Reactive Extrusion and Magnesium (II) N-Heterocyclic Carbene Catalyst in Continuous PLA Production
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Syntheses
Synthesis of the NHC
2.3. Batch Polymerization
2.4. Reactive Extrusion
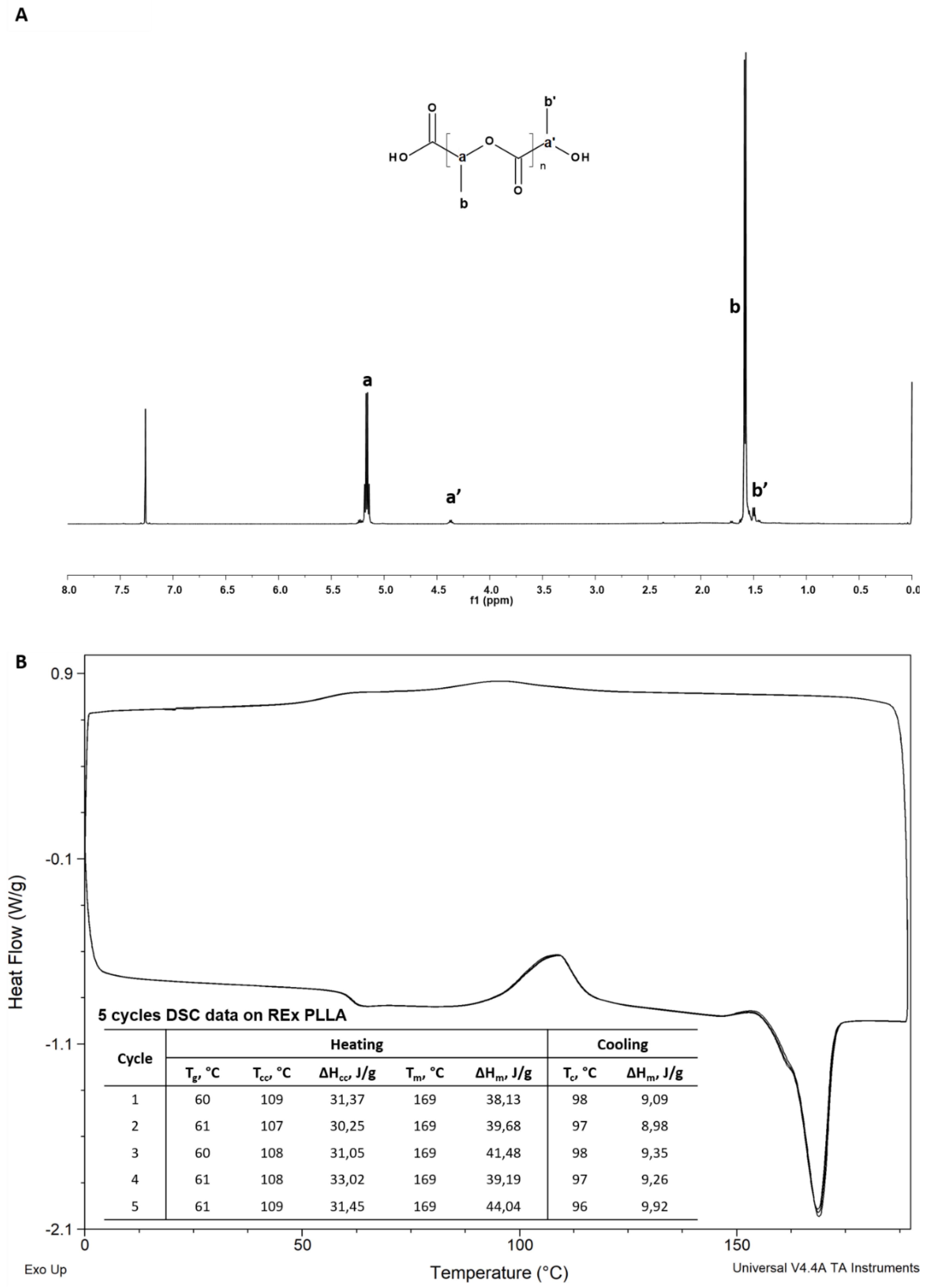
3. Characterizations
4. Results and Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dubois, P.; Coulembier, O.; Raquez, J.M. (Eds.) Handbook of Ring-Opening Polymerization; Wiley-VCH: Hoboken, NJ, USA, 2009. [Google Scholar]
- Itävaara, M.; Karjomaa, S.; Selin, J.F. Biodegradation of polylactide in aerobic and anaerobic thermophilic conditions. Chemosphere 2002, 46, 879–885. [Google Scholar] [CrossRef]
- Raquez, J.M.; Narayan, R.; Dubois, P. Recent Advances in Reactive Extrusion Processing of Biodegradable Polymer-Based Compositions. Macromol. Mater. Eng. 2008, 293, 447–470. [Google Scholar] [CrossRef]
- Jacobsen, S.; Fritz, H.G.; Degée, P.; Dubois, P.; Jérôme, R. New developments on the ring opening polymerisation of polylactide. Ind. Crops Prod. 2000, 11, 265–275. [Google Scholar] [CrossRef]
- Banu, I.; Puaux, J.P.; Bozga, G.; Nagy, I. Modeling of L-lactide polymerization by reactive extrusion. Macromol. Symp. 2010, 289, 108–118. [Google Scholar] [CrossRef]
- Nachtergael, A.; Coulembier, O.; Dubois, P.; Helvenstein, M.; Duez, P.; Blankert, B.; Mespouille, L. Organocatalysis paradigm revisited: Are metal-free catalysts really harmless? Biomacromolecules 2015, 16, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Arends, I.W.C.E.; Hanefeld, U. Green Chemistry and Catalysis; Wiley-VCH: Hoboken, NJ, USA, 2007; Chapter 1; pp. 1–47. [Google Scholar]
- Mezzasalma, L.; Dove, A.P.; Coulembier, O. Organocatalytic ring-opening polymerization of L-lactide in bulk: A long standing challenge. Eur. Polym. J. 2017, 95, 628–634. [Google Scholar] [CrossRef]
- Raquez, J.M.; Ramy-Ratiarison, R.; Murariu, M.; Dubois, P. Reactive extrusion of PLA-based materials: From synthesis to reactive melt-blending. RSC Polym. Chem. Ser. 2015, 12, 101–123. [Google Scholar]
- Rosen, T.; Goldberg, I.; Navarra, W.; Venditto, V.; Kol, M. Block–Stereoblock Copolymers of Poly(ϵ-Caprolactone) and Poly(Lactic Acid). Angew. Chem. Int. Ed. 2018, 57, 7191–7195. [Google Scholar] [CrossRef]
- D’Auria, I.; Tedesco, C.; Mazzeo, M.; Pellecchia, C. New homoleptic bis(pyrrolylpyridiylimino) Mg(II) and Zn(II) complexes as catalysts for the ring opening polymerization of cyclic esters: Via an “activated monomer” mechanism. Dalt. Trans. 2017, 46, 12217–12225. [Google Scholar] [CrossRef]
- Fliedel, C.; Vila-Viçosa, D.; Calhorda, M.J.; Dagorne, S.; Avilés, T. Dinuclear zinc-N-heterocyclic carbene complexes for either the controlled ring-opening polymerization of lactide or the controlled degradation of polylactide under mild conditions. Chem. Cat. Chem. 2014, 6, 1357–1367. [Google Scholar] [CrossRef]
- Arduengo, A.J.; Davidson, F.; Krafczyk, R.; Marshall, W.J.; Tamm, M. Adducts of Carbenes with Group II and XII. Organometallics 1998, 7, 3375–3382. [Google Scholar] [CrossRef]
- Schumann, H.; Gottfriedsen, J.; Glanz, M.; Dechert, S.; Demtschuk, J.J. Metallocenes of the alkaline earth metals and their carbene complexes. Organomet. Chem. 2001, 617, 588–600. [Google Scholar] [CrossRef]
- Mungur, S.A.; Liddle, S.T.; Wilson, C.; Sarsfield, M.J.; Arnold, P.L. Bent metal carbene geometries in amido N-heterocyclic carbene complexes. Chem. Commun. 2004, 2, 2738–2739. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.L.; Edworthy, I.S.; Carmichael, C.D.; Blake, A.J.; Wilson, C. Magnesium amido N-heterocyclic carbene complexes. Dalt. Trans. 2008, 3739–3746. [Google Scholar] [CrossRef]
- Arnold, P.L.; Casely, I.J.; Turner, Z.R.; Bellabarba, R.; Tooze, R.B. Magnesium and zinc complexes of functionalised, saturated N-heterocyclic carbene ligands: carbene lability and functionalisation, and lactide polymerisation catalysis. Dalt. Trans. 2009, 6903–6914. [Google Scholar] [CrossRef]
- Brulé, E.; Guérineau, V.; Vermaut, P.; Prima, F.; Balogh, J.; Maron, L.; Slawin, A.M.Z.; Nolan, S.P.; Thomas, C.M. Polymerization of Cyclic Esters Using N- Heterocyclic Carbene Carboxylate Catalysts. Polym. Chem. 2013, 4871–4877. [Google Scholar] [CrossRef]
- Dove, A.P.; Pratt, R.C.; Lohmeijer, B.G.G.; Culkin, D.A.; Hagberg, E.C.; Nyce, G.W.; Waymouth, R.M.; Hedrick, J.L. N-Heterocyclic carbenes: Effective organic catalysts for living polymerization. Polymer 2006, 47, 4018–4025. [Google Scholar] [CrossRef]
- Van Ausdall, B.R.; Glass, J.L.; Wiggins, K.M.; Aarif, A.M.; Louie, J.J. A systematic investigation of factors influencing the decarboxylation of imidazolium carboxylates. Org. Chem. 2009, 74, 7935–7942. [Google Scholar] [CrossRef]
- Fèvre, M.; Pinaud, J.; Gnanou, Y.; Vignolle, J.; Taton, D. N-Heterocyclic carbenes (NHCs) as organocatalysts and structural components in metal-free polymer synthesis. Chem. Soc. Rev. 2013, 42, 2142–2172. [Google Scholar] [CrossRef]
- Delaude, L.; Demonceau, A.; Wouters, J. Assessing the potential of zwitterionic NHOCS2 adducts for probing the stereoelectronic parameters of N-heterocyclic carbenes. Eur. J. Inorg. Chem. 2009, 1882–1891. [Google Scholar] [CrossRef]
- Naumann, S.; Schmidt, F.G.; Speiser, M.; Böhl, M.; Epple, S.; Bonten, C.; Buchmeiser, M.R. Anionic ring-opening homo- and copolymerization of lactams by latent, protected N-heterocyclic carbenes for the preparation of PA 12 and PA 6/12. Macromolecules 2013, 46, 8426–8433. [Google Scholar] [CrossRef]
- Nyce, G.W.; Glauser, T.; Connor, E.F.; Möck, A.; Waymouth, R.M.; Hedrick, J.L.J. In situ generation of carbenes: a general and versatile platform for organocatalytic living polymerization. Am. Chem. Soc. 2003, 125, 3046–3056. [Google Scholar] [CrossRef] [PubMed]
- Bantu, B.; Pawar, G.M.; Decker, U.; Wurst, K.; Schmidt, A.M.; Buchmeiser, M.R. CO2 and Sn II adducts of N-heterocyclic carbenes as delayed-action catalysts for polyurethane synthesis. Chem. A Eur. J. 2009, 15, 3103–3109. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.T.; Scheidt, K.A. Cooperative Lewis acid/N-heterocyclic carbene catalysis. Chem. Sci. 2012, 3, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, A.; Enders, D. N-heterocyclic carbene catalyzed domino reactions. Angew. Chem. Int. Ed. 2012, 51, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Naumann, S.; Scholten, P.B.V.; Wilson, J.A.; Dove, A.P.J. Dual Catalysis for Selective Ring-Opening Polymerization of Lactones: Evolution toward Simplicity. Am. Chem. Soc. 2015, 137, 14439–14445. [Google Scholar] [CrossRef] [PubMed]
- Csihony, S.; Culkin, D.A.; Sentman, A.C.; Dove, A.P.; Waymouth, R.M.; Hedrick, J.L.J. Single-component catalyst/initiators for the organocatalytic ring-opening polymerization of lactide. Am. Chem. Soc. 2005, 127, 9079–9084. [Google Scholar] [CrossRef]
- Culkin, D.A.; Jeong, W.; Csihony, S.; Gomez, E.D.; Balsara, N.P.; Hedrick, J.L.; Waymouth, R.M. Zwitterionic polymerization of lactide to cyclic poly(lactide) by using N-heterocyclic carbene organocatalysts. Angew. Chem. Int. Ed. 2007, 46, 2627–2630. [Google Scholar] [CrossRef]
- De Winter, J.; Coulembier, O.; Gerbaux, P.; Dubois, P. High molecular weight poly(α,α′,β-trisubstituted β-lactones) as generated by metal-free phosphazene catalysts. Macromolecules 2010, 43, 10291–10296. [Google Scholar] [CrossRef]
- Coulembier, O.; Moins, S.; Raquez, J.M.; Meyer, F.; Mespouille, L.; Duquesne, E.; Dubois, P. Thermal degradation of poly(l-lactide): Accelerating effect of residual DBU-based organic catalysts. Polym. Degrad. Stab. 2011, 96, 739–744. [Google Scholar] [CrossRef]
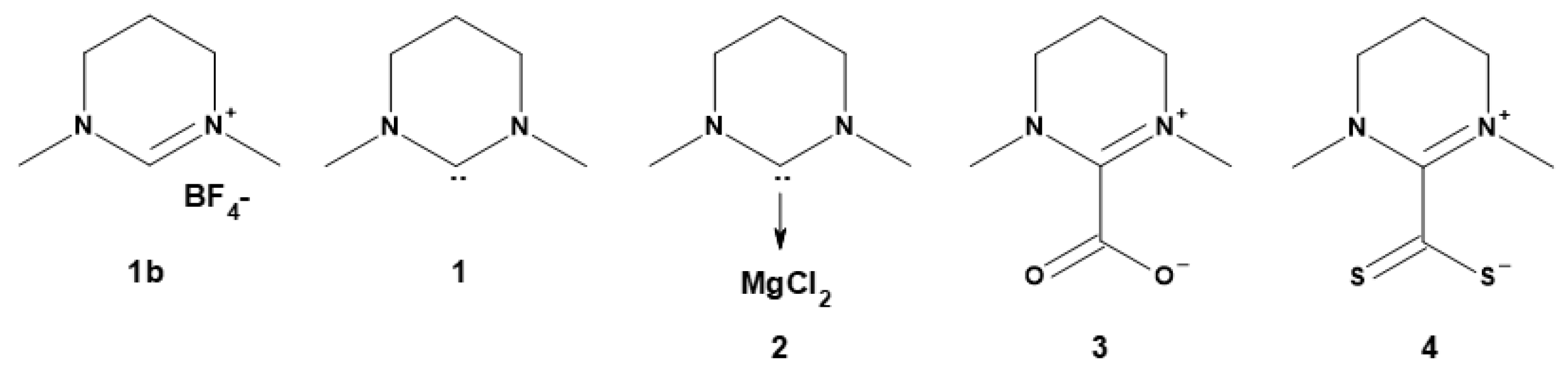
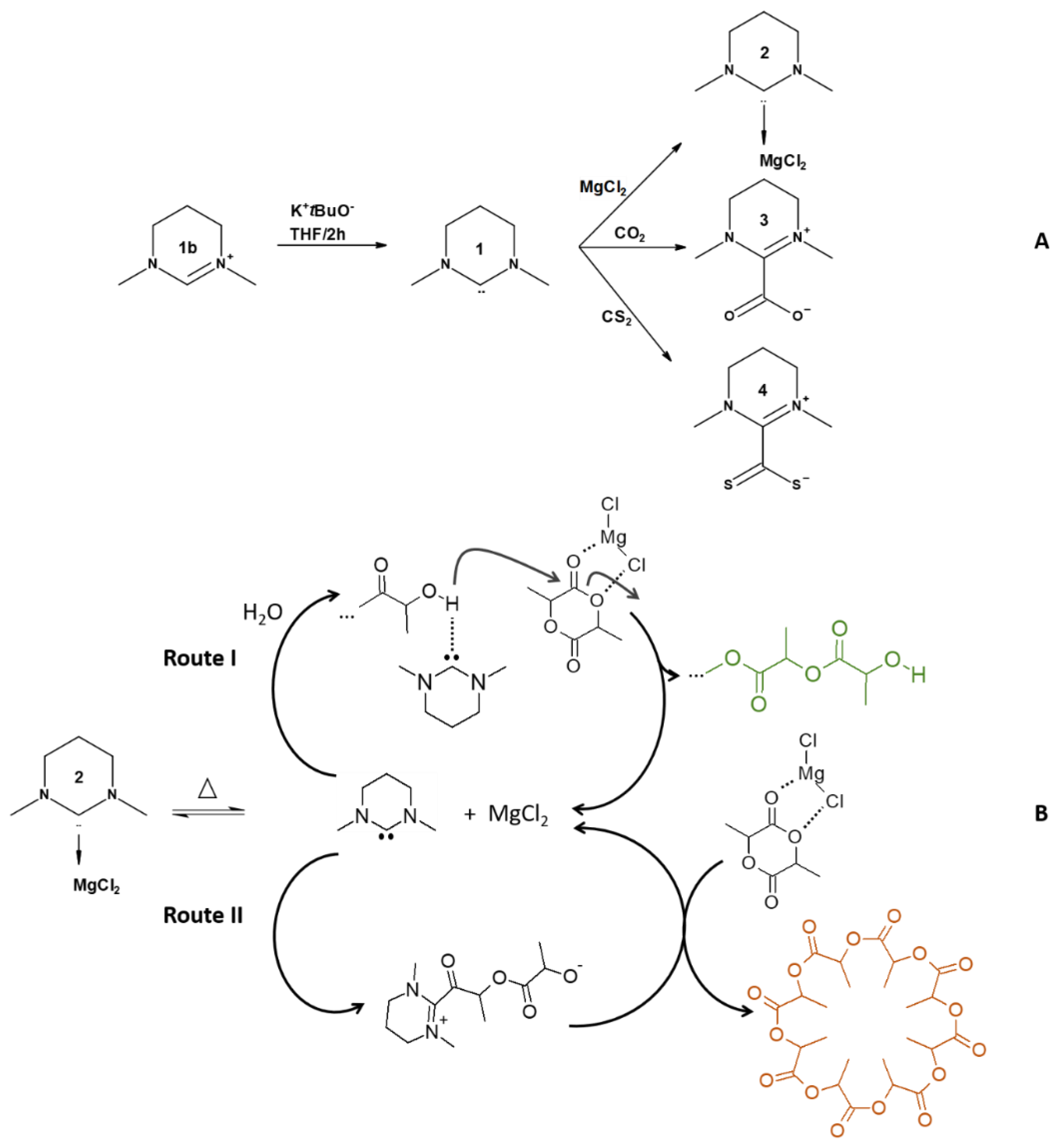
| Entry | Adduct | t (h) | Conv. (%)a | ed (%)a | Mn (g mol−1)b | Mw (g mol−1)b | Ðb |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 0.5 | 28 | 22 | 5,700 | 11,000 | 1.91 |
| 2 | 2 | 0.5 | 94 | 5 | 32,000 | 54,000 | 1.94 |
| 3 | 3 | 17 | 92 | 38 | 8,100 | 9,400 | 1.25 |
| 4 | 4 (or MgCl2) | 1 | - | - | - | - | - |
| Entry | T (°C) | T (min) | Conv (/%)a | Ed (%)a | Mn (g mol−1)b | Ðb |
|---|---|---|---|---|---|---|
| 1 | 170 | 5 | 64 | 3 | 39.000 | 1.61 |
| 2 | 15 | 76 | 6 | 38.000 | 1.70 | |
| 3 | 30 | 80 | 6 | 41.000 | 1.76 | |
| 4 | 45 | 83 | 8 | 35.000 | 1.88 | |
| 5 | 60 | 85 | 8 | 33.000 | 1.94 | |
| 6 | 190 | 5 | 72 | 5 | 33.000 | 1.73 |
| 7 | 15 | 80 | 8 | 27.000 | 1.95 | |
| 8 | 30 | 90 | 10 | 24.000 | 2.01 | |
| 9 | 45 | 92 | 10 | 19.500 | 2.30 | |
| 10 | 60 | 95 | 15 | 18.300 | 2.14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mincheva, R.; Narayana Murthy Chilla, S.; Todd, R.; Guillerm, B.; De Winter, J.; Gerbaux, P.; Coulembier, O.; Dubois, P.; Raquez, J.-M. Reactive Extrusion and Magnesium (II) N-Heterocyclic Carbene Catalyst in Continuous PLA Production. Polymers 2019, 11, 1987. https://doi.org/10.3390/polym11121987
Mincheva R, Narayana Murthy Chilla S, Todd R, Guillerm B, De Winter J, Gerbaux P, Coulembier O, Dubois P, Raquez J-M. Reactive Extrusion and Magnesium (II) N-Heterocyclic Carbene Catalyst in Continuous PLA Production. Polymers. 2019; 11(12):1987. https://doi.org/10.3390/polym11121987
Chicago/Turabian StyleMincheva, Rosica, Satya Narayana Murthy Chilla, Richard Todd, Brieuc Guillerm, Julien De Winter, Pascal Gerbaux, Olivier Coulembier, Philippe Dubois, and Jean-Marie Raquez. 2019. "Reactive Extrusion and Magnesium (II) N-Heterocyclic Carbene Catalyst in Continuous PLA Production" Polymers 11, no. 12: 1987. https://doi.org/10.3390/polym11121987
APA StyleMincheva, R., Narayana Murthy Chilla, S., Todd, R., Guillerm, B., De Winter, J., Gerbaux, P., Coulembier, O., Dubois, P., & Raquez, J.-M. (2019). Reactive Extrusion and Magnesium (II) N-Heterocyclic Carbene Catalyst in Continuous PLA Production. Polymers, 11(12), 1987. https://doi.org/10.3390/polym11121987









