Synthesis of Monoacryloxypropyl-POSS-based Hybrid Epoxyacrylate Copolymers and Their Application in Thermally Curable Structural Self-Adhesive Tapes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of the EA-POSS Hybrid Copolymers
2.3. Preparation and Characterization of Self-Adhesive Tapes (SATs) and Al/SAT/Al Joints
3. Results
3.1. Properties of A-POSS-Modified Epoxyacrylate Copolymers
3.2. Properties of Thermally Uncured SATs with A-POSS-Based Epoxyacrylate Copolymers.
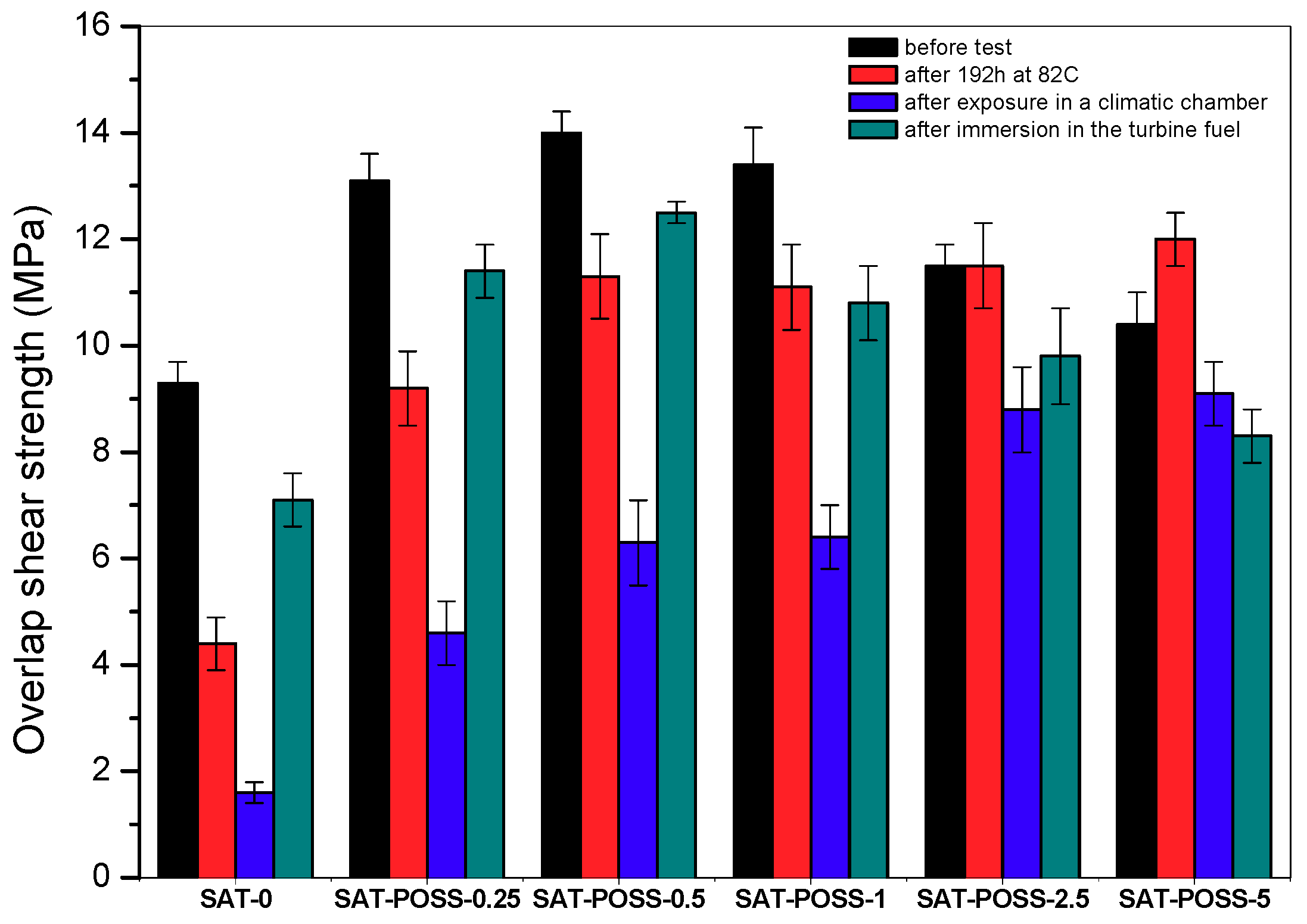
3.3. Properties of Thermally Cured SATs with A-POSS-Based Epoxyacrylate Copolymers
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, H.; Ye, Q.; Xu, J. Polyhedral oligomeric silsesquioxane-based hybrid materials and their applications. Mater. Chem. Front. 2017, 1, 212–230. [Google Scholar] [CrossRef]
- Li, G.; Pittman, C.U. Polyhedral Oligomeric Silsesquioxane (POSS) Polymers, copolymers, and resin nanocomposites. In Macromolecules Containing Metal and Metal-Like Elements; Abd-El-Aziz, A.S., Carraher, C.E., Pittman, C.U., Zeldin, M., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2005; Volume 3, pp. 89–131. [Google Scholar]
- Lichtenhan, J.D.; Otonari, Y.A.; Carr, M.J. Linear hybrid polymer building blocks: Methacrylate-functionalized polyhedral oligomeric silsesquioxane monomers and polymers. Macromolecules 1995, 28, 8435–8437. [Google Scholar] [CrossRef]
- Schiavo, S.L.; Mineo, P.; Cardiano, P.; Piraino, P. Novel propylmethacrylate-monofunctionalized polyhedral oligomeric silsesquioxanes homopolymers prepared via radical bulk free polymerization. Eur. Polym. J. 2007, 43, 4898–4904. [Google Scholar] [CrossRef]
- Kanehashi, S.; Tomita, Y.; Obokata, K.; Kidesaki, T.; Sato, S.; Miyakoshi, T.; Nagai, K. Effect of substituted groups on characterization and water vapor sorption property of polyhedral oligomeric sislsesquioxane (POSS)-containing methacryl polymer membranes. Polymer 2013, 54, 2315–2323. [Google Scholar] [CrossRef]
- Shim, J.; Kim, D.G.; Lee, J.H.; Baik, J.H.; Lee, J.C. Synthesis and properties of organic/inorganic hybrid branched-graft copolymers and their application to solid-state electrolytes for high-temperature lithium-ion batteries. Polym. Chem. 2014, 5, 3432–3442. [Google Scholar] [CrossRef]
- Zhang, W.; Yuan, J.; Weiss, S.; Ye, X.; Li, C.; Müller, A.H.E. Telechelic hybrid poly(acrylic acid)s containing polyhedral oligomeric silsesquioxane (POSS) and their self-assembly in water. Macromolecules 2011, 44, 6891–6898. [Google Scholar] [CrossRef]
- Xue, Y.; Wang, H.; Yu, D.; Feng, L.; Dai, L.; Wang, X.; Lin, T. Superhydrophobic electrospun POSS-PMMA copolymer fibres with highly ordered nanofibrillar and surface structures. Chem. Commun. 2009, 6418–6420. [Google Scholar] [CrossRef]
- Hanifeh, M.; Zandi, M.; Shokrollahi, P.; Ataie, M.; Ghafarzadeh, E.; Askari, F. Compositional design and taguchi optimization ofhardness properties in silicone based ocular lenses. Prog. Biomater. 2017, 6, 67–74. [Google Scholar] [CrossRef]
- Liu, B.; Wang, H.; Guo, X.; Yang, R.; Li, X. Effects of an organic-inorganic hybrid containing allyl benzoxazine and POSS on thermal properties and flame retardancy of epoxy resin. Polymers 2019, 11, 770. [Google Scholar] [CrossRef]
- Li, M.; Wu, W.; Li, M.; Xu, Y.; Chen, G.; Dai, L. A novel POSS-based copolymer functionalized grapheme: An effective flame retardant for reducing the flammability of epoxy resin. Polymers 2019, 11, 241. [Google Scholar] [CrossRef]
- Cordes, B.D.; Lickiss, P.D.; Rataboul, F. Recent developments in the chemistry of cubic polyhedral ligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowska, A.; Przadka, D.; Andrzejewska, E. POSS functionalized with mixed fluoroalkyl and methacryloxysubstituents as modifiers for UV-curable coatings. J. Coat. Technol. Res. 2019, 16, 167–178. [Google Scholar] [CrossRef]
- Marcinkowska, A.; Przadka, D.; Andrzejewska, E. Modification of poly-HEMA with nonreactive POSS derivatives by in situ photopolymerization. J. Therm. Anal. Calorim. 2019, 182, 1033–1047. [Google Scholar] [CrossRef]
- Andrzejewska, E.; Marcinkowska, A.; Szcześniak, B. Nanocomposites obtained by photopolymerization of (methacrylate monomer)/(methacrylate functionalized polyhedral oligomeric silsesquioxane) system. Polimery-Warsaw 2011, 56, 63–66. [Google Scholar] [CrossRef]
- Franczyk, A.; He, H.; Burdyńska, J.; Hui, C.M.; Matyjaszewski, K.; Marciniec, B. Synthesis of high molecular weight polymethacrylates with polyhedral oligomeric silsesquioxane moieties by atom transfer radical polymerization. Macro Lett. 2014, 3, 799–802. [Google Scholar] [CrossRef]
- Caydamli, Y.; Yildirim, E.; Shen, J.; Fang, X.; Pasquinelli, M.A.; Spontak, R.J.; Tonelli, A.E. Nanoscale considerations responsible for diverse macroscopic phase behavior in monosubstituted isobutyl-POSS/poly(ethylene oxide) blends. Soft Matter 2017, 13, 8672–8677. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Xue, X. Morphology and properties of UV-curing epoxy acrylate coatings modiefied with methacryl-POSS. Prog. Org. Coat. 2015, 78, 404–410. [Google Scholar] [CrossRef]
- Norouzi, S.; Mohseni, M.; Yahyaei, H. Preparation and characterization of an acrylic acid modified polyhedral oligomeric silsesquioxane and investigating its effect in a UV curable coating. Prog. Org. Coat. 2016, 99, 1–10. [Google Scholar] [CrossRef]
- Prządka, D.; Marcinkowska, A.; Andrzejewska, E. POSS-modified UV-curable coatings with improved scratch hardness and hydrophobicity. Prog. Org. Coat. 2016, 100, 165–172. [Google Scholar] [CrossRef]
- Strachota, A.; Kroutilová, I.; Kovářová, J.; Matejka, L. Epoxy networks reinforced with polyhedral oligomeric silsesquioxanes (POSS). Thermomechanical properties. Macromolecules 2004, 37, 9457–9464. [Google Scholar] [CrossRef]
- Wu, K.; Song, L.; Hu, Y.; Lu, H.; Kandola, B.K.; Kandare, E. Synthesis and characterization of functional polyhedral oligomeric silsesquioxane and its flame retardancy in epoxy resin. Prog. Org. Coat. 2009, 65, 490–497. [Google Scholar] [CrossRef]
- Jones, I.K.; Zhou, Y.X.; Jeelani, S.; Mabry, J.M. Effect of polyhedral-oligomeric-sil-sesquioxanes on thermal and mechanical behaviour of SC-15- epoxy. eXPRESS Polym. Lett. 2008, 2, 494–501. [Google Scholar] [CrossRef]
- Zaioncz, S.; Dahmouche, K.; Paranhos, C.M.; San Gil, R.A.S.; Soares, B.G. Reletionships between nanostructure and dynamic-mechanical properties of epoxy network containing PMMA-modified silsesquioxane. eXPRESS Polym. Lett. 2009, 3, 340–351. [Google Scholar] [CrossRef]
- Akopova, T.A.; Osichik, V.S.; Olikhova, Y.V.; Ivanov, G.A.; Zhukov, A.F. The modification of epoxy binders with oligomeric silsesquioxanes. Plasticheskie Massy 2013, 7, 6–8. [Google Scholar] [CrossRef]
- Longhi, M.; Phandolphi Zini, L.; Kunst, S.R.; Zattera, A.J. Influence of the type of epoxy resin and concentration of glycidylisobutyl-POSS in the properties of nanocomposites. Polym. Polym. Compos. 2017, 8, 593–602. [Google Scholar] [CrossRef]
- Barra, G.; Vartuccio, L.; Vietri, U.; Naddeo, C.; Hadavinia, H.; Guadagno, L. Toughening of epoxy adhesives by combined interaction of carbon nanotubes and silsesquioxanes. Materials 2017, 10, 1131. [Google Scholar] [CrossRef]
- Marcinkowska, A.; Prządka, D.; Dudziec, B.; Szczesniak, K.; Andrzejewska, E. Anchor effect in polymerization kinetics: Case of monofunctionalized POSS. Polymers 2019, 11, 515. [Google Scholar] [CrossRef]
- Prządka, D.; Andrzejewska, E.; Marcinkowska, A. Multimethacryloxy-POSS as crosslinkers for hydrogel materials. Europ. Polym. J. 2015, 72, 34–49. [Google Scholar] [CrossRef]
- Raut, H.K.; Dinachali, S.S.; He, A.Y.; Ganesh, A.; Saifullah, M.; Law, J.; Ramakrishna, S. Robust and durable polyhedral oligomericsilsesquioxane-based anti-reflective nanostructureswith broadband quasi-omnidirectional properties. Energy Environ. Sci. 2013, 6, 1929. [Google Scholar] [CrossRef]
- Toepfer, O.; Neumann, D.; Choudhury, N.R.; Whittaker, A.; Matisons, J. Organic–inorganic poly(methyl methacrylate) hybrids with confined polyhedraloligosilsesquioxane macromonomers. Chem. Mater. 2005, 17, 1027–1035. [Google Scholar] [CrossRef]
- Sastre, R.; Martin, V.; Garrido, L.; Chiara, J.L.; Trastoy, B.; Garcia, O.; Costela, A.; Garcia-Moreno, I. Dye-doped polyhedral oligomeric silsesquioxane (POSS)-modified polymeric matrices for highly efficient and photostable solid-state lasers. Adv. Funct. Mater. 2009, 19, 3307–3316. [Google Scholar] [CrossRef]
- Chen, C.; Liang, X.; Wang, J.; Yang, S.; Yan, Z.; Cai, Q.; Yao, S. Development of a highly robust solid phase microextraction fiber based on crosslinked methylmethacrylate–polyhedral oligomeric silsesquioxane hybrid polymeric coating. Anal. Chim. Acta 2013, 792, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Bi, Y.; Ren, H.; Zhu, F.; Luo, M.; Zhang, L. Poly(methyl methacrylate)/methacryl-POSS nanocomposites with excellent thermal properties. Chin. J. Chem. 2010, 28, 2527–2532. [Google Scholar] [CrossRef]
- Shokrolahi, F.; Zandi1, M.; Shokrollahi, P.; Atai, M.; Ghafarzadeh, E.; Hanifeh, M. Cure kinetic study of methacrylate-POSS copolymers for ocularlens. Prog. Biomater. 2017, 6, 147–156. [Google Scholar] [CrossRef]
- Pramudya, I.; Rico, C.G.; Lee, C.; Chung, H. POSS-containing bioinspired adhesives with enhanced mechanical and optical properties for biomedical applications. Biomacromolecules 2016, 17, 3853–3861. [Google Scholar] [CrossRef]
- Chian, W.; Mallampalli, C.; Winter, R.M. Nano-reinforcement of epoxy adhesives with POSS. NSTI-Nanotech 2005, 2, 107. [Google Scholar]
- Fadaie, P.; Atai, M.; Imani, M.; Karkhaneh, A.; Ghasaban, S. Cyanoacrylate–POSS nanocomposites: Novel adhesives with improved properties for dental applications. Dent. Mater. 2013, 29, 61–69. [Google Scholar] [CrossRef]
- Higgins, A. Adhesive bonding of aircraft structures. Int. J. Adhes. Adhes. 2000, 20, 367–376. [Google Scholar] [CrossRef]
- Weglewski, J.; Pastirik, D. Structural bonding tapes and articles containing the same. Patent Application WO 079337, 10 November 2002. [Google Scholar]
- Kowalczyk, A.; Kowalczyk, K.; Czech, Z. Synthesis and properties of solid structural adhesives modified in-situ using 1D and 2D-type microfillers. Int. J. Adhes. Adhes. 2012, 32, 76–81. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Kowalczyk, K.; Weisbrodt, M. Influence of a phosphorus-based methacrylate monomer on features of thermally curable self-adhesive structural tapes. Int. J. Adhes. Adhes. 2018, 85, 286–292. [Google Scholar] [CrossRef]
- Lachenal, G.; Pierre, A.; Poison, N. FT-NIR spectroscopy: Trends and application to the kinetic study of epoxy/triamine system (comparison with DSC and SEC results). Micron 1996, 27, 329–334. [Google Scholar] [CrossRef]
- Kazicyna, L.; Kupletska, N. Metody Spektroskopowe Wyznaczania Struktury Związków Organicznych (Spectroscopic Methods for Chemical Structure Determination of Organic Compounds); PWN: Warsaw, Poland, 1976. [Google Scholar]
- Prządka, D. Polimery Hybrydowe i Kompozyty Polimerowe Zawierające Funkcjonalizowane Poliedryczne Oligomeryczne Silseskwioksany (Hybride Polymers and Polymer Composites with Functionalized Polyhedral Oligomeric Silsesquioxanes). Ph.D. thesis, Poznan University of Technology, Poznań, Poland, 2014. [Google Scholar]
- Chiou, C.W.; Lin, Y.C.; Wang, L.; Hirano, C.; Suzuki, Y.; Hayakawa, T.; Kuo, S.W. Strong screening effect of polyhedral oligomeric silsesquioxanes (POSS) nanoparticles on hydrogen bonded polymer blends. Polymers 2014, 6, 926–948. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Kowalczyk, K.; Gziut, K.; Nowakowski, D.; Sałaciński, M. Influence of a wollastonite microfiller and a halloysite nanofiller onproperties of thermally curable pressure-sensitive structural adhesives. J. Adhes. Adhes. 2019, 95, 102397. [Google Scholar] [CrossRef]
- Urbaniak, M. A relationship between the glass transition temperature and the conversion degree in the curing reaction of the EPY® epoxy system. Polimery 2011, 56, 240–243. [Google Scholar] [CrossRef]
- Zhang, C.; Li, T.; Song, H.; Han, Y.; Su, H.; Wang, Y.; Wang, Q. Epoxy Resin/POSS Nanocomposites with toughness and thermal stability. J. Photopolym. Sci. Technol. 2017, 30, 25–31. [Google Scholar] [CrossRef][Green Version]
- Penzel, E.; Rieger, J.; Schneider., H.A. The glass transition temperature of random copolymers: 1. Experimental data and the Gordon-Taylor equation. Polymer. 1997, 38, 325–337. [Google Scholar] [CrossRef]
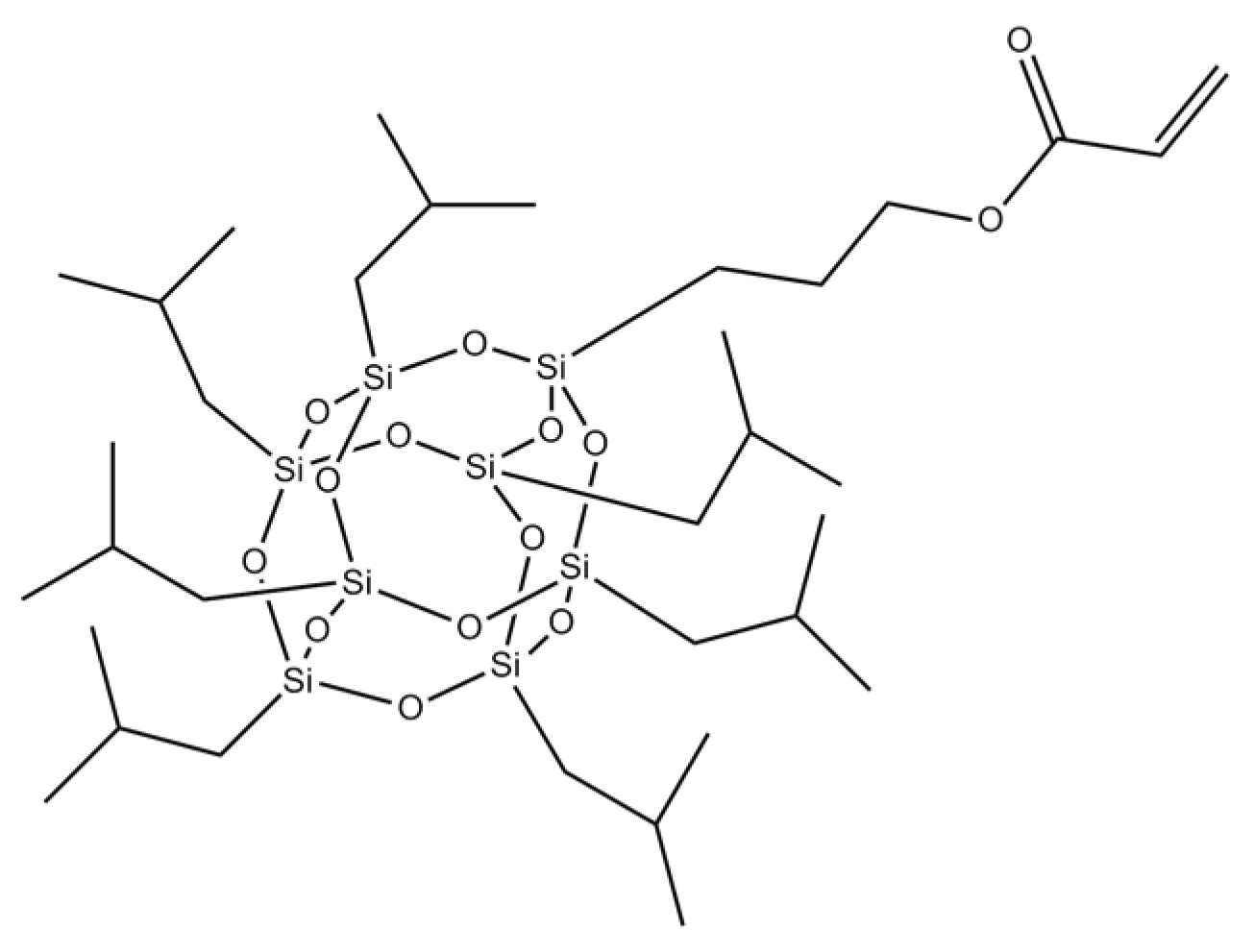
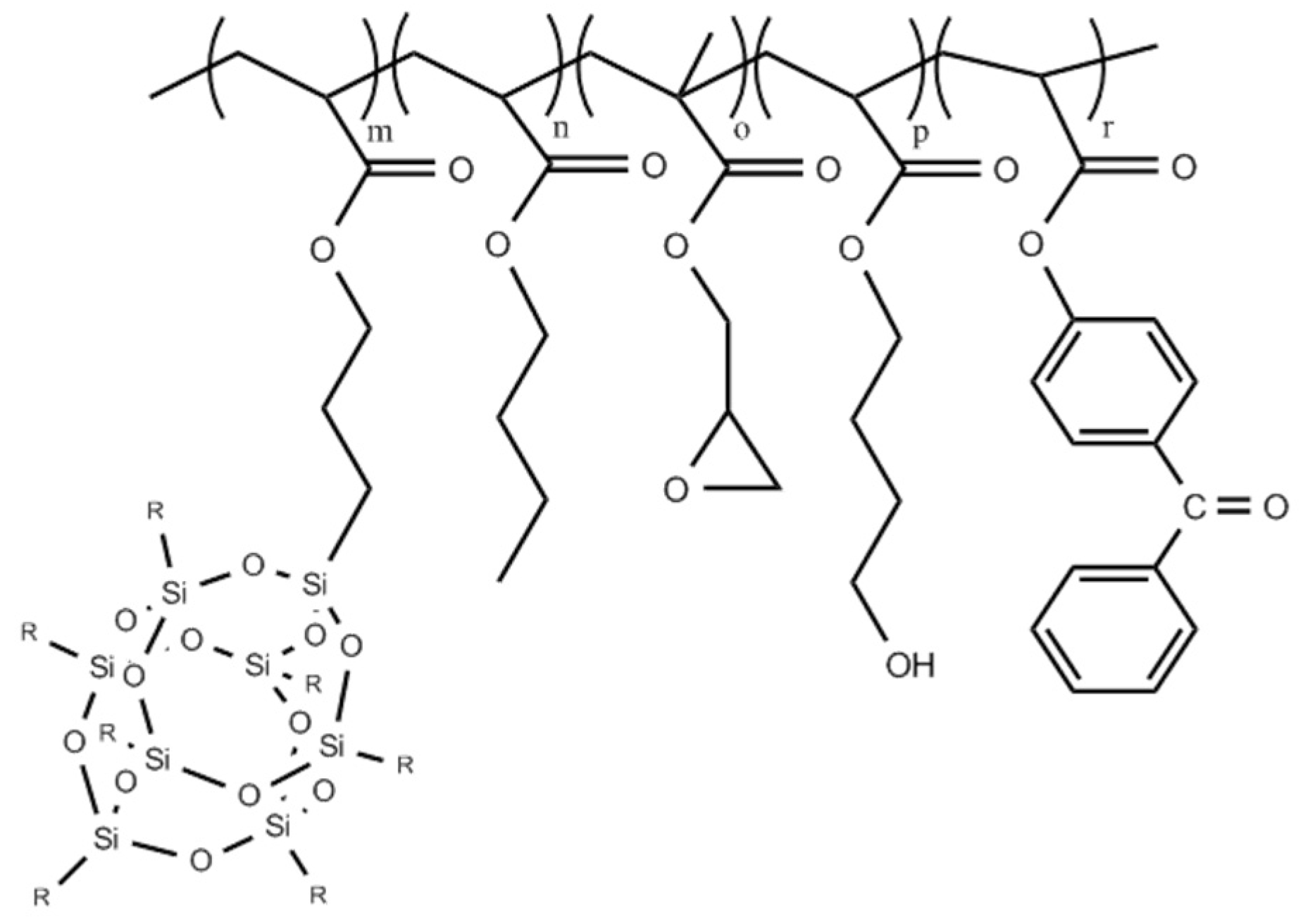

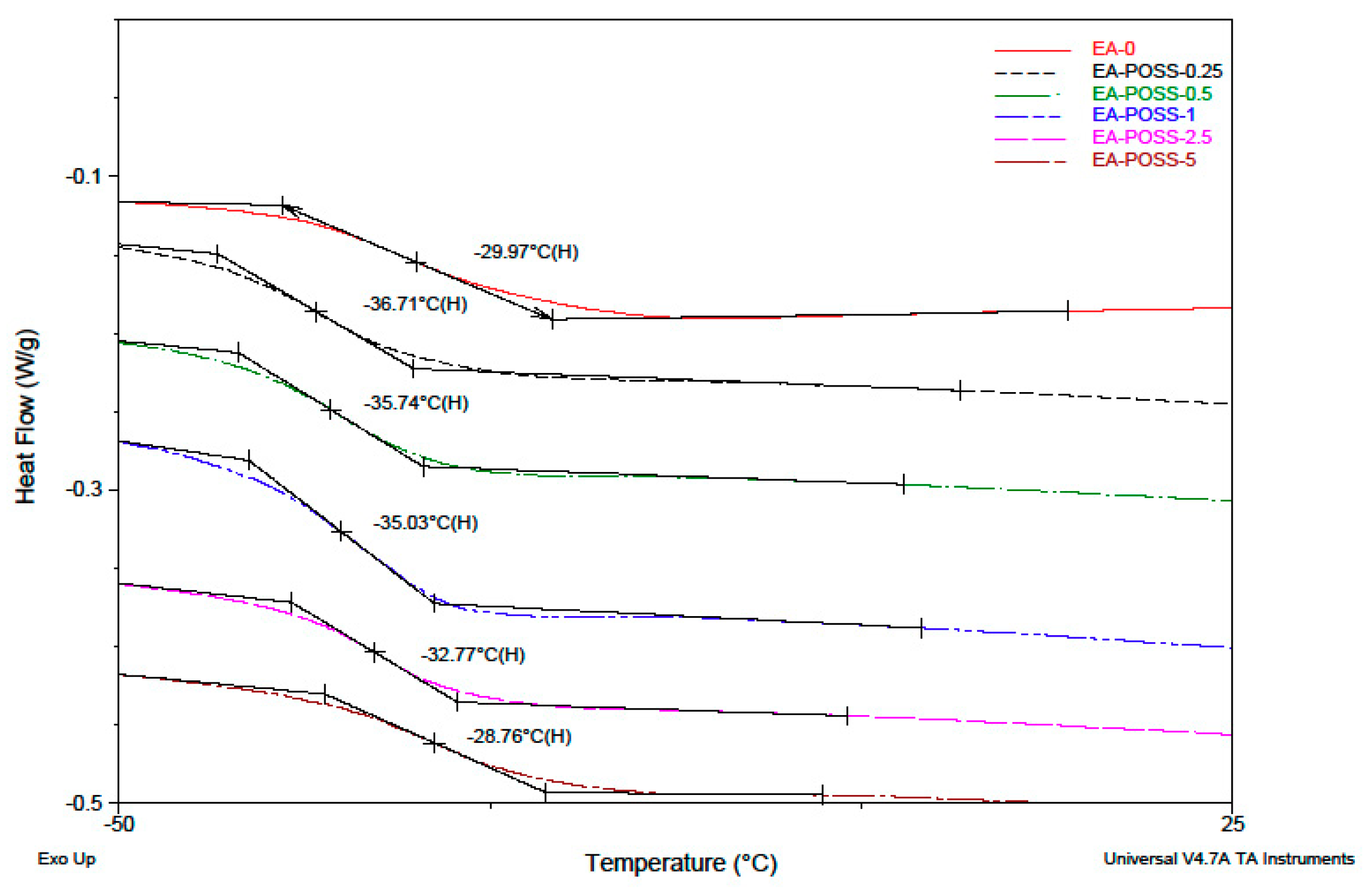
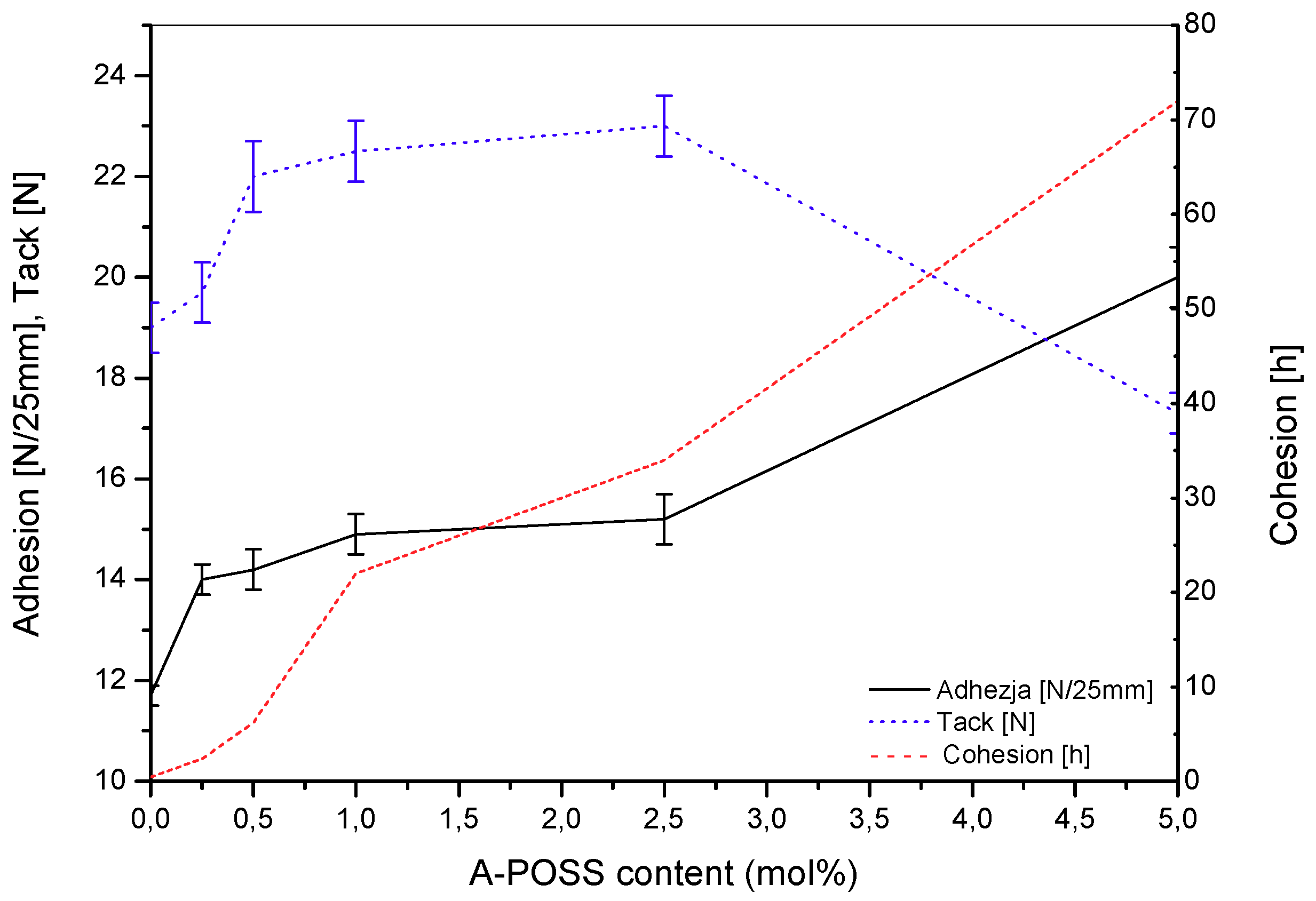

| Copolymer Symbol | Monomers (mol %) | ||||
|---|---|---|---|---|---|
| A-POSS | BA | GMA | HBA | ABP | |
| EA-0 | 0 (0 1) | 79.99 | 10.0 | 10.0 | 0.01 |
| EA-POSS-0.25 | 0.25 (1.8) | 79.74 | |||
| EA-POSS-0.5 | 0.5 (3.4) | 79.49 | |||
| EA-POSS-1 | 1 (6.7) | 78.99 | |||
| EA-POSS-2.5 | 2.5 (15.4) | 77.49 | |||
| EA-POSS-5.0 | 5.0 (27.3) | 74.99 | |||
| EA Acronym | η (Pa∙s) | Mn (g/mol) | Mw (g/mol) | PDI | Tg (°C) |
|---|---|---|---|---|---|
| EA-0 | 12.7 | 135 500 | 436 800 | 3.22 | −21 |
| EA-POSS-0.25 | 19.0 | 105 900 | 702 700 | 6.63 | −37 |
| EA-POSS-0.5 | 25.9 | 114 700 | 635 800 | 5.54 | −36 |
| EA-POSS-1 | 15.6 | 105 600 | 604 600 | 5.72 | −35 |
| EA-POSS-2.5 | 3.3 | 128 100 | 415 400 | 3.24 | −33 |
| EA-POSS-5 | 1.5 | 85 900 | 331 700 | 3.86 | −29 |
| SAT Acronym | EA Type | Tg (°C) | Ti (°C) | Tp (°C) | ΔH (J/g) |
|---|---|---|---|---|---|
| SAT-0 | EA-0 | −19 | 112 | 130/140/163 | 205 |
| SAT-POSS-0.25 | EA-POSS-0.25 | −18 | 121 | 145/167 | 185 |
| SAT-POSS-0.5 | EA-POSS-0.5 | −23, −12 | 122 | 143/176 | 193 |
| SAT-POSS-1 | EA-POSS-1 | −25, −8 | 114 | 144/176 | 207 |
| SAT-POSS-2.5 | EA-POSS-2.5 | −26, +4 | 110 | 145/173 | 212 |
| SAT-POSS-5 | EA-POSS-5 | −26, +2 | 107 | 143/174 | 215 |
| SAT acronym | T5a (°C) | T50b (°C) | Calcination residue at 700 °C (wt %) |
|---|---|---|---|
| SAT-0 | 321 | 404 | 0 |
| SAT-POSS-0.25 | 345 | 436 | 0.7 |
| SAT-POSS-0.5 | 338 | 434 | 1.0 |
| SAT-POSS-1 | 329 | 433 | 4.1 |
| SAT-POSS-2.5 | 331 | 427 | 5.3 |
| SAT-POSS-5 | 340 | 438 | 9.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, A.; Kowalczyk, K.; Gziut, K. Synthesis of Monoacryloxypropyl-POSS-based Hybrid Epoxyacrylate Copolymers and Their Application in Thermally Curable Structural Self-Adhesive Tapes. Polymers 2019, 11, 2058. https://doi.org/10.3390/polym11122058
Kowalczyk A, Kowalczyk K, Gziut K. Synthesis of Monoacryloxypropyl-POSS-based Hybrid Epoxyacrylate Copolymers and Their Application in Thermally Curable Structural Self-Adhesive Tapes. Polymers. 2019; 11(12):2058. https://doi.org/10.3390/polym11122058
Chicago/Turabian StyleKowalczyk, Agnieszka, Krzysztof Kowalczyk, and Konrad Gziut. 2019. "Synthesis of Monoacryloxypropyl-POSS-based Hybrid Epoxyacrylate Copolymers and Their Application in Thermally Curable Structural Self-Adhesive Tapes" Polymers 11, no. 12: 2058. https://doi.org/10.3390/polym11122058
APA StyleKowalczyk, A., Kowalczyk, K., & Gziut, K. (2019). Synthesis of Monoacryloxypropyl-POSS-based Hybrid Epoxyacrylate Copolymers and Their Application in Thermally Curable Structural Self-Adhesive Tapes. Polymers, 11(12), 2058. https://doi.org/10.3390/polym11122058






