A Novel Class of Cost Effective and High Performance Composites Based on Terephthalate Salts Reinforced Polyether Ether Ketone
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Terephthalate Salts
2.2. Production of Composite Materials
2.3. Characterization of Terephthalate Salts and PEEK/CATAS Composites
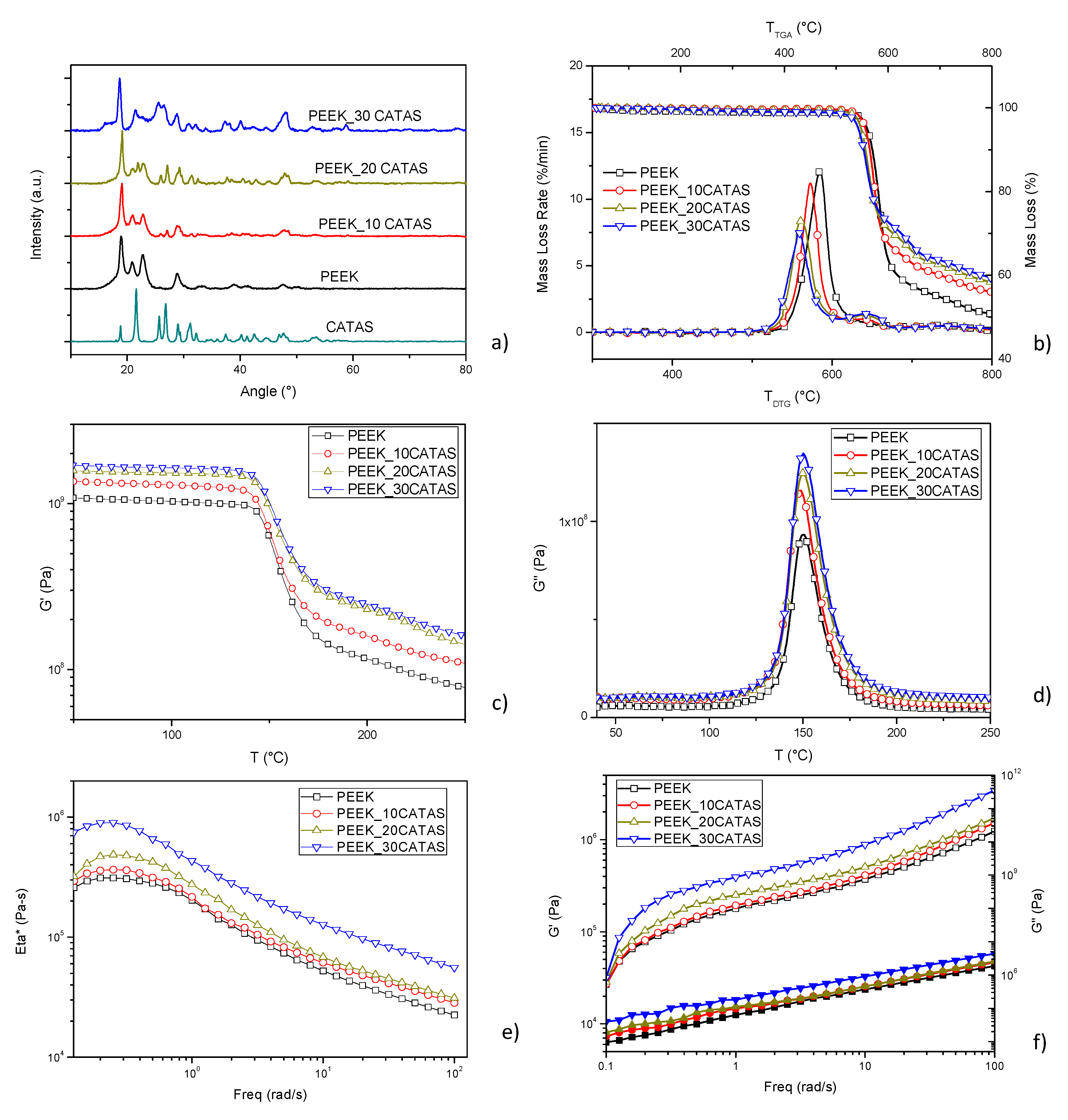
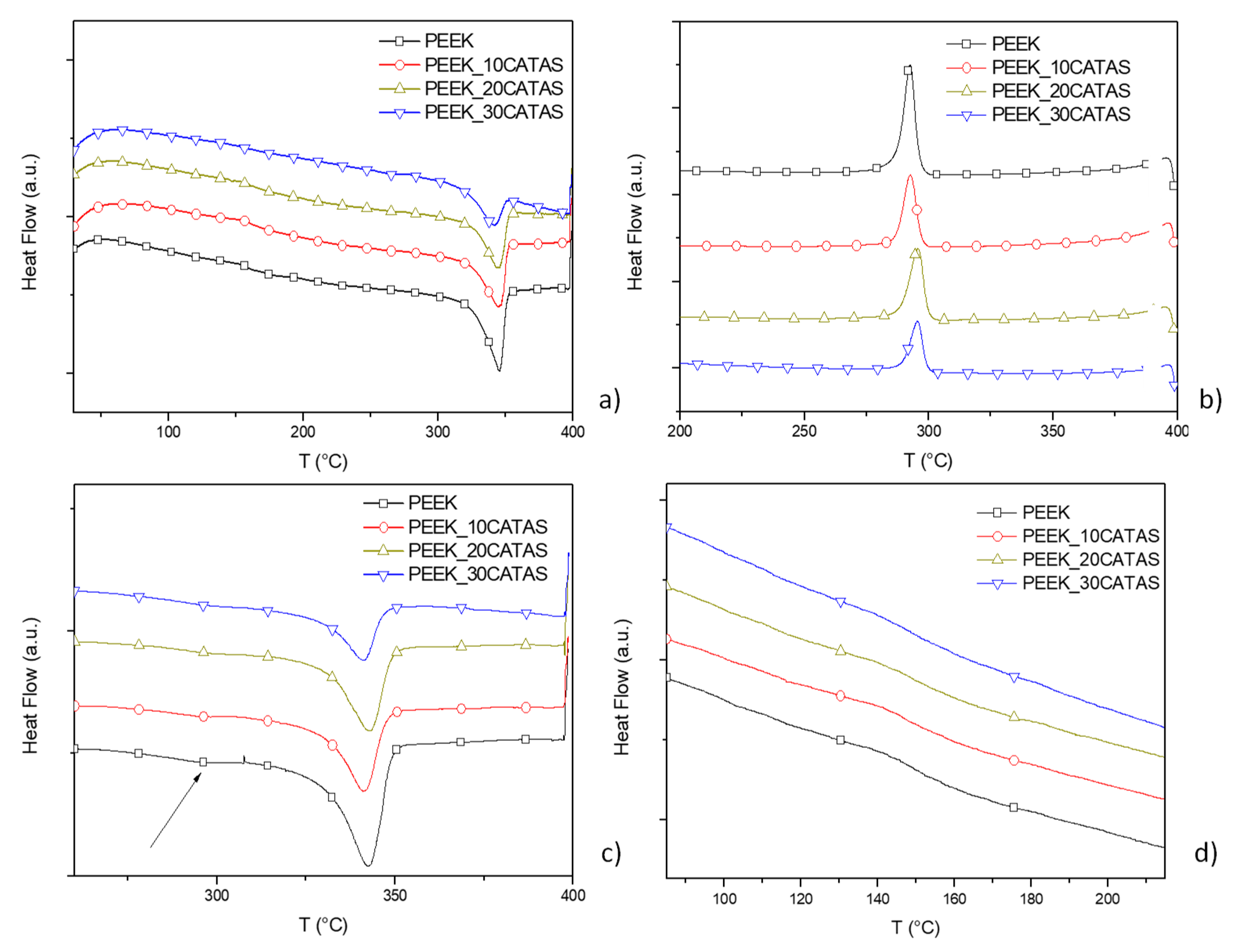
3. Results and Discussion
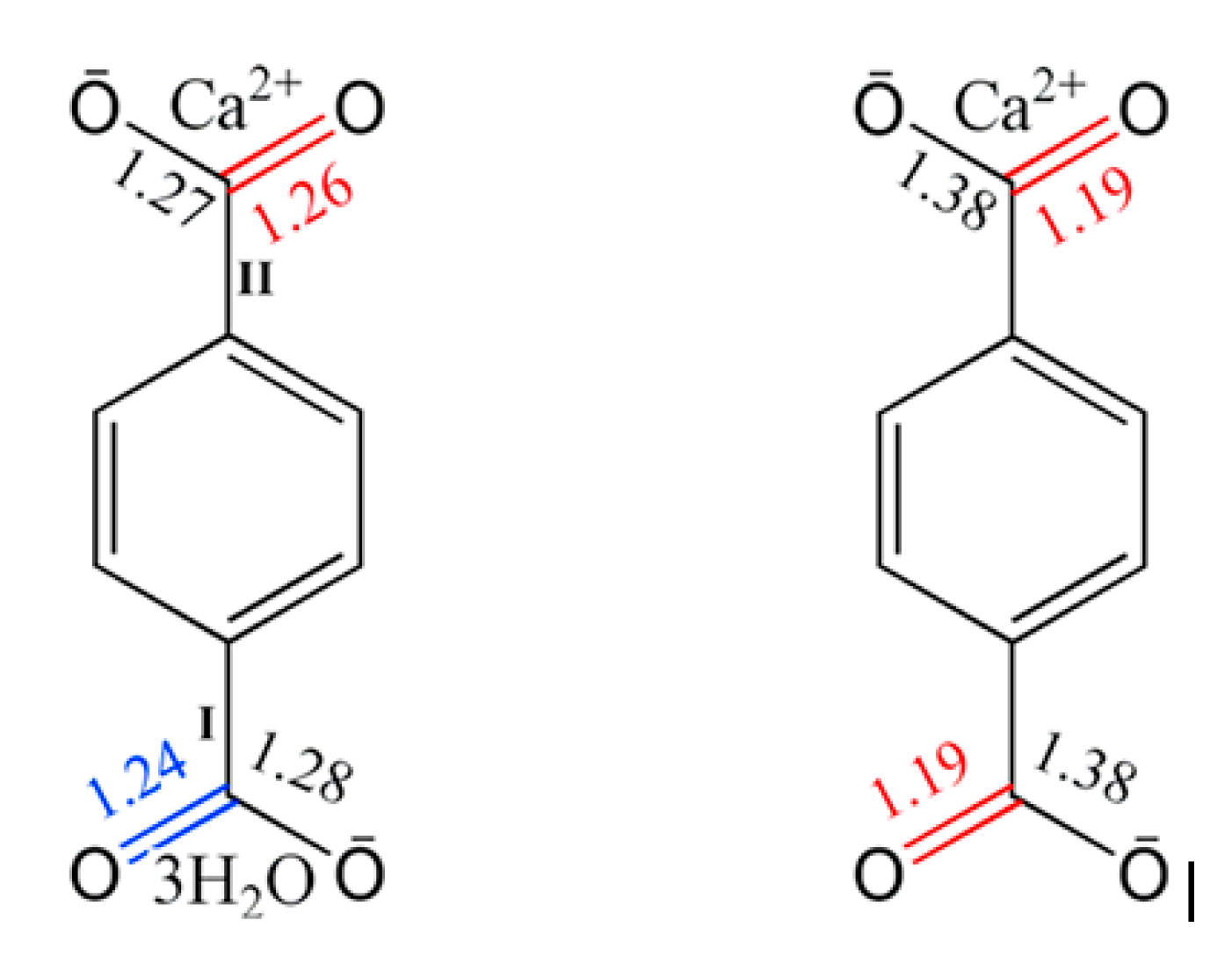
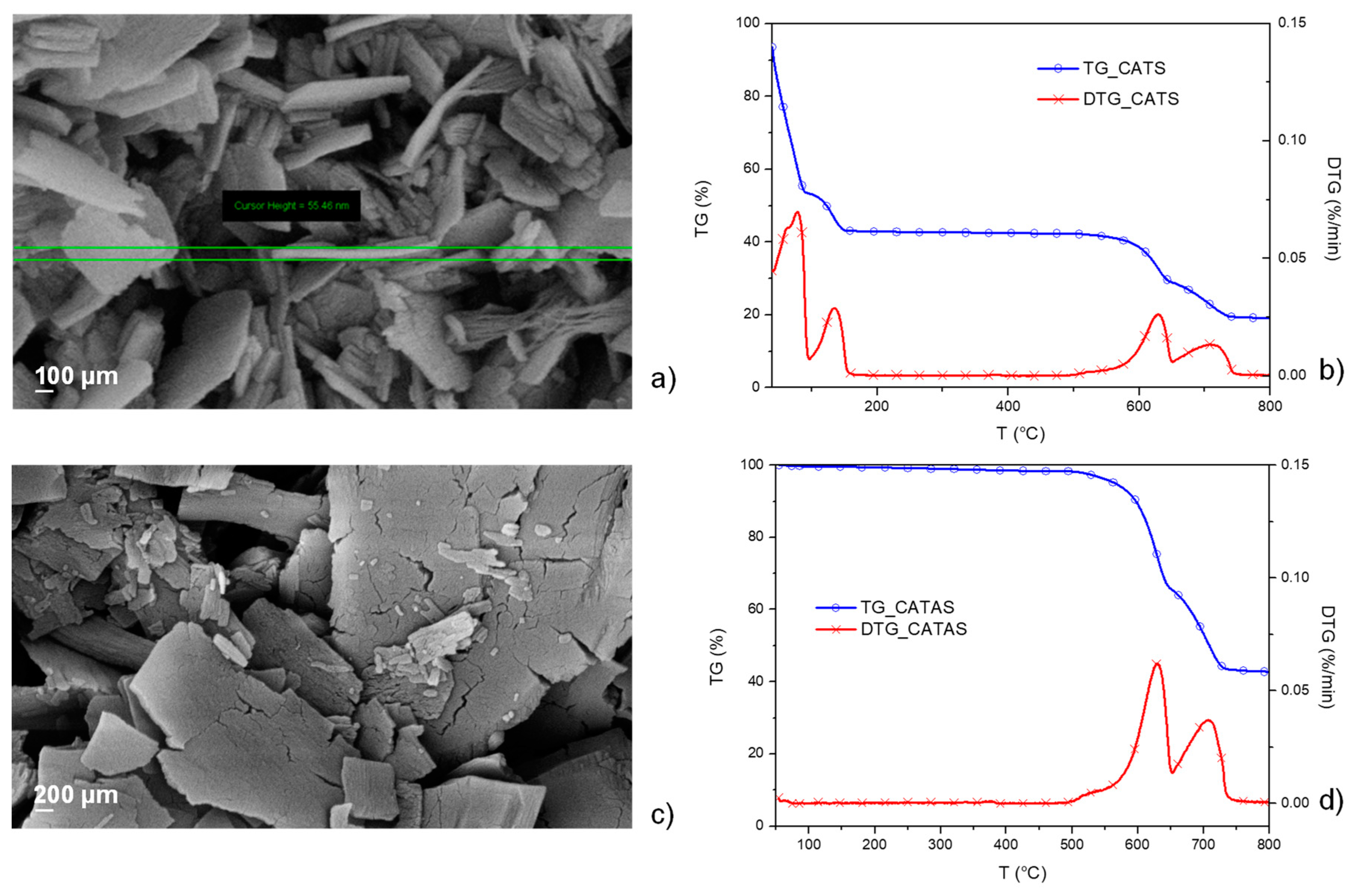
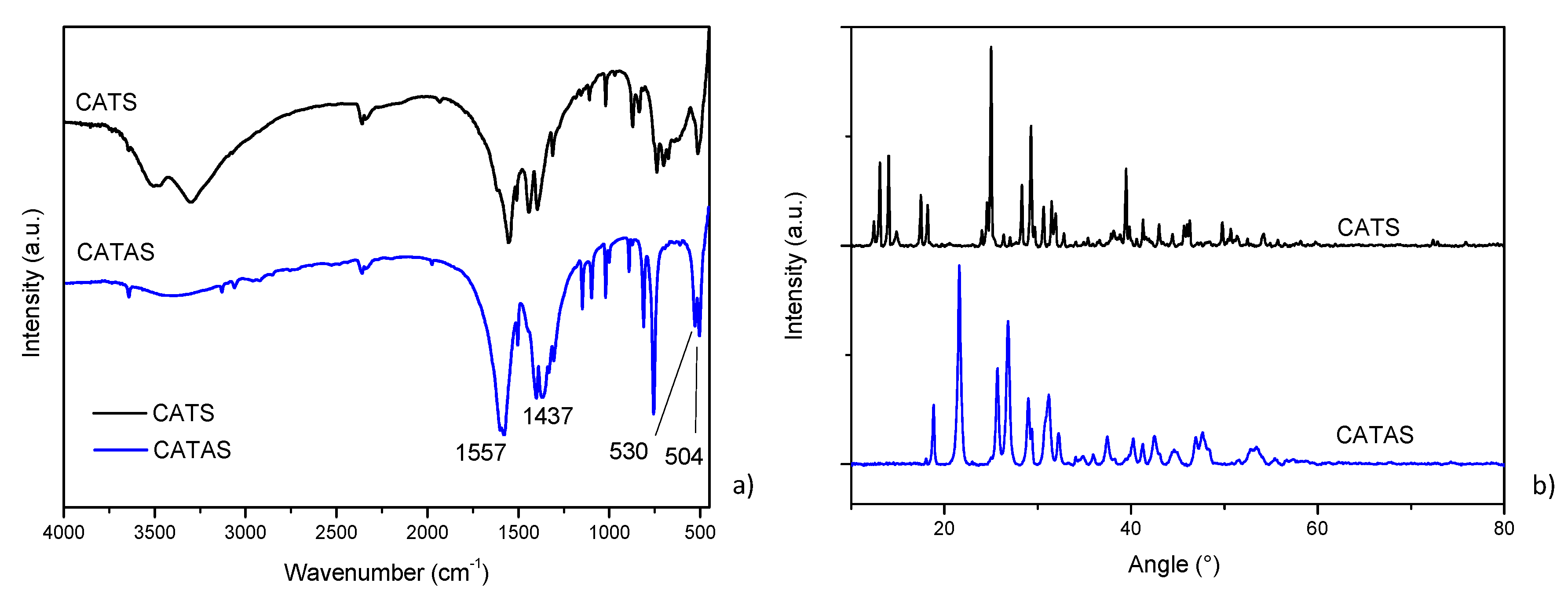
3.1. Characterization of Calcium Terephthalate Salts
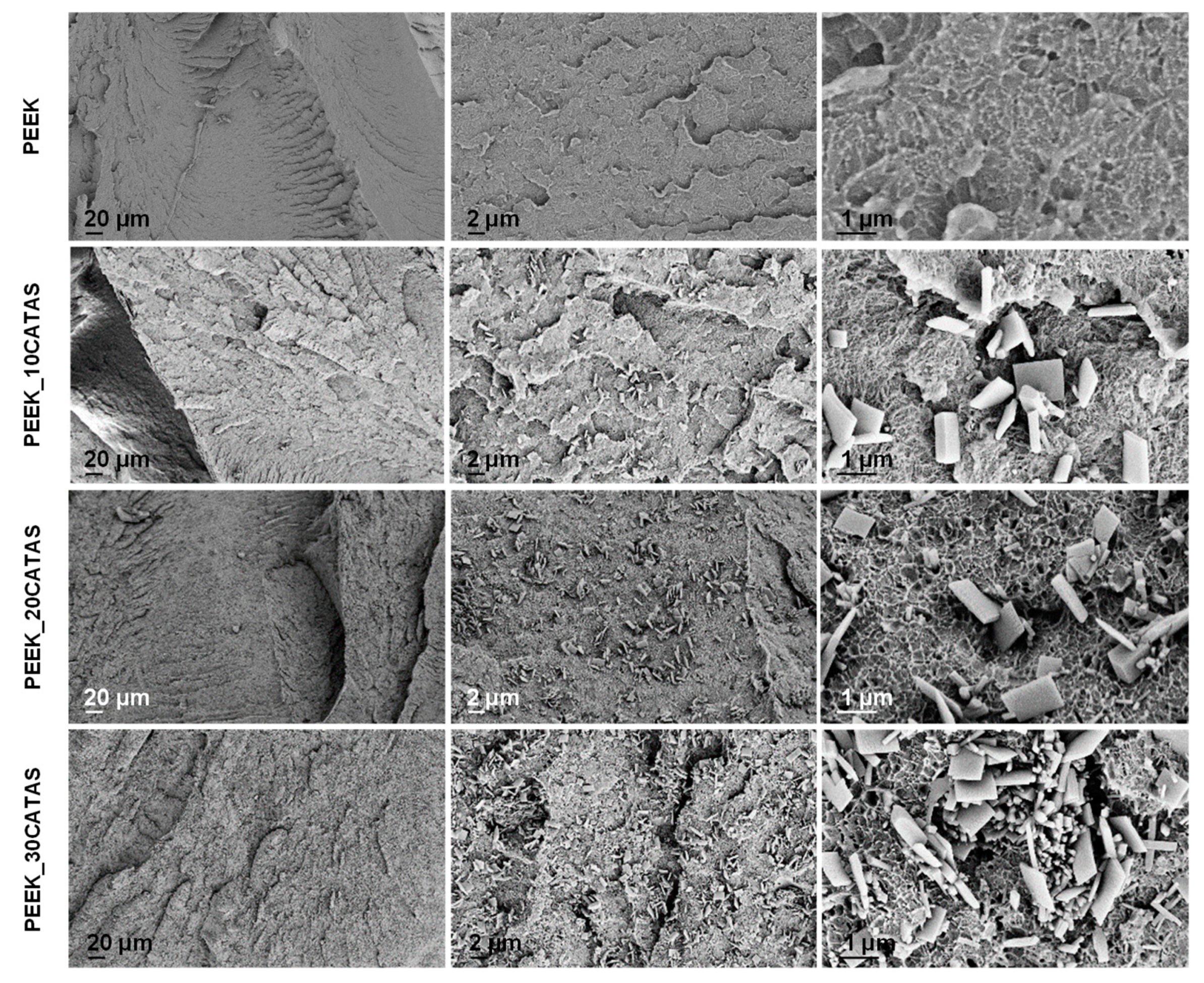
3.2. Characteristics of Composites PEEK/Calcium Terephthalate Salts
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ma, R.; Zhu, B.; Zeng, Q.; Wang, P.; Wang, Y.; Liu, C.; Shen, C. Melt-Processed Poly (Ether Ether Ketone)/Carbon Nanotubes/Montmorillonite Nanocomposites with Enhanced Mechanical and Thermomechanical Properties. Materials 2019, 12, 525. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, K.; Zhou, C.; Ren, M.; Gu, Y.; Li, T. Engineering the mechanical properties of CNT/PEEK nanocomposites. RSC Adv. 2019, 9, 12836–12845. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Naffakh, M.; Marco, C.; Ellis, G.; Gómez-Fatou, M.A. High-performance nanocomposites based on polyetherketones. Prog. Mater. Sci. 2012, 57, 1106–1190. [Google Scholar] [CrossRef]
- Balaji, V.; Tiwari, A.N.; Goyal, R.K. Fabrication and properties of high performance PEEK/Si3N4 nanocomposites. J. Appl. Polym. Sci. 2011, 119, 311–318. [Google Scholar] [CrossRef]
- Patki, A.M.; Goyal, R.K. High performance polyetherketone-hexagonal boron nitride nanocomposites for electronic applications. J. Mater. Sci. Mater. Electron. 2019, 30, 3899–3908. [Google Scholar] [CrossRef]
- Hao, L.; Hu, Y.; Zhang, Y.; Wei, W.; Hou, X.; Guo, Y.; Hu, X.; Jiang, D. Enhancing the mechanical performance of poly (ether ether ketone)/zinc oxide nanocomposites to provide promising biomaterials for trauma and orthopedic implants. RSC Adv. 2018, 8, 27304–27317. [Google Scholar] [CrossRef]
- Knör, N.; Gebhard, A.; Haupert, F.; Schlarb, A.K. Polyetheretherketone (PEEK) nanocomposites for extreme mechanical and tribological loads. Mech. Compos. Mater. 2009, 45, 199–206. [Google Scholar] [CrossRef]
- Mou, C.; Wang, L.; Deng, Q.; Huang, Z.; Li, J. Calcium terephthalate/graphite composites as anode materials for lithium-ion batteries. Ionics 2015, 21, 1893–1899. [Google Scholar] [CrossRef]
- Jones, P.G.; Ossowski, J.; Kus, P.; Dix, I. Three Crystal Structures of Terephthalic Acid Salts of Simple Amines. Z. Nat. B 2009, 64, 865–870. [Google Scholar] [CrossRef]
- Panasyuk, G.P.; Azarova, L.A.; Budova, G.P.; Izotov, A.D. Synthesis and characterization of ammonium terephthalates. Inorg. Mater. 2002, 38, 385–389. [Google Scholar] [CrossRef]
- Panasyuk, G.P.; Khaddaj, M.; Privalov, V.I.; Miroshnichenko, I.V. Poly (ethylene terephthalate) Transformations during Autoclaving in Water Vapor. Plast. Massy 2002, 2, 27–31. [Google Scholar]
- Panasyuk, G.P.; Azarova, L.A. Mishal Khaddaj, Structural Transformation of Terephthalic Acid in Water Fluids. Proc. Viii Meet. Supercrit. Fluids 2002, 225. [Google Scholar]
- Panasyuk, G.P.; Azarova, L.A.; Khaddaj, M.; Voroshilov, I.L.; Boudova, G.P. Hydrothemal Synthesis and Investigation of Aluminum Terephthalate. Inorg. Mater. 41, 1201–1205. Available online: bibliothek.fzk.de/zb/verlagspublikationen/AIRAPT_EHPRG2005/Orals/O193.pdf (accessed on 27 November 2019). [CrossRef]
- Matsuzaki, T.; Iitaka, Y. The crystal structure of calcium terephthalate trihydrate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1972, 28, 1977–1981. [Google Scholar] [CrossRef]
- Alavi, M.A.; Morsali, A. Alkaline-earth metal carbonate, hydroxide and oxide nano-crystals synthesis methods, size and morphologies consideration. In Nanocrystal; Masuda, Y., Ed.; InTech: Rijeka, Croatia, 2011; Chapter 9; pp. 237–262. [Google Scholar]
- Wang, L.; Mou, C.; Sun, Y.; Liu, W.; Deng, Q.; Li, J. Structure-property of metal organic frameworks calcium terephthalates anodes for lithium-ion batteries. Electrochim. Acta 2015, 173, 235–241. [Google Scholar] [CrossRef]
- Liang, P.-C.; Liu, H.-K.; Yeh, C.-T.; Lin, C.-H.; Zima, V.T.Z. Supramolecular Assembly of Calcium Metal—Organic Frameworks with Structural Transformations. Cryst. Growth Des. 2011, 11, 699–708. [Google Scholar] [CrossRef]
- Panasyuk, G.P.; Azarova, L.A.; Khaddaj, M.; Budova, G.P.; Voroshilov, I.L.; Grusha, T.V.; Izotov, A.D. Preparation and properties of sodium, potassium, magnesium, calcium, and aluminum terephthalates. Inorg. Mater. 2003, 39, 1292–1297. [Google Scholar] [CrossRef]
- Mazaj, M.; Mali, G.; Rangus, M.; Zunkovic, E.; Kaucic, V.E.; Zabukovec Logar, N.A. Spectroscopic studies of structural dynamics induced by heating and hydration: A case of calcium-terephthalate metal–organic framework. J. Phys. Chem. C 2013, 117, 7552–7564. [Google Scholar] [CrossRef]
- Blundell, D.J.; Osborn, B.N. The morphology of poly (aryl-ether-ether-ketone). Polymer 1983, 24, 953–958. [Google Scholar] [CrossRef]
- Giants, T.W. Crystallinity and dielectric properties of PEEK, poly (ether ether ketone). IEEE Trans. Dielectr. Electr. Insul. 1994, 1, 991–999. [Google Scholar] [CrossRef]
- Karacan, I. X-ray Diffraction Studies of Poly (aryl ether ether ketone) Fibers with Different Degrees of Crystallinity and Orientation. Fibers Polym. 2005, 6, 206–218. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Liu, M.; Li, Z.; Vallés, C.; Young, R.J.; Kinloch, I.A. Hybrid poly (ether ether ketone) composites reinforced with a combination of carbon fibres and graphene nanoplatelets. Compos. Sci. Technol. 2019, 175, 60–68. [Google Scholar] [CrossRef]
- Alvaredo, Á.; Martín, M.I.; Castell, P.; de Guzmán Villoria, R.; Fernández-Blázquez, J.P. Non-Isothermal Crystallization Behavior of PEEK/Graphene Nanoplatelets Composites from Melt and Glass States. Polymers 2019, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Bangarusampath, D.S.; Ruckdäschel, H.; Altstädt, V.; Sandler, J.K.W.; Garray, D.; Shaffer, M.S.P. Rheology and properties of melt-processed poly (ether ether ketone)/multi-wall carbon nanotube composites. Polymer 2009, 50, 5803–5811. [Google Scholar] [CrossRef]
- Wu, D.; Wu, L.; Sun, Y.; Zhang, M. Rheological properties and crystallization behavior of multi-walled carbon nanotube/poly (ε-caprolactone) composites. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 3137–3147. [Google Scholar] [CrossRef]
- Zhan, Y.; Long, Z.; Wan, X.; He, Y.; Liu, X. Exfoliated graphite nanoplatelets/poly (arylene ether nitrile) nanocomposites: In situ synthesis, characterization, and enhanced properties. High Perform. Polym. 2017, 29, 1121–1129. [Google Scholar] [CrossRef]
- Nair, S.T.; Vijayan, P.P.; Xavier, P.; Bose, S.; George, S.C.; Thomas, S. Selective localisation of multi walled carbon nanotubes in polypropylene/natural rubber blends to reduce the percolation threshold. Compos. Sci. Technol. 2015, 116, 9–17. [Google Scholar] [CrossRef]
- Cheng, S.Z.D.; Cao, M.Y.; Wunderlich, B. Glass transition and melting behavior of poly (oxy-1, 4-phenyleneoxy-1, 4-phenylenecarbonyl-1, 4-phenylene)(PEEK). Macromolecules 1986, 19, 1868–1876. [Google Scholar] [CrossRef]
- De Candia, F.; Vittoria, V. Permeability of PEEK to carbon dioxide. J. Appl. Polym. Sci. 1994, 51, 2103–2107. [Google Scholar] [CrossRef]
- Huo, P.; Cebe, P. Temperature-dependent relaxation of the crystal-amorphous interphase in poly (ether ether ketone). Macromolecules 1992, 25, 902–909. [Google Scholar] [CrossRef]
- Krishnaswamy, R.K.; Kalika, D.S. Glass transition characteristics of poly (aryl ether ketone ketone) and its copolymers. Polymer 1996, 37, 1915–1923. [Google Scholar] [CrossRef]
- Reyna-Valencia, A.; Kaliaguine, S.; Bousmina, M. Structural and mechanical characterization of poly (ether ether ketone) (PEEK) and sulfonated PEEK films: Effects of thermal history, sulfonation, and preparation conditions. J. Appl. Polym. Sci. 2006, 99, 756–774. [Google Scholar] [CrossRef]
- Saleem, A.; Frormann, L.; Iqbal, A. High performance thermoplastic composites: Study on the mechanical, thermal, and electrical resistivity properties of carbon fiber-reinforced polyetheretherketone and polyethersulphone. Polym. Compos. 2007, 28, 785–796. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, W.N. Glass transition temperatures and rigid amorphous fraction of poly (ether ether ketone) and poly (ether imide) blends. Polymer 1997, 38, 2657–2663. [Google Scholar]
- Saiello, S.; Kenny, J.; Nicolais, L. Interface morphology of carbon fibre/PEEK composites. J. Mater. Sci. 1990, 25, 3493–3496. [Google Scholar] [CrossRef]
- Vasconcelos, G.D.C.; Mazur, R.L.; Botelho, E.C.; Rezende, M.C.; Costa, M.L. Evaluation of crystallization kinetics of poly (ether-ketone-ketone) and poly (ether-ether-ketone) by DSC. J. Aerosp. Technol. Manag. 2010, 2, 155–162. [Google Scholar] [CrossRef]
- Jonas, A.M.; Ivanov, D.A.; Yoon, D.Y. The Semicrystalline Morphology of Poly (ether− ether− ketone) Blends with Poly (ether− imide). Macromolecules 1998, 31, 5352–5362. [Google Scholar] [CrossRef]
- Crevecoeur, G.; Groeninckx, G. Binary blends of poly (ether ether ketone) and poly (ether imide): Miscibility, crystallization behavior and semicrystalline morphology. Macromolecules 1991, 24, 1190–1195. [Google Scholar] [CrossRef]
| Sample Name | PEEK wt % | CATAS wt % |
|---|---|---|
| PEEK | 100 | --- |
| PEEK_10CATAS | 90 | 10 |
| PEEK_20CATAS | 80 | 20 |
| PEEK_30CATAS | 70 | 30 |
| Sample Name | G’ [MPa] @100 °C | G’ [MPa] @200 °C | % ΔG’ 100 vs PEEK | % ΔG’ 200 vs PEEK |
|---|---|---|---|---|
| PEEK | 1.03 × 109 | 1.15 × 108 | ||
| PEEK_10CATAS | 1.29 × 109 | 1.59 × 108 | +25.24 | +38.26 |
| PEEK_20CATAS | 1.53 × 109 | 2.29 × 109 | +48.54 | +99.13 |
| PEEK_30CATAS | 1.64 × 109 | 2.48 × 109 | +59.22 | +115.65 |
| Phase Fractions | PEEK | PEEK_30CATAS | |
|---|---|---|---|
| First heating | ΔHm (J/g) | 47.5 ± 1.6 | 30.5 ± 2.3 |
| Xc | 36.6 ± 1.2 | 33.5 ± 2.6 | |
| Xa | 47.9 ± 0.9 | 40.2 ± 0.8 | |
| Xraf | 15.6 ± 0.3 | 26.4 ± 3.4 | |
| cooling | ΔHc (J/g) | 43.5 ± 2.1 | 32.2 ± 1.6 |
| Xc | 33.5 ± 1.6 | 35.4 ± 1.7 | |
| Xa | 45.9 ± 0.6 | 12.9 ± 0.9 | |
| Xraf | 20.6 ± 1.0 | 51.8 ± 2.6 | |
| Second heating | ΔHm (J/g) | 45.4 ± 1.3 | 32.4 ± 1.7 |
| Xc | 34.9 ± 1.0 | 35.6 ± 1.9 | |
| Xa | 52.8 ± 1.1 | 16.9 ± 0.4 | |
| Xraf | 12.3 ± 2.1 | 47.6 ± 1.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dominici, F.; Puglia, D.; Luzi, F.; Sarasini, F.; Rallini, M.; Torre, L. A Novel Class of Cost Effective and High Performance Composites Based on Terephthalate Salts Reinforced Polyether Ether Ketone. Polymers 2019, 11, 2097. https://doi.org/10.3390/polym11122097
Dominici F, Puglia D, Luzi F, Sarasini F, Rallini M, Torre L. A Novel Class of Cost Effective and High Performance Composites Based on Terephthalate Salts Reinforced Polyether Ether Ketone. Polymers. 2019; 11(12):2097. https://doi.org/10.3390/polym11122097
Chicago/Turabian StyleDominici, Franco, Debora Puglia, Francesca Luzi, Fabrizio Sarasini, Marco Rallini, and Luigi Torre. 2019. "A Novel Class of Cost Effective and High Performance Composites Based on Terephthalate Salts Reinforced Polyether Ether Ketone" Polymers 11, no. 12: 2097. https://doi.org/10.3390/polym11122097
APA StyleDominici, F., Puglia, D., Luzi, F., Sarasini, F., Rallini, M., & Torre, L. (2019). A Novel Class of Cost Effective and High Performance Composites Based on Terephthalate Salts Reinforced Polyether Ether Ketone. Polymers, 11(12), 2097. https://doi.org/10.3390/polym11122097










