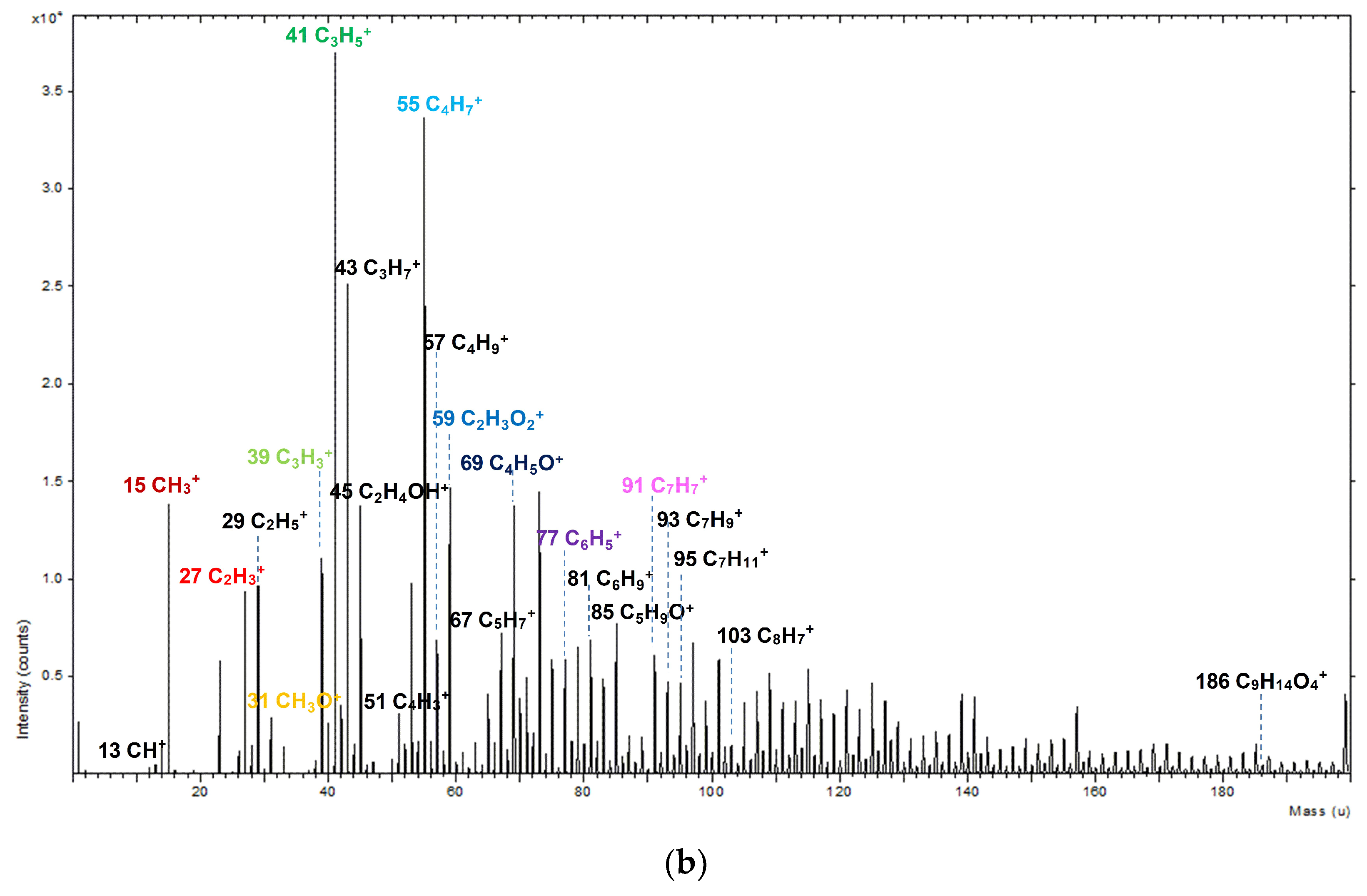
2.4. Time of Flight-Secondary Ion Mass Spectrometry Analysis
2.5. Surface Morphology Study
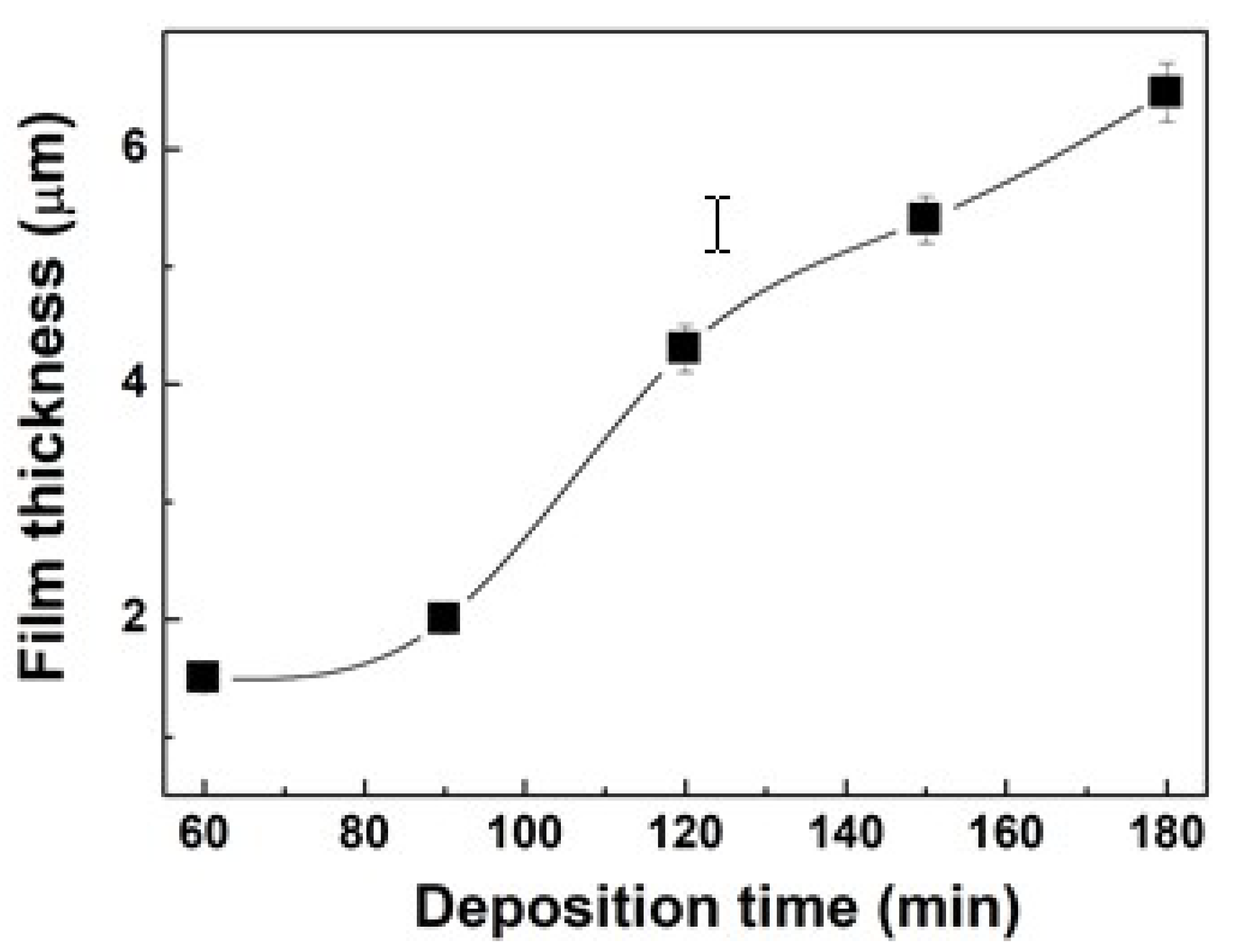
2.6. Thickness Measurement
2.7. Optical Transmittance
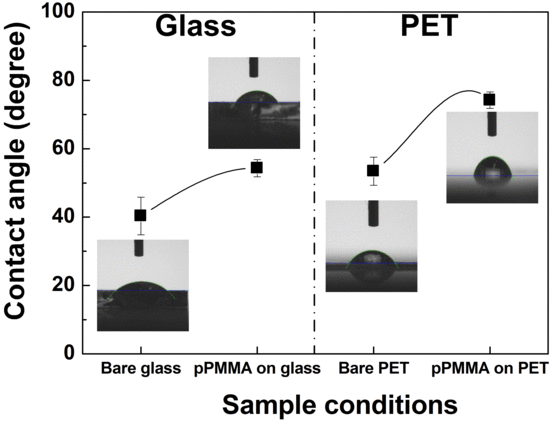
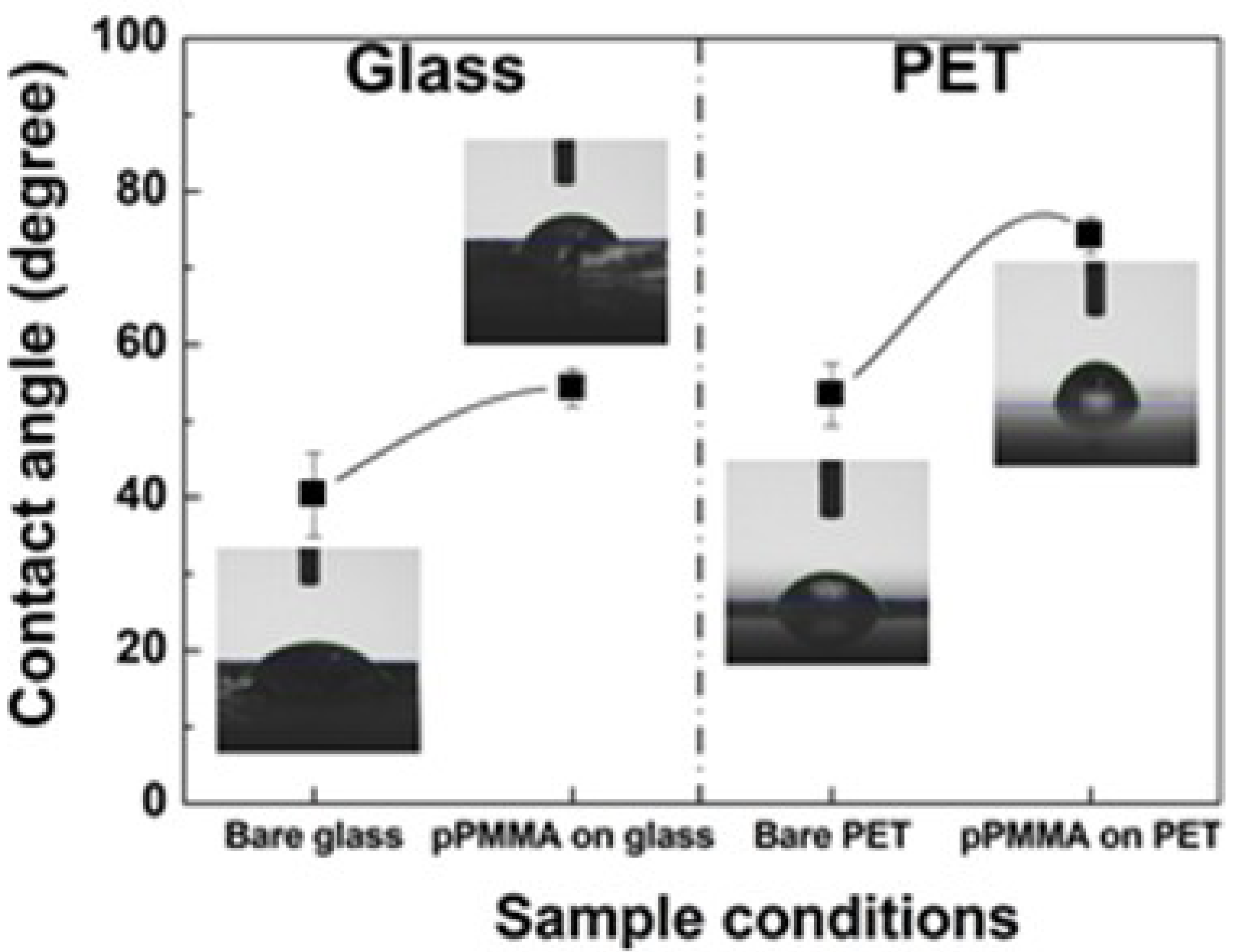
2.8. Water Contact Angle Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koizumi, Y.; Shida, N.; Ohira, M.; Nishiyama, H.; Tomita, I.; Inagi, S. Electropolymerization on wireless electrodes towards conducting polymer microfibre networks. Nat. Commun. 2016, 7, 10404. [Google Scholar] [CrossRef] [PubMed]
- Berkes, B.B.; Bandarenka, A.S.; Inzelt, G. Electropolymerization: Further insight into the formation of conducting polyindole thin films. J. Phys. Chem. C 2015, 119, 1996–2003. [Google Scholar] [CrossRef]
- Thanh, D.V.; Li, L.-J.; Chu, C.-W.; Yen, P.-J.; Wei, K.-H. Plasma-assisted electrochemical exfoliation of graphite for rapid production of graphene sheets. RSC Adv. 2014, 4, 6946–6949. [Google Scholar] [CrossRef]
- Kareem, A.T.; Kaliani, A.A. ZnS nanoparticle synthesis and stability of 1-butyl-3-methylimidazolium tetrafluoroborate ([BMIM][BF4]) under glow discharge plasma. Ionics 2013, 19, 1559–1565. [Google Scholar] [CrossRef]
- Rogov, A.B.; Yerokhin, A.; Matthews, A. The role of cathodic current in plasma electrolytic oxidation of aluminum: Phenomenological concepts of the “soft sparking” mode. Langmuir 2017, 33, 11059–11069. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, C.; Lu, Y.; Wei, G.; Ye, G.; Sun, T.; Chen, J. Microplasma-assisted rapid, chemical oxidant-free and controllable polymerization of dopamine for surface modification. Polym. Chem. 2017, 8, 4388–4392. [Google Scholar] [CrossRef]
- Phillips, J.; Luhrs, C.; Richard, M. Review: Engineering particles using the aerosol-through-plasma method. IEEE Trans. Plasma Sci. 2009, 37, 726–739. [Google Scholar] [CrossRef]
- Luhrs, C.; Phillips, J.; Fanson, P.T. Production of unique structures using the Aerosol-Through-Plasma (A-T-P) process. WIT Trans. Built Environ. 2008, 97, 63–70. [Google Scholar]
- Huang, T.-S.; Su, Y.-K.; Wang, P.-C. Study of organic thin film transistor with polymethylmethacrylate as a dielectric layer. Appl. Phys. Lett. 2007, 91, 092116. [Google Scholar] [CrossRef]
- Yáñez-Pacios, A.J.; Martín-Martínez, J.M. Comparative adhesion, ageing resistance, and surface properties of wood plastic composite treated with low pressure plasma and atmospheric pressure plasma jet. Polymers 2018, 10, 643. [Google Scholar] [CrossRef]
- Chang, C.-K.; Wang, H.-M.D.; Lan, J.C.-W. Investigation and characterization of plasma-treated poly(3-hydroxybutyrate) and poly(3-hydroxybutyrateco-3-hydroxyvalerate) biopolymers for an in vitro cellular study of mouse adipose-derived stem cells. Polymers 2018, 10, 355. [Google Scholar] [CrossRef]
- Kan, C.-W.; Man, W.-S. Parametric study of effects of atmospheric pressure plasma treatment on the wettability of cotton fabric. Polymers 2018, 10, 233. [Google Scholar] [CrossRef]
- Chen, K.-S.; Chang, S.-J.; Feng, C.-K.; Lin, W.-L.; Liao, S.-C. Plasma deposition and UV light induced surface grafting polymerization of NIPAAm on stainless steel for enhancing corrosion resistance and its drug delivery property. Polymers 2018, 10, 1009. [Google Scholar] [CrossRef]
- Lin, F.; Li, W.; Tang, Y.; Shao, H.; Su, C.; Jiang, J.; Chen, N. High-performance polyimide filaments and composites improved by O2 plasma treatment. Polymers 2018, 10, 695. [Google Scholar] [CrossRef]
- Deynse, A.V.; Cools, P.; Leys, C.; Geyter, N.D.; Morent, R. Surface activation of polyethylene with an argon atmospheric pressure plasma jet: Influence of applied power and flow rate. Appl. Surf. Sci. 2015, 328, 269–278. [Google Scholar] [CrossRef]
- Cools, P.; Sainz-Garcı, E.; Geyter, N.D.; Nikiforov, A.; Blajan, M.; Shimizu, K.; Alba-Elıas, F.; Leys, C.; Morent, R. Influence of DBD inlet geometry on the homogeneity of plasma-polymerized acrylic acid films: The use of a microplasma–electrode inlet configuration. Plasma Process. Polym. 2015, 12, 1153–1163. [Google Scholar] [CrossRef]
- Vrekhema, S.V.; Cools, P.; Declercq, H.; Tongel, A.V.; Vercruysse, C.; Cornelissen, M.; Geyter, N.D.; Morent, R. Application of atmospheric pressure plasma on polyethylene for increased prosthesis adhesion. Thin Solid Films 2015, 596, 256–263. [Google Scholar] [CrossRef]
- Morent, R.; Geyter, N.D.; Trentesaux, M.; Gengembre, L.; Dubruel, P.; Leys, C.; Payen, E. Stability study of polyacrylic acid films plasma-polymerized on polypropylene substrates at medium pressure. Appl. Surf. Sci. 2010, 257, 372–380. [Google Scholar] [CrossRef]
- Geyter, N.D.; Morent, R.; Vlierberghe, S.V.; Dubruel, P.; Leys, C.; Gengembre, L.; Schacht, E.; Payen, E. Deposition of polymethyl methacrylate on polypropylene substrates using an atmospheric pressure dielectric barrier discharge. Prog. Org. Coat. 2009, 64, 230–237. [Google Scholar] [CrossRef]
- Geyter, N.D.; Morent, R.; Leys, C.; Gengembre, L.; Payen, E. Treatment of polymer films with a dielectric barrier discharge in air, helium and argon at medium pressure. Surf. Coat. Technol. 2007, 201, 7066–7075. [Google Scholar] [CrossRef]
- Casserly, T.B.; Gleason, K.K. Effect of substrate temperature on the plasma polymerization of poly(methyl methacrylate). Chem. Vap. Depos. 2006, 12, 59–66. [Google Scholar] [CrossRef]
- Kasih, T.P.; Kuroda, S.-I.; Kubota, H. Poly(methyl methacrylate) films deposited via non-equilibrium atmospheric pressure plasma polymerization using argon as working gas. Plasma Process. Polym. 2007, 4, 648–653. [Google Scholar] [CrossRef]
- Vrekhem, S.V.; Morent, R.; Geyter, N.D. Deposition of a PMMA coating with an atmospheric pressure plasma jet. J. Coat. Technol. Res. 2018, 15, 679–690. [Google Scholar] [CrossRef]
- Cools, P.; Geyter, N.D.; Vanderleyden, E.; Barberis, F.; Dubruel, P.; Morent, R. Adhesion improvement at the PMMA bone cement-titanium implant interface using methyl methacrylate atmospheric pressure plasma polymerization. Surf. Coat. Technol. 2016, 294, 201–209. [Google Scholar] [CrossRef]
- Vrekhem, S.V.; Vloebergh, K.; Asadian, M.; Vercruysse, C.; Declercq, H.; Tongel, A.V.; Wilde, L.D.; Geyter, N.D.; Morent, R. Improving the surface properties of an UHMWPE shoulder implant with an atmospheric pressure plasma jet. Sci. Rep. 2018, 8, 4720. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-S.; Kim, D.H.; Shin, B.J.; Tae, H.-S. Synthesis and characterization of nanofibrous polyaniline thin film prepared by novel atmospheric pressure plasma polymerization technique. Materials 2016, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-S.; Jung, E.Y.; Kim, D.H.; Kim, D.Y.; Lee, H.-K.; Shin, B.J.; Lee, D.H.; Tae, H.-S. Atmospheric pressure plasma polymerization synthesis and characterization of polyaniline films doped with and without iodine. Materials 2017, 10, 1272. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-S.; Kim, D.Y.; Kim, D.H.; Lee, H.-K.; Shin, B.J.; Tae, H.-S. Humidity-independent conducting polyaniline films synthesized using advanced atmospheric pressure plasma polymerization with in-situ iodine doping. Appl. Phys. Lett. 2017, 110, 033502. [Google Scholar] [CrossRef]
- Kim, D.H.; Park, C.-S.; Kim, W.H.; Shin, B.J.; Hong, J.G.; Park, T.S.; Seo, J.H.; Tae, H.-S. Influences of guide-tube and bluff-body on advanced atmospheric pressure plasma source for single-crystalline polymer nanoparticle synthesis at low temperature. Phys. Plasmas 2017, 24, 023506. [Google Scholar] [CrossRef]
- Park, C.-S.; Jung, E.Y.; Kim, D.H.; Cho, B.-G.; Shin, B.J.; Tae, H.-S. TOF-SIMS study on nano size conducting polymer prepared by simple atmospheric pressure plasma polymerization technique for display applications. Mol. Cryst. Liq. Cryst. 2017, 651, 16–25. [Google Scholar] [CrossRef]
- Phan, L.T.; Yoon, S.M.; Moon, M.-W. Plasma-based nanostructuring of polymers: A Review. Polymers 2017, 9, 417. [Google Scholar] [CrossRef]
- Lv, S.; Zhao, X.; Shi, L.; Zhang, G.; Wang, S.; Kang, W.; Zhuang, X. Preparation and properties of sc-PLA/PMMA transparent nanofiber air filter. Polymers 2018, 10, 996. [Google Scholar] [CrossRef]
- Ting, Y.-H.; Liu, C.-C.; Park, S.-M.; Jiang, H.; Nealey, P.F.; Wendt, A.E. Surface roughening of polystyrene and poly(methyl methacrylate) in Ar/O2 plasma etching. Polymers 2010, 2, 649–663. [Google Scholar] [CrossRef]
- Buruaga, L.; Pomposo, J.A. Metal-free polymethyl methacrylate (PMMA) nanoparticles by enamine “Click” chemistry at room temperature. Polymers 2011, 3, 1673–1683. [Google Scholar] [CrossRef]
- Burrows, P.E.; Graff, G.L.; Gross, M.E.; Martin, P.M.; Shi, M.K.; Hall, M.; Mast, E.; Bonham, C.; Bennett, W.; Sullivan, M.B. Ultra barrier flexible substrates for flat panel displays. Display 2001, 22, 65–69. [Google Scholar] [CrossRef]
- Yamashita, K.; Mori, T.; Mizutani, T. Encapsulation of organic light-emitting diode using thermal chemical-vapour-deposition polymer film. J. Phys. D 2001, 34, 740–743. [Google Scholar] [CrossRef]
- Alghunaim, N.S. Spectroscopic analysis of PMMA/PVC blends containing CoCl2. Results Phys. 2015, 5, 331–336. [Google Scholar] [CrossRef]
- Jasim, R.I.; Jameel, A.N.; Alwan, T.J. Synthesis and characterization the optical properties and FT-IR spectroscopy of PMMA/CR2O3 blend films. GESJ Phys. 2016, 1, 45–53. [Google Scholar]
- Thakur, V.K.; Vennerberg, D.; Madbouly, S.A.; Kessler, M.R. Bio-inspired green surface functionalization of PMMA for multifunctional capacitors. RSC Adv. 2014, 4, 6677–6684. [Google Scholar] [CrossRef]
- Vijayakumari, G.; Selvakumar, N.; Jeyasubramanian, K.; Mala, R. Investigation on the electrical properties of polymer metal nanocomposites for physiological sensing applications. Phys. Proc. 2013, 49, 67–78. [Google Scholar] [CrossRef]
- Ma, Y.; Cao, X.; Feng, X.; Ma, Y.; Zou, H. Fabrication of super-hydrophobic film from PMMA with intrinsic water contact angle below 90°. Polymer 2007, 48, 7455–7460. [Google Scholar] [CrossRef]
- Raptis, I.; Kovač, J.; Chatzichristidi, M.; Sarantopoulou, E.; Kollia, Z.; Kobe, S.; Cefalas, A.C. Enhancement of sensing properties of thin poly(methyl methacrylate) films by VUV modification. J. Laser Micro/Nanoeng. 2007, 2, 200–205. [Google Scholar] [CrossRef]
- Naderi-Gohar, S.; Huang, K.M.H.; Wu, Y.; Lau, W.M.; Nie, H.-Y. Depth profiling cross-linked poly(methyl methacrylate) films: a time-of-flight secondary ion mass spectrometry approach. Rapid Commun. Mass Spect. 2017, 31, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, C.M.; Fahey, A.J.; Gillen, G. Temperature-controlled depth profiling of poly(methyl methacrylate) using cluster secondary ion mass spectrometry. 1. Investigation of depth profile characteristics. Anal. Chem. 2007, 79, 828–836. [Google Scholar] [CrossRef]
- Dekeyser, C.M.; Biltresse, S.; Marchand-Brynaert, J.; Rouxhet, P.G.; Dupont-Gillain, C.C. Submicrometer-scale heterogeneous surfaces by PS–PMMA demixing. Polymer 2004, 45, 2211–2219. [Google Scholar] [CrossRef]
- Endo, K.; Kobayashi, N.; Aida, M.; Hoshi, T. Spectral analysis of polystyrene, polypropylene, and poly(methyl methacrylate) polymers in TOF SIMS and XPS by MO calculations using the model oligomers. Polym. J. 1996, 28, 901–910. [Google Scholar] [CrossRef]
- Sathish, S.; Chandar, S.B. Dip and spin coated nanoscale transparent PMMA thin films for field effect thin film transistors and optoelectronic devices. J. Optoelectron. Adv. Mater. 2013, 15, 139–144. [Google Scholar]
- Sathish, S.; Chandar, S.B. Preparation and characterization of nano scale PMMA thin films. Ind. J. Pure Appl. Phys. 2014, 52, 64–67. [Google Scholar]
- Wang, B.; Lin, Q.; Shen, C.; Han, Y.; Tang, J.; Chen, H. Synthesis of MA POSS–PMMA as an intraocular lens material with high light transmittance and good cytocompatibility. RSC Adv. 2014, 4, 52959–52966. [Google Scholar] [CrossRef]
- Choi, B.K.; Lee, I.H.; Kim, J.H.; Chang, Y.J. Tunable wetting property in growth mode-controlled WS2 thin films. Nanoscale Res. Lett. 2017, 12, 262. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Bazaka, K.; Oelgemöller, M.; Jacob, M.V. Wetting, solubility and chemical characteristics of plasma-polymerized 1-isopropyl-4-methyl-1,4-cyclohexadiene thin films. Coatings 2014, 4, 527–552. [Google Scholar] [CrossRef]
- Patel, K.H.; Rawal, S.K. Exploration of wettability and optical aspects of ZnO nano thin films synthesized by radio frequency magnetron sputtering. Nanomater. Nanotechnol. 2016, 6, 22. [Google Scholar] [CrossRef]



| Sample | Concentrations of Correlative Functional Group (%) | ||||
|---|---|---|---|---|---|
| C 1s | O 1s | ||||
| C–C/C–H (285.0 eV) | C–O (286.8 eV) | O–C=O (289.1 eV) | C=O (531.0 eV) | C–O (533.2 eV) | |
| pPMMA | 54.9 | 29.4 | 15.7 | 68.9 | 31.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.-S.; Jung, E.Y.; Jang, H.J.; Bae, G.T.; Shin, B.J.; Tae, H.-S. Synthesis and Properties of Plasma-Polymerized Methyl Methacrylate via the Atmospheric Pressure Plasma Polymerization Technique. Polymers 2019, 11, 396. https://doi.org/10.3390/polym11030396
Park C-S, Jung EY, Jang HJ, Bae GT, Shin BJ, Tae H-S. Synthesis and Properties of Plasma-Polymerized Methyl Methacrylate via the Atmospheric Pressure Plasma Polymerization Technique. Polymers. 2019; 11(3):396. https://doi.org/10.3390/polym11030396
Chicago/Turabian StylePark, Choon-Sang, Eun Young Jung, Hyo Jun Jang, Gyu Tae Bae, Bhum Jae Shin, and Heung-Sik Tae. 2019. "Synthesis and Properties of Plasma-Polymerized Methyl Methacrylate via the Atmospheric Pressure Plasma Polymerization Technique" Polymers 11, no. 3: 396. https://doi.org/10.3390/polym11030396
APA StylePark, C.-S., Jung, E. Y., Jang, H. J., Bae, G. T., Shin, B. J., & Tae, H.-S. (2019). Synthesis and Properties of Plasma-Polymerized Methyl Methacrylate via the Atmospheric Pressure Plasma Polymerization Technique. Polymers, 11(3), 396. https://doi.org/10.3390/polym11030396