The Effects of Inorganic Salts with Different Anions on the Structure and Properties of Starch/Poly (Butylene Succinate) Blends Plasticized with Ionic Liquid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterizations
2.3.1. Fourier Transform Infrared (FT-IR) Spectroscopy
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Thermal Gravity Analysis (TGA)
2.3.4. Differential Scanning Calorimetry (DSC)
2.3.5. X-ray Diffraction (XRD) Measurements
2.3.6. Mechanical Properties Test
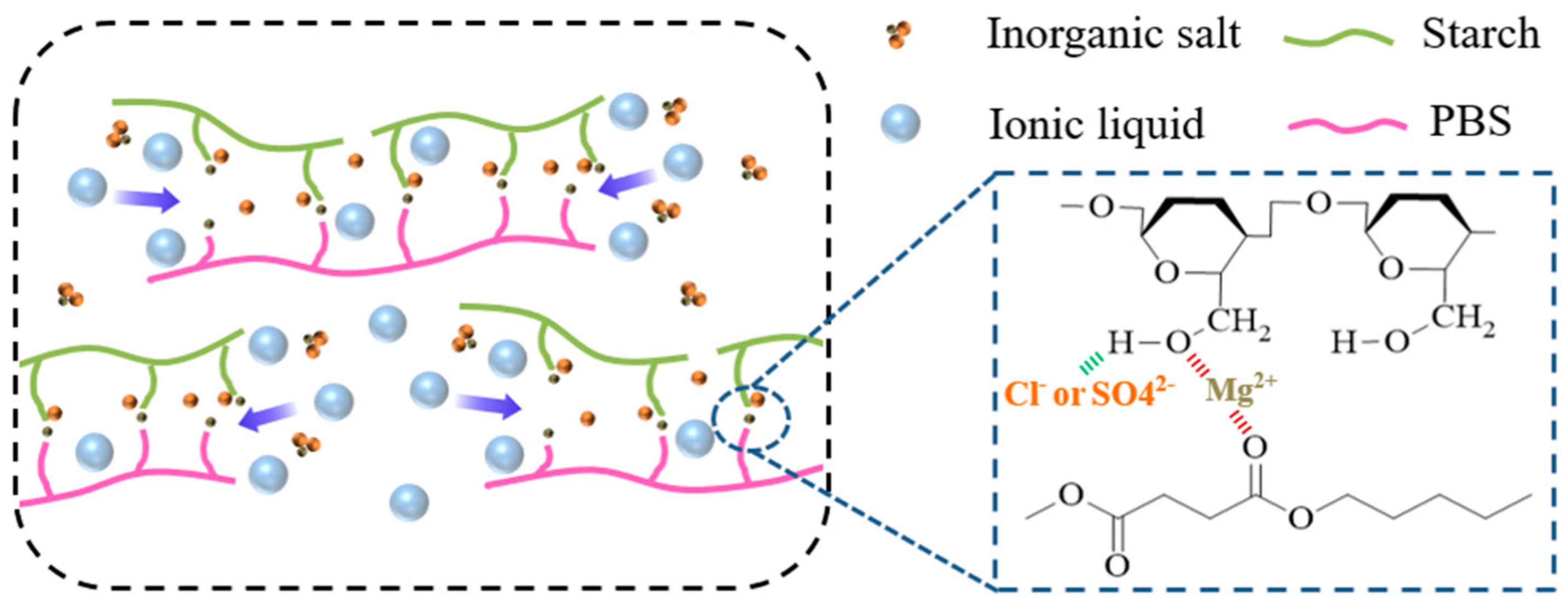
3. Results and Discussion
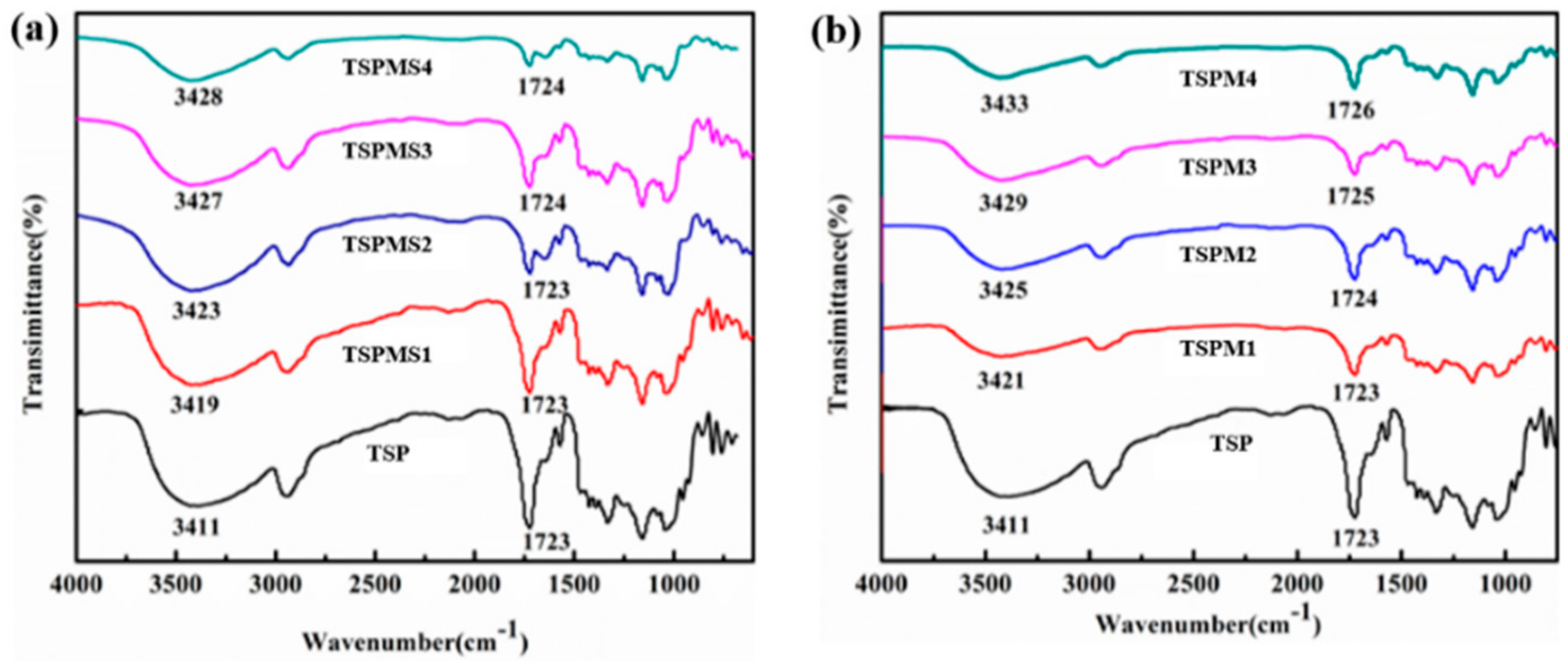
3.1. FT-IR Analysis
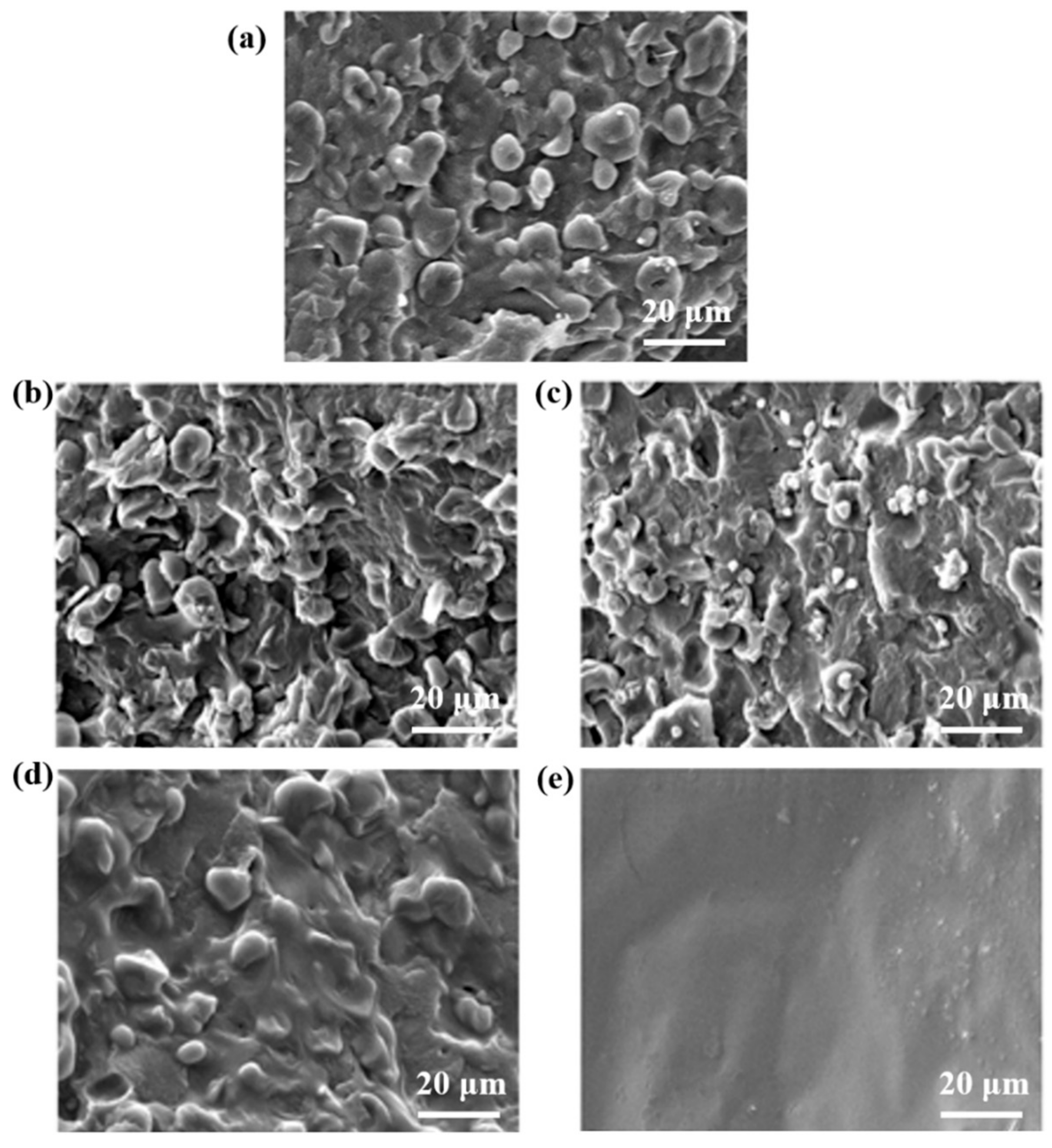
3.2. Scanning Electron Microscopy
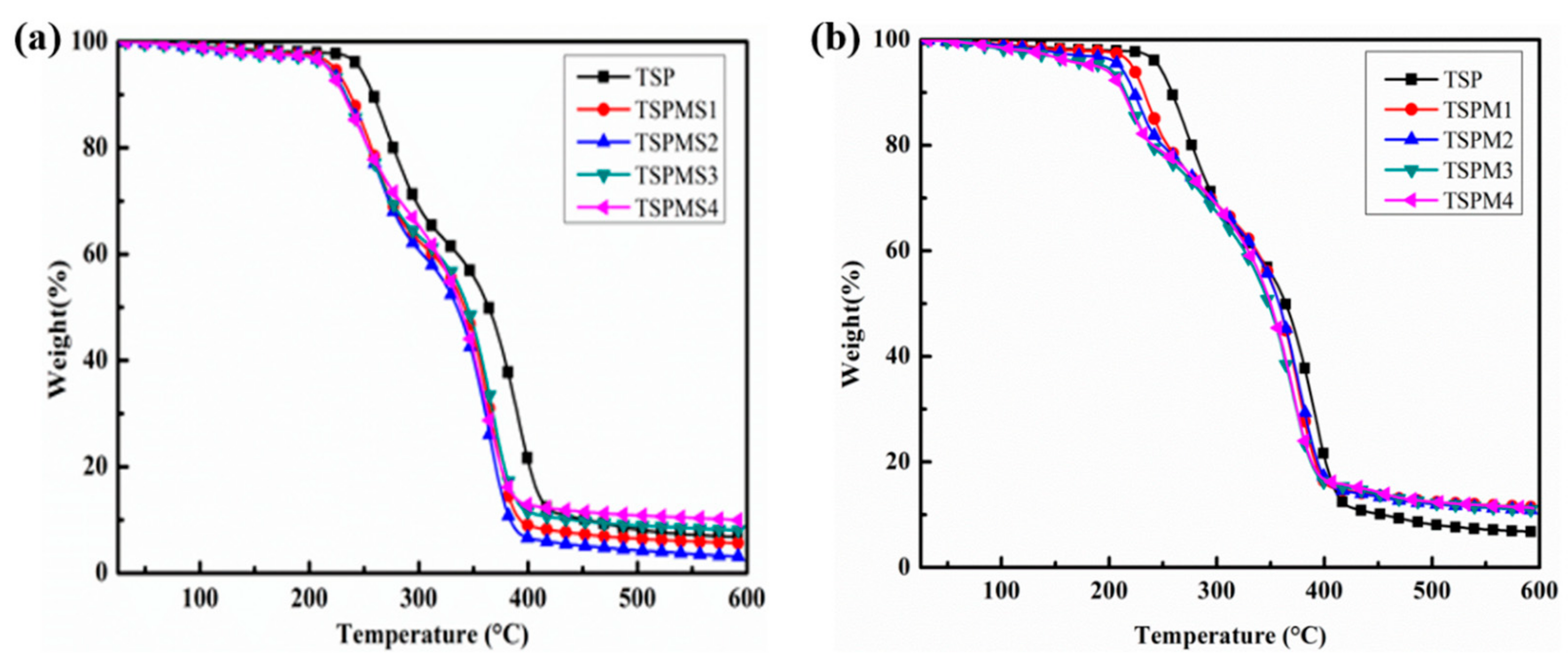
3.3. Thermal Stability Analysis
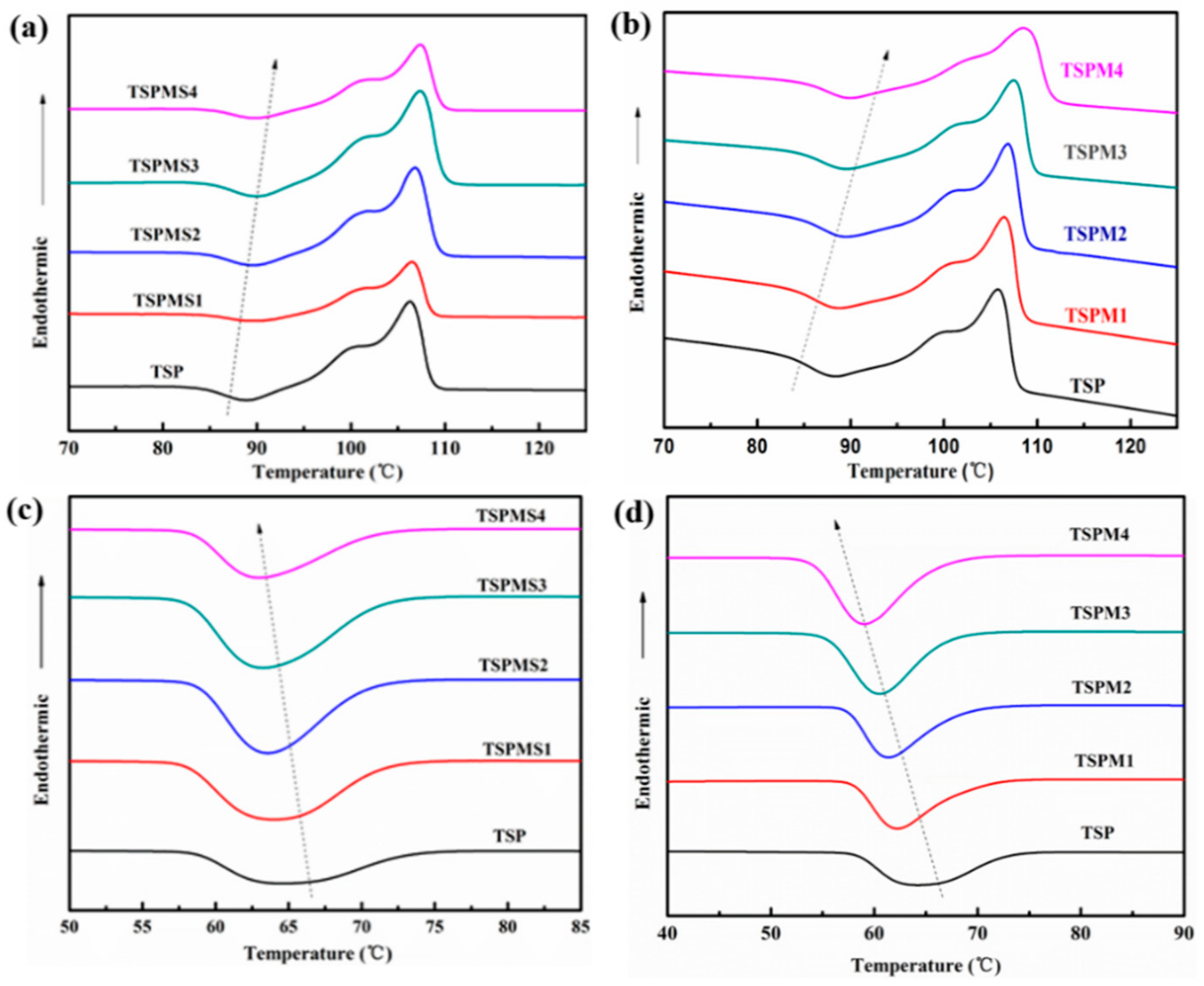
3.4. Thermal Properties
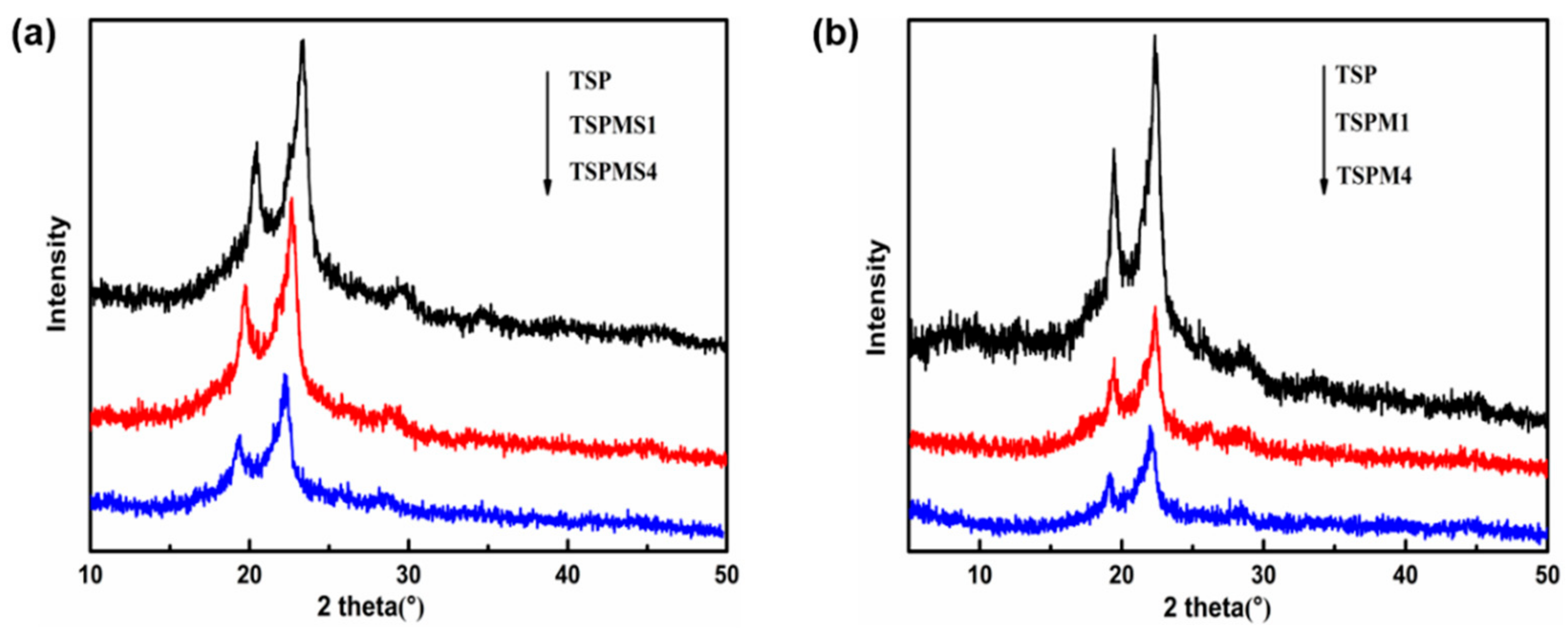
3.5. Crystalline Properties
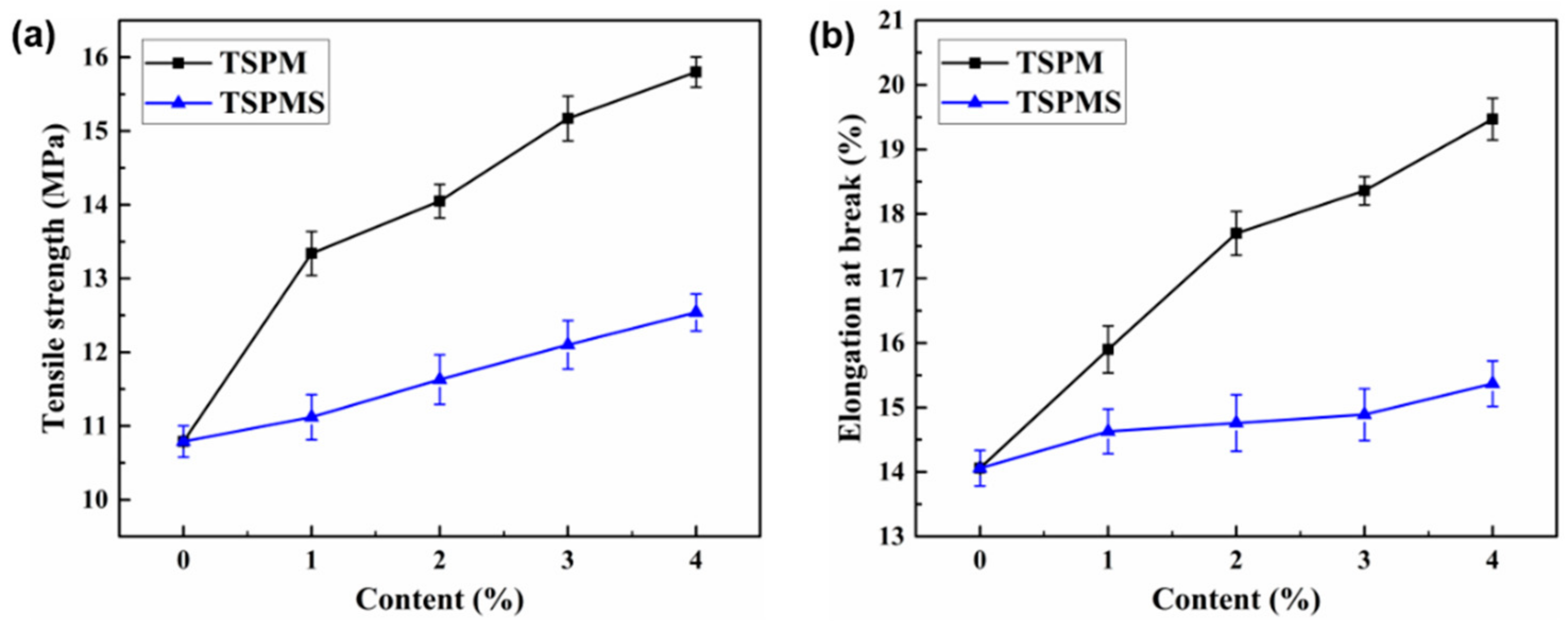
3.6. Mechanical Properties
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Iwata, T. Biodegradable and Bio-Based Polymers: Future Prospects of Eco-Friendly Plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef]
- Muller, J.; Gonzalez-Martinez, C.; Chiralt, A. Combination of Poly(lactic) Acid and Starch for Biodegradable Food Packaging. Materials 2017, 10, 952. [Google Scholar] [CrossRef] [PubMed]
- Peres, A.M.; Pires, R.R.; Orefice, R.L. Evaluation of the effect of reprocessing on the structure and properties of low density polyethylene/thermoplastic starch blends. Carbohydr. Polym. 2016, 136, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Elsawy, M.A.; Kim, K.-H.; Park, J.-W.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Karamanlioglu, M.; Preziosi, R.; Robson, G.D. Abiotic and biotic environmental degradation of the bioplastic polymer poly(lactic acid): A review. Polym. Degrad. Stab. 2017, 137, 122–130. [Google Scholar] [CrossRef]
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Perspective on Polylactic Acid (PLA) based Sustainable Materials for Durable Applications: Focus on Toughness and Heat Resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Chen, D.R.; Bei, J.Z.; Wang, S.G. Polycaprolactone microparticles and their biodegradation. Polym. Degrad. Stab. 2000, 67, 455–459. [Google Scholar] [CrossRef]
- Kweon, H.; Yoo, M.K.; Park, I.K.; Kim, T.H.; Lee, H.C.; Lee, H.S.; Oh, J.S.; Akaike, T.; Cho, C.S. A novel degradable polycaprolactone networks for tissue engineering. Biomaterials 2003, 24, 801–808. [Google Scholar] [CrossRef]
- Xu, X.; Fan, P.; Ren, J.; Cheng, Y.; Ren, J.; Zhao, J.; Song, R. Self-healing thermoplastic polyurethane (TPU)/polycaprolactone (PCL) /multi-wall carbon nanotubes (MWCNTs) blend as shape-memory composites. Compos. Sci. Technol. 2018, 168, 255–262. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Garcia-Sanoguera, D.; Fombuena, V.; Lopez-Martinez, J.; Balart, R. Improvement of mechanical and thermal properties of poly(3-hydroxybutyrate) (PHB) blends with surface-modified halloysite nanotubes (HNT). Appl. Clay Sci. 2018, 162, 487–498. [Google Scholar] [CrossRef]
- Godbole, S.; Gote, S.; Latkar, M.; Chakrabarti, T. Preparation and characterization of biodegradable poly-3-hydroxybutyrate-starch blend films. Bioresour. Technol. 2003, 86, 33–37. [Google Scholar] [CrossRef]
- Kai, D.; Chong, H.M.; Chow, L.P.; Jiang, L.; Lin, Q.; Zhang, K.; Zhang, H.; Zhang, Z.; Loh, X.J. Strong and biocompatible lignin /poly (3-hydroxybutyrate) composite nanofibers. Compos. Sci. Technol. 2018, 158, 26–33. [Google Scholar] [CrossRef]
- Deng, Y.; Thomas, N.L. Blending poly(butylene succinate) with poly(lactic acid): Ductility and phase inversion effects. Eur. Polym. J. 2015, 71, 534–546. [Google Scholar] [CrossRef]
- Fortunati, E.; Puglia, D.; Iannoni, A.; Terenzi, A.; Kenny, J.M.; Torre, L. Processing Conditions, Thermal and Mechanical Responses of Stretchable Poly (Lactic Acid)/Poly (Butylene Succinate) Films. Materials 2017, 10, 809. [Google Scholar] [CrossRef] [PubMed]
- Tserki, V.; Matzinos, P.; Pavlidou, E.; Vachliotis, D.; Panayiotou, C. Biodegradable aliphatic polyesters. Part I. Properties and biodegradation of poly(butylene succinate-co-butylene adipate). Polym. Degrad. Stab. 2006, 91, 367–376. [Google Scholar] [CrossRef]
- Averous, L. Biodegradable multiphase systems based on plasticized starch: A review. J. Macromol. Sci. Pol. R. 2004, 44, 231–274. [Google Scholar] [CrossRef]
- Copeland, L.; Blazek, J.; Salman, H.; Tang, M.C. Form and functionality of starch. Food Hydrocoll. 2009, 23, 1527–1534. [Google Scholar] [CrossRef]
- Jobling, S. Improving starch for food and industrial applications. Curr. Opin. Plant Biol. 2004, 7, 210–218. [Google Scholar] [CrossRef]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Farran, A.; Cai, C.; Sandoval, M.; Xu, Y.; Liu, J.; Hernaiz, M.J.; Linhardt, R.J. Green solvents in carbohydrate chemistry: From raw materials to fine chemicals. Chem. Rev. 2015, 115, 6811–6853. [Google Scholar] [CrossRef]
- Lichtenthaler, F.W.; Peters, S. Carbohydrates as green raw materials for the chemical industry. C. R. Chim. 2004, 7, 65–90. [Google Scholar] [CrossRef]
- Sheldon, R.A. Engineering a more sustainable world through catalysis and green chemistry. J. R. Soc. Interface 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, R.A.; Karman, A.P.; Janssen, L.P.B.M. Material properties and glass transition temperatures of different thermoplastic starches after extrusion processing. Starch Starke 2003, 55, 80–86. [Google Scholar] [CrossRef]
- De Menezes, A.J.; Pasquini, D.; Curvelo, A.A.S.; Gandini, A. Novel thermoplastic materials based on the outer-shell oxypropylation of corn starch granules. Biomacromolecules 2007, 8, 2047–2050. [Google Scholar] [CrossRef]
- Nabar, Y.; Narayan, R.; Schindler, M. Twin-screw extrusion production and characterization of starch foam products for use in cushioning and insulation applications. Polym. Eng. Sci. 2006, 46, 438–451. [Google Scholar] [CrossRef]
- Ning, W.; Yu, J.G.; Han, C.M. Influence of citric acid on the properties of glycerol-plasticised Cornstarch extrusion blends. Polym. Polym. Compos. 2007, 15, 545–552. [Google Scholar] [CrossRef]
- Ratto, J.A.; Stenhouse, P.J.; Auerbach, M.; Mitchell, J.; Farrell, R. Processing, performance and biodegradability of a thermoplastic aliphatic polyester/starch system. Polymer 1999, 40, 6777–6788. [Google Scholar] [CrossRef]
- Li, J.W.; Luo, X.G.; Lin, X.Y.; Zhou, Y. Comparative study on the blends of PBS/thermoplastic starch prepared from waxy and normal corn starches. Starch Starke 2013, 65, 831–839. [Google Scholar] [CrossRef]
- Zeng, J.-B.; Jiao, L.; Li, Y.-D.; Srinivasan, M.; Li, T.; Wang, Y.-Z. Bio-based blends of starch and poly(butylene succinate) with improved miscibility, mechanical properties, and reduced water absorption. Carbohydr. Polym. 2011, 83, 762–768. [Google Scholar] [CrossRef]
- Sankri, A.; Arhaliass, A.; Dez, I.; Gaumont, A.C.; Grohens, Y.; Lourdin, D.; Pillin, I.; Rolland-Sabaté, A.; Leroy, E. Thermoplastic starch plasticized by an ionic liquid. Carbohydr. Polym. 2010, 82, 256–263. [Google Scholar] [CrossRef]
- Cai, Y.M.; Lv, J.G.; Feng, J.M. Spectral Characterization of Four Kinds of Biodegradable Plastics: Poly (Lactic Acid), Poly (Butylenes Adipate-Co-Terephthalate), Poly (Hydroxybutyrate-Co-Hydroxyvalerate) and Poly (Butylenes Succinate) with FTIR and Raman Spectroscopy. J. Polym. Environ. 2013, 21, 108–114. [Google Scholar] [CrossRef]
- Zullo, R.; Iannace, S. The effects of different starch sources and plasticizers on film blowing of thermoplastic starch: Correlation among process, elongational properties and macromolecular structure. Carbohydr. Polym. 2009, 77, 376–383. [Google Scholar] [CrossRef]
- Nofar, M.; Oguz, H.; Ovali, D. Effects of the matrix crystallinity, dispersed phase, and processing type on the morphological, thermal, and mechanical properties of polylactide-based binary blends with poly[(butylene adipate)-co-terephthalate] and poly[(butylene succinate)-co-adipate]. J. Appl. Polym. Sci. 2019, 136. [Google Scholar] [CrossRef]
- Liu, G.; Zheng, L.; Zhang, X.; Li, C.; Jiang, S.; Wang, D. Reversible Lamellar Thickening Induced by Crystal Transition in Poly(butylene succinate). Macromolecules 2012, 45, 5487–5493. [Google Scholar] [CrossRef]
- Zeng, J.-B.; Li, Y.-D.; Zhu, Q.-Y.; Yang, K.-K.; Wang, X.-L.; Wang, Y.-Z. A novel biodegradable multiblock poly(ester urethane) containing poly(l-lactic acid) and poly(butylene succinate) blocks. Polymer 2009, 50, 1178–1186. [Google Scholar] [CrossRef]
| Samples | Starch/g | PBS/g | (BMIM)Cl/g | MgSO4/g |
|---|---|---|---|---|
| TSP | 16 | 24 | 4 | 0 |
| TSPMS1 | 16 | 24 | 4 | 0.4 |
| TSPMS2 | 16 | 24 | 4 | 0.8 |
| TSPMS3 | 16 | 24 | 4 | 1.2 |
| TSPMS4 | 16 | 24 | 4 | 1.6 |
| Samples | Starch/g | PBS/g | (BMIM)Cl/g | MgCl2/g |
|---|---|---|---|---|
| TSP | 16 | 24 | 4 | 0 |
| TSPM1 | 16 | 24 | 4 | 0.4 |
| TSPM2 | 16 | 24 | 4 | 0.8 |
| TSPM3 | 16 | 24 | 4 | 1.2 |
| TSPM4 | 16 | 24 | 4 | 1.6 |
| Sample | Ti (°C) | Residue at 600 °C (%) |
|---|---|---|
| TSP | 243 | 6.8 |
| TSPMS1 | 224 | 8.1 |
| TSPMS2 | 221 | 5.6 |
| TSPMS3 | 219 | 3.1 |
| TSPMS4 | 215 | 9.9 |
| TSPM1 | 218 | 11.4 |
| TSPM2 | 214 | 10.7 |
| TSPM3 | 204 | 12.5 |
| TSPM4 | 203 | 12.6 |
| Sample | ΔHm (J/g) | Xc (%) | Tc (°C) | Tcc (°C) |
|---|---|---|---|---|
| TSP | 28.6 | 47.6 | 64.2 | 88.3 |
| TSPMS1 | 26.9 | 45.1 | 64.0 | 88.9 |
| TSPMS2 | 25.3 | 42.8 | 63.6 | 89.3 |
| TSPMS3 | 24.4 | 41.6 | 63.4 | 89.7 |
| TSPMS4 | 23.0 | 39.5 | 63.2 | 89.8 |
| TSPM1 | 25.1 | 42.1 | 62.2 | 89.0 |
| TSPM2 | 24.4 | 41.3 | 61.4 | 89.7 |
| TSPM3 | 23.0 | 39.3 | 60.4 | 90.1 |
| TSPM4 | 22.0 | 37.8 | 59.2 | 91.7 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|
| TSP | 10.8 ± 0.21 | 14.1 ± 0.27 | 687 ± 5.36 |
| TSPMS1 | 11.1 ± 0.30 | 14.6 ± 0.34 | 768 ± 6.68 |
| TSPMS2 | 11.6 ± 0.33 | 14.8 ± 0.43 | 839 ± 8.36 |
| TSPMS3 | 12.1 ± 0.32 | 14.9 ± 0.40 | 951 ± 7.52 |
| TSPMS4 | 12.5 ± 0.25 | 15.4 ± 0.35 | 1052 ± 6.57 |
| TSPM1 | 13.3 ± 0.29 | 15.9 ± 0.36 | 827 ± 5.54 |
| TSPM2 | 14.1 ± 0.22 | 17.7 ± 0.33 | 943 ± 9.34 |
| TSPM3 | 15.2 ± 0.30 | 18.4 ± 0.21 | 1001 ± 6.92 |
| TSPM4 | 15.8 ± 0.20 | 19.5 ± 0.32 | 1183 ± 6.66 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Lei, B.; Du, W.; Zhang, X. The Effects of Inorganic Salts with Different Anions on the Structure and Properties of Starch/Poly (Butylene Succinate) Blends Plasticized with Ionic Liquid. Polymers 2019, 11, 2004. https://doi.org/10.3390/polym11122004
Zhao Z, Lei B, Du W, Zhang X. The Effects of Inorganic Salts with Different Anions on the Structure and Properties of Starch/Poly (Butylene Succinate) Blends Plasticized with Ionic Liquid. Polymers. 2019; 11(12):2004. https://doi.org/10.3390/polym11122004
Chicago/Turabian StyleZhao, Zhixin, Bei Lei, Wenhao Du, and Xi Zhang. 2019. "The Effects of Inorganic Salts with Different Anions on the Structure and Properties of Starch/Poly (Butylene Succinate) Blends Plasticized with Ionic Liquid" Polymers 11, no. 12: 2004. https://doi.org/10.3390/polym11122004
APA StyleZhao, Z., Lei, B., Du, W., & Zhang, X. (2019). The Effects of Inorganic Salts with Different Anions on the Structure and Properties of Starch/Poly (Butylene Succinate) Blends Plasticized with Ionic Liquid. Polymers, 11(12), 2004. https://doi.org/10.3390/polym11122004






