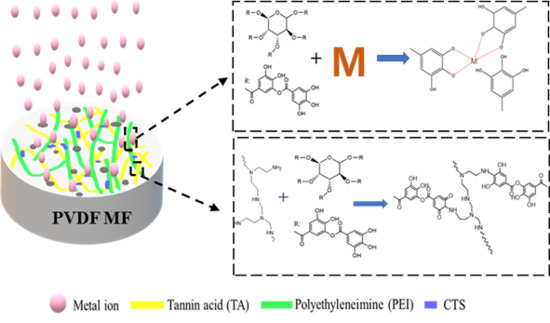
A Stable Anti-Fouling Coating on PVDF Membrane Constructed of Polyphenol Tannic Acid, Polyethyleneimine and Metal Ion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
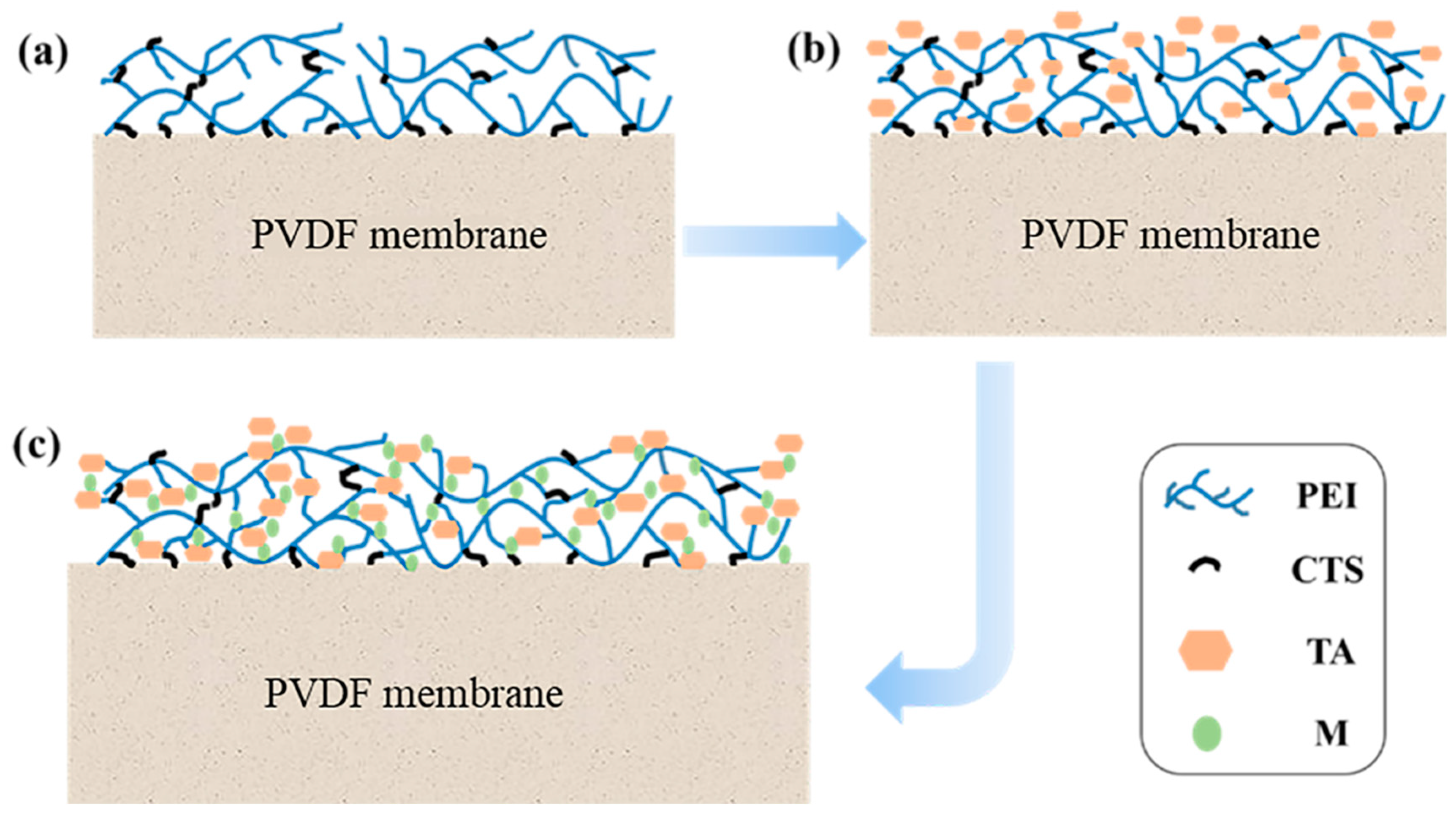
2.2. Preparation of TA/PEI/M Modified PVDF MF Membranes
2.3. Physicochemical Properties of Modified PVDF MF Membranes
2.4. Hydrophilicity and Oleophobicity of Modified PVDF MF Membranes
2.5. Hydrophilic Stability of Modified PVDF MF Membranes
3. Results and Discussion
3.1. Surface Characterization of the Chemical Elements
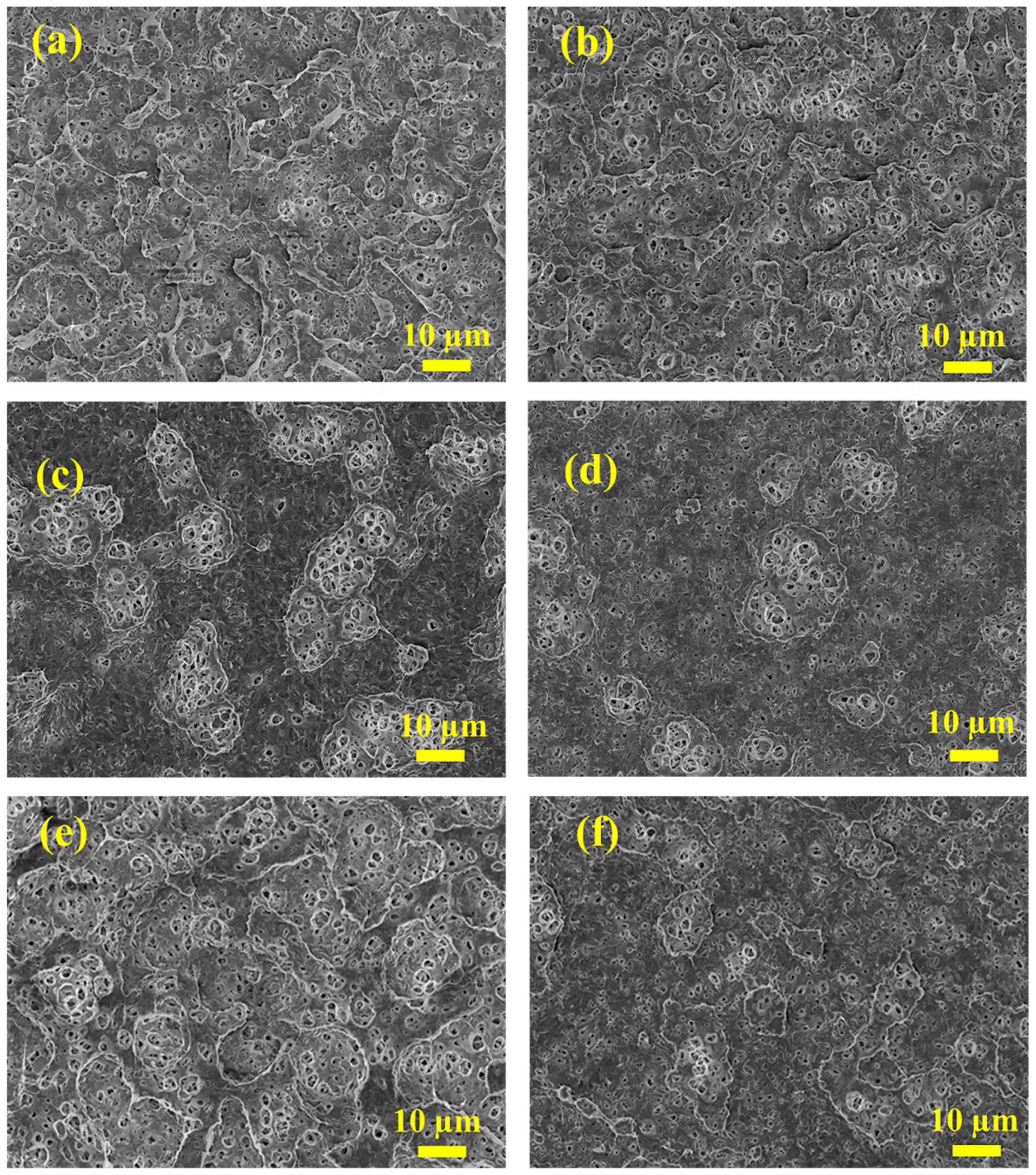
3.2. Surface Morphologies of the TA/PEI/M Modified Membranes
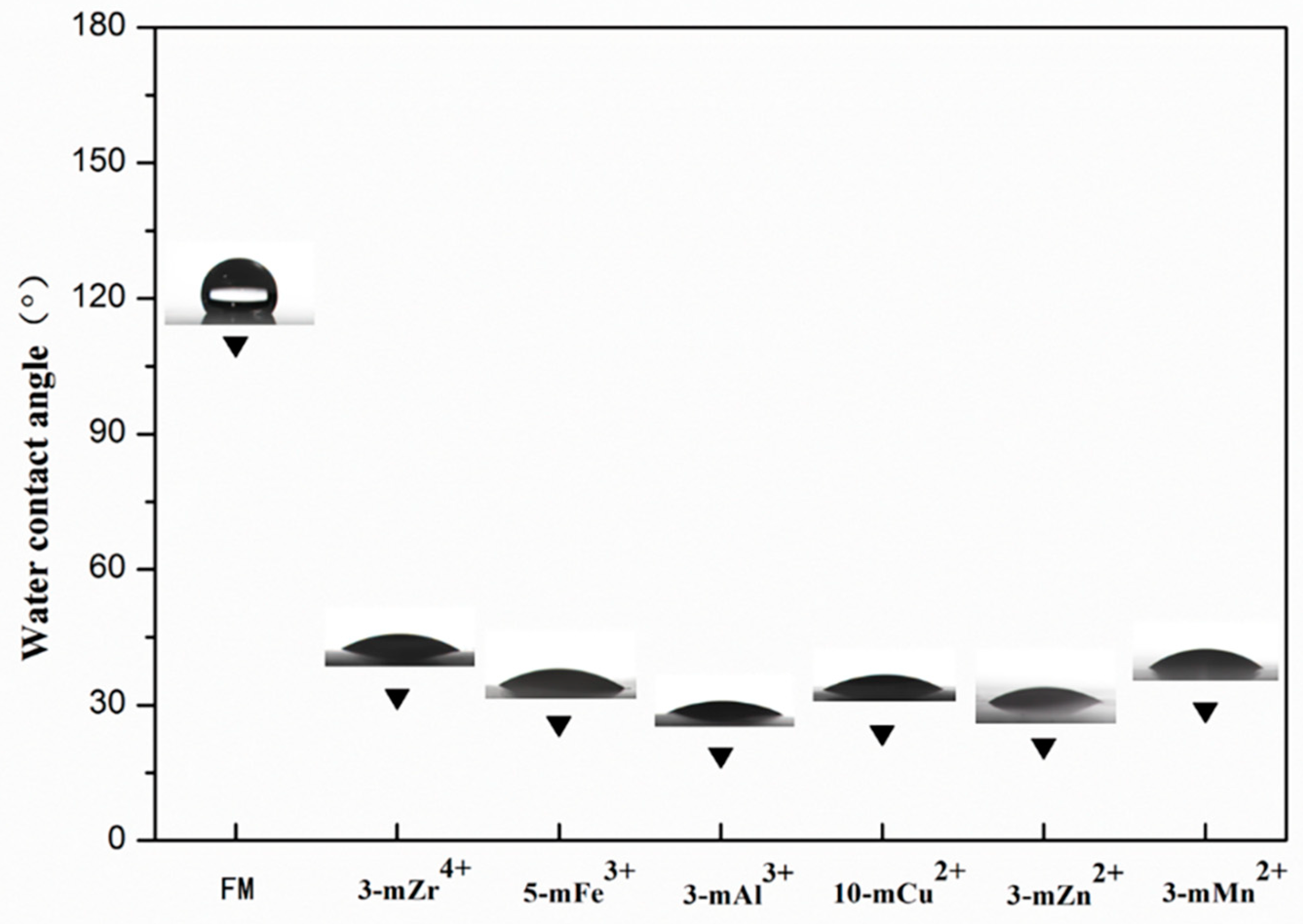
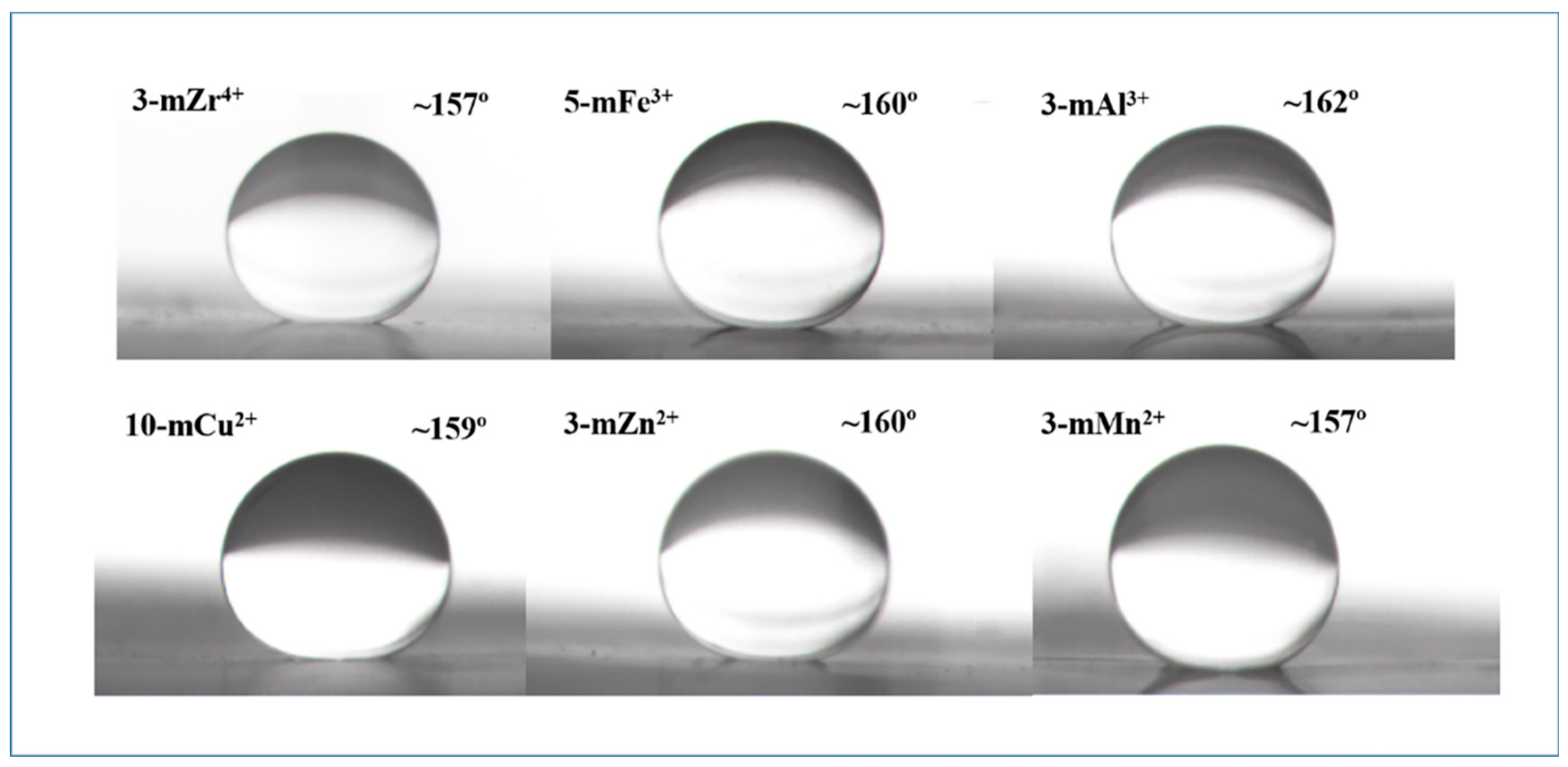
3.3. Hydrophilicity of the TA/PEI/M Modified Membranes
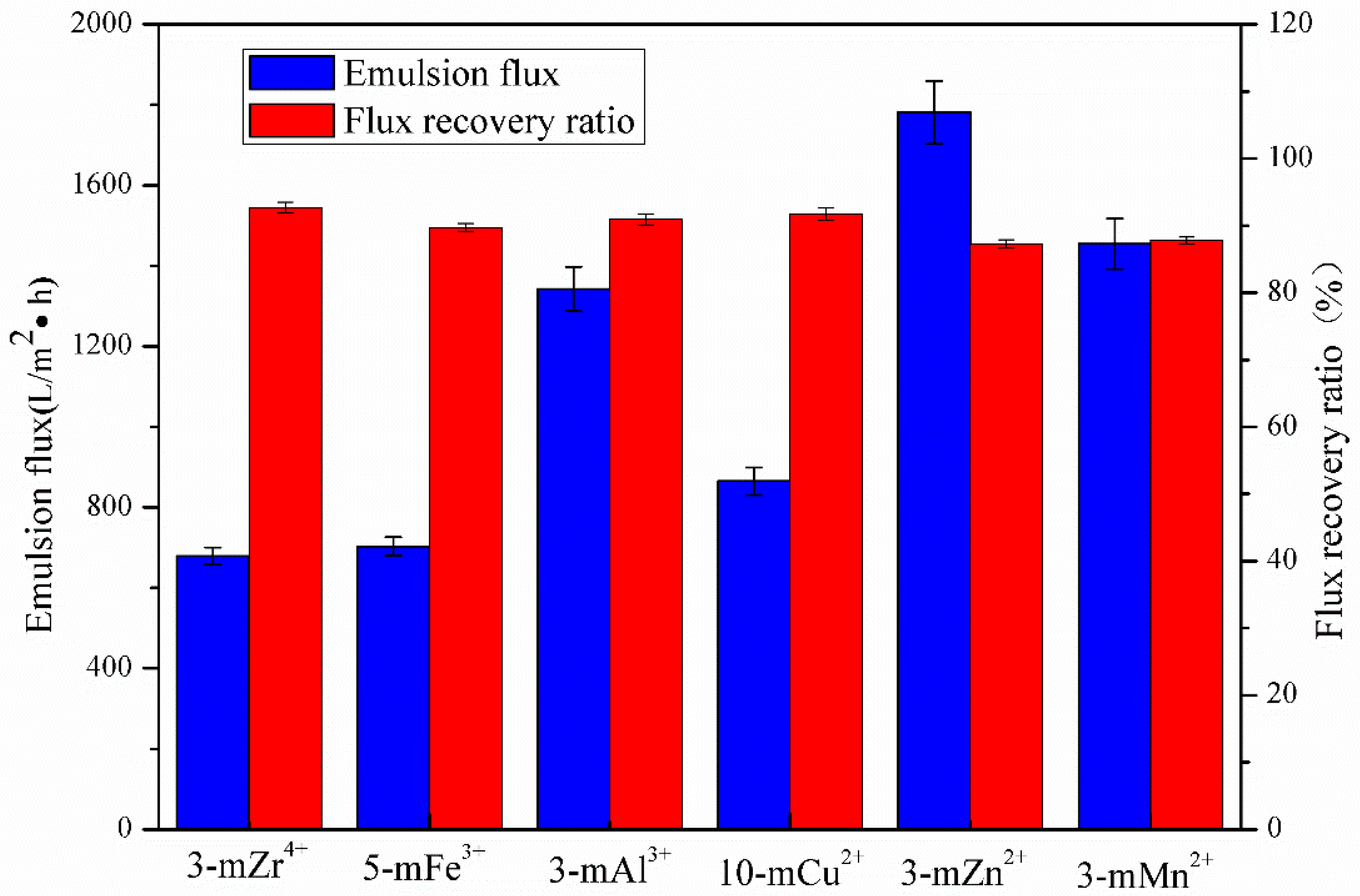
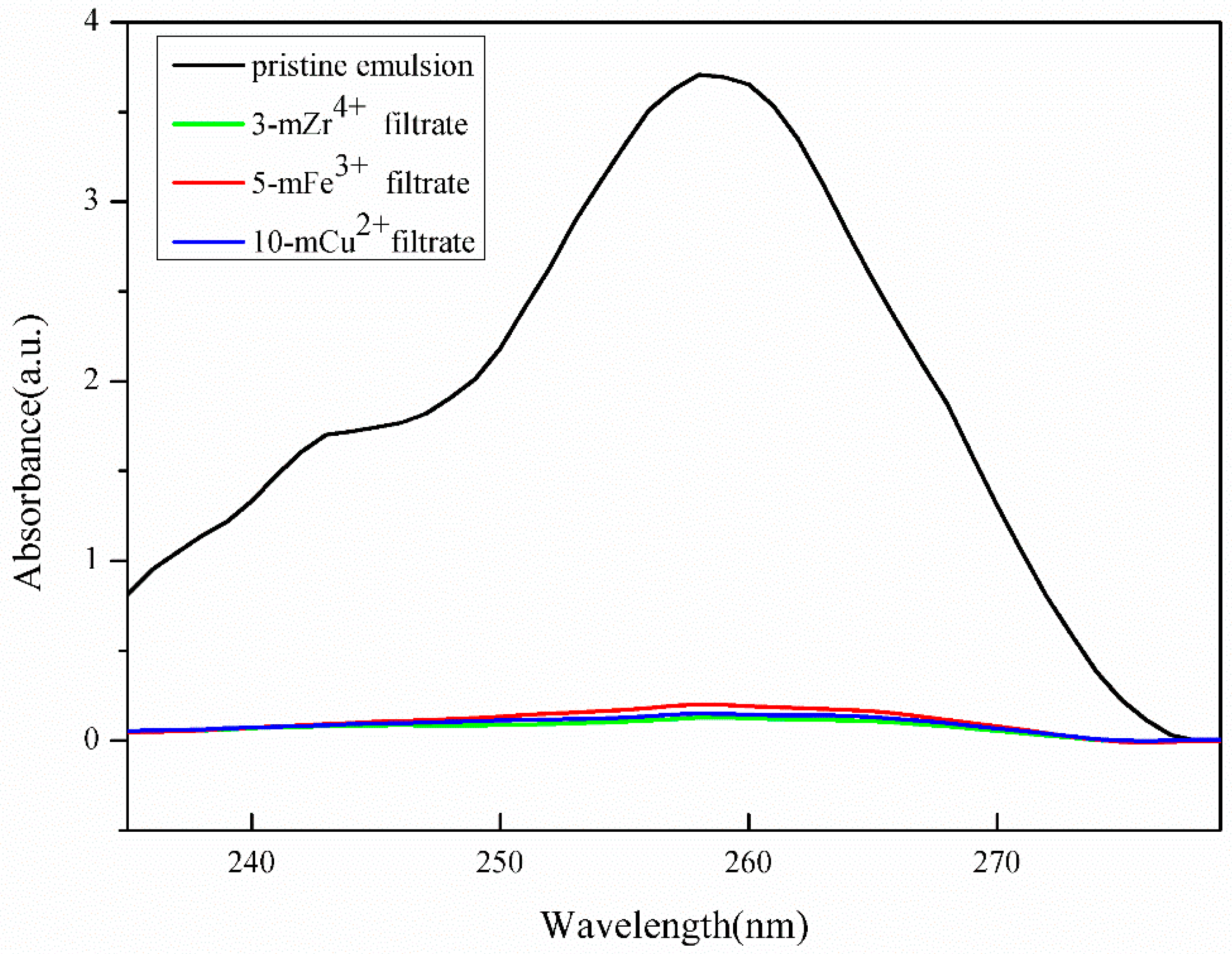
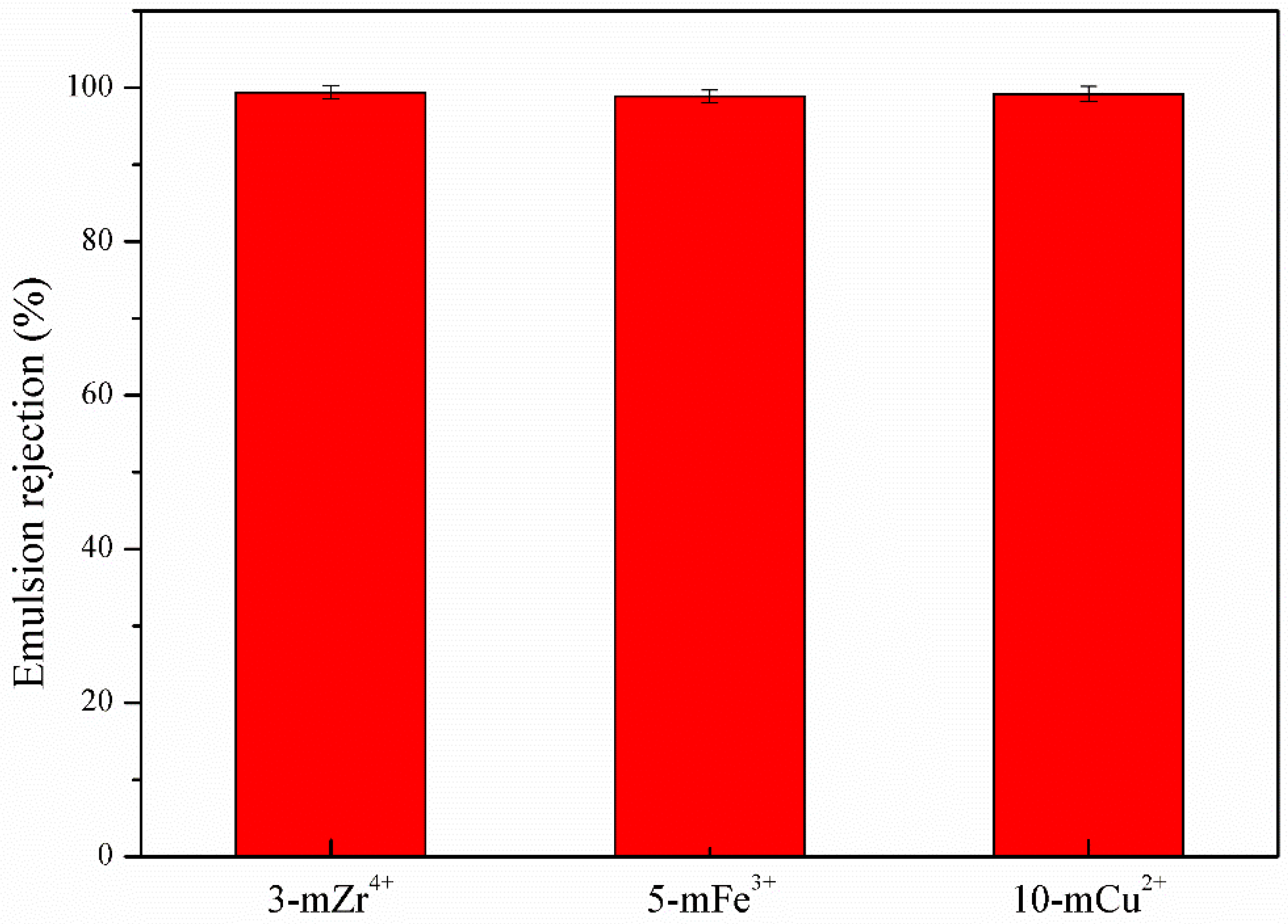
3.4. Anti-Fouling Performance of TA/PEI/M Modified Membranes
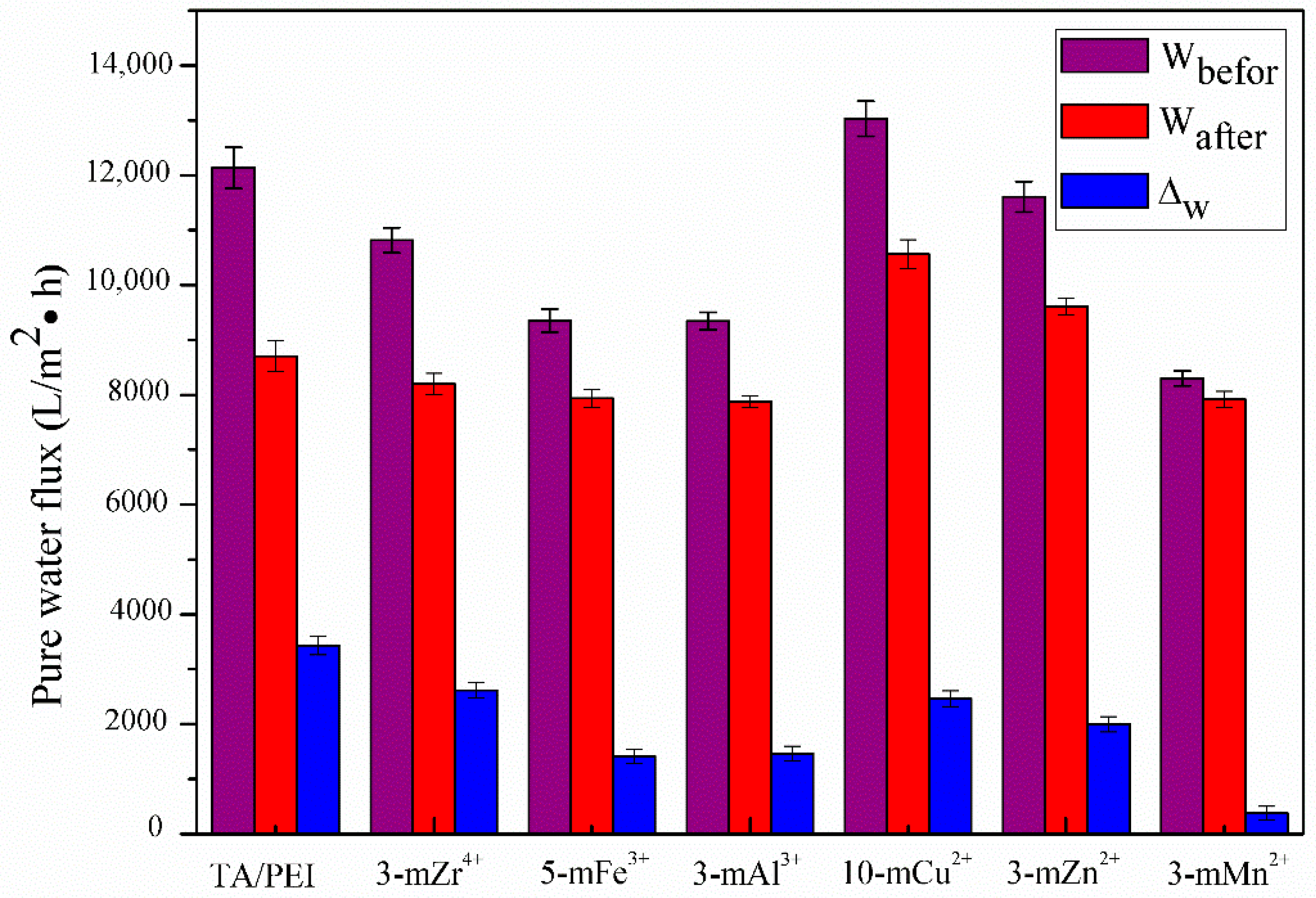
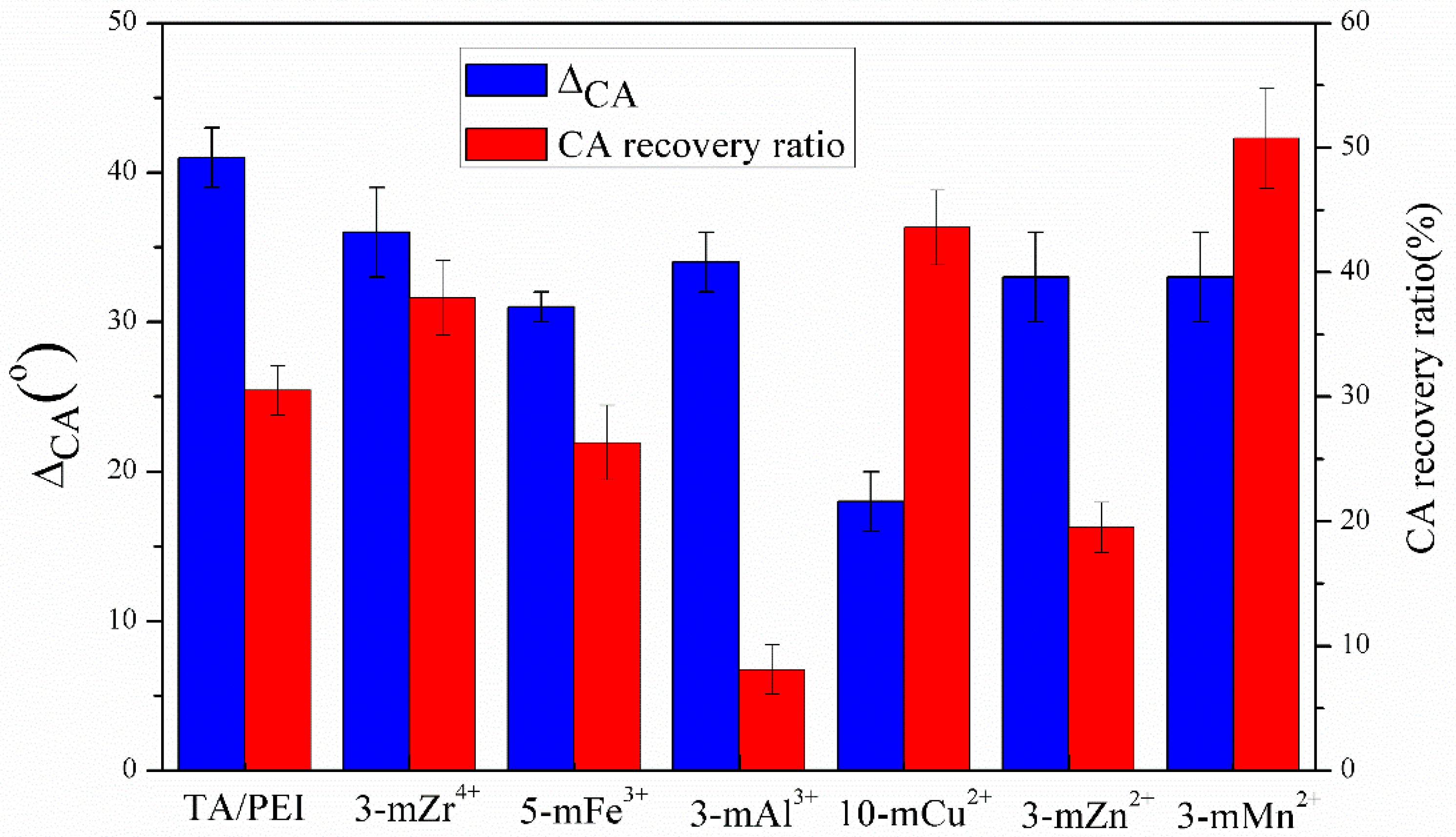
3.5. Stability of TA/PEI/M Modified Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhao, X.; Zhang, R.; Liu, Y.; He, M.; Su, Y.; Gao, C.; Jiang, Z. Antifouling membrane surface construction: Chemistry plays a critical role. J. Membr. Sci. 2018, 551, 145–171. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Escobar, I.C. Microfiltration and ultrafiltration membrane science and technology. J. Appl. Polym. Sci. 2015, 132, 1. [Google Scholar] [CrossRef]
- Yan, L.; Wang, J. Development of new polymer membrane-PVB/PVD blended membrane. Desalination 2011, 281, 455–461. [Google Scholar] [CrossRef]
- Lin, X.-C.; An, S. Preparation and properties of PVDF/PVA hollow fiber membranes. Desalination 2010, 250, 530–537. [Google Scholar]
- Jiang, J.-H.; Zhu, L.-P.; Zhang, H.-T.; Zhu, B.-K.; Xu, Y.-Y. Improved hydrodynamic permeability and antifouling properties of poly(vinylidene fluoride) membranes using polydopamine nanoparticles as additives. J. Membr. Sci. 2014, 457, 73–81. [Google Scholar] [CrossRef]
- Qin, A.-W.; Li, X.; Zhao, X.-Z.; Liu, D.-P.; He, C.-J. Engineering a highly hydrophilic PVDF membrane via binding TiO2 nanoparticles and a PVA layer onto a membrane surface. ACS Appl. Mater. Interfaces 2015, 7, 8427–8436. [Google Scholar] [CrossRef]
- Zhao, X.-Z.; Xuan, H.-X.; Chen, Y.-L.; He, C.-J. Preparation and characterization of superior antifouling PVDF membrane with extremely ordered and hydrophilic surface layer. J. Membr. Sci. 2015, 494, 48–56. [Google Scholar] [CrossRef]
- Liu, J.; Shen, X.; Zhao, Y.-P.; Chen, L. Acryloyl morpholine-grafted PVDF membrane with improved protein fouling resistance. Ind. Eng. Chem. Res. 2013, 52, 18392–18400. [Google Scholar] [CrossRef]
- Yang, X.-X.; Deng, B.; Liu, Z.-Y.; Shi, L.-Q.; Bian, X.-K.; Yu, M. Microfiltration membranes prepare from acryl amide grafted poly(vinylidene fluoride) powder and their pH sensitive behaviour. J. Membr. Sci. 2010, 362, 298–305. [Google Scholar] [CrossRef]
- Shao, L.; Wang, Z.-X.; Zhang, Y.-L.; Jiang, Z.-X.; Liu, Y.-Y. A facile strategy to enhance PVDF ultrafiltration membrane performance via self-polymerized polydopamine followed by hydrolysis of ammonium fluotitanate. J. Membr. Sci. 2014, 461, 10–21. [Google Scholar] [CrossRef]
- Xiang, Y.-H.; Liu, F.; Xue, L.-X. Under seawater superoleophobic PVDF membrane inspired by olydopamine for efficient oil/seawater separation. J. Membr. Sci. 2015, 476, 321–329. [Google Scholar] [CrossRef]
- Shi, H.; He, Y.; Pan, Y.; Di, H.-H.; Zeng, G.-Y.; Zhang, L.; Zhang, C.-L. A modified mussel-inspired method to fabricate TiO2 decorated superhydrophilic PVDF membrane for oil/water separation. J. Membr. Sci. 2016, 506, 60–70. [Google Scholar] [CrossRef]
- Zin, G.; Wu, J.-J.; Rezzadori, K.; Petrus, J.C.C.; DiLuccio, M. Modification of hydrophobic commercial PVDF microfiltration membranes into superhydrophilic membranes by the mussel-inspired method with dopamine and polyethyleneimine. Sep. Purif. Technol. 2019, 212, 641–649. [Google Scholar] [CrossRef]
- Kangn, G.D.; Cao, Y.-M. Application and modification of poly(vinylidene fluoride)(PVDF)membranes—A review. J. Membr. Sci. 2014, 63, 145–165. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Silverman, H.G.; Roberto, F.F. Understanding Marine Mussel Adhesion. Mar. Biotechnol. 2007, 9, 661–681. [Google Scholar] [CrossRef]
- Ryu, H.J.; Messersmith, P.B.; Lee, H. Polydopamine Surface Chemistry: A Decade of Discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef]
- Zhao, C.-Q.; Zhang, G.-Q.; Xu, X.-C.; Yang, F.-L.; Yang, Y.-S. Rapidly self-assembled polydopamine coating membranes with polyhexamethylene guanidine: Formation, characterization and antifouling evaluation. Colloids Surf. A 2017, 512, 41–50. [Google Scholar] [CrossRef]
- Guo, X.-Y.; Fan, S.-G.; Hu, Y.-D.; Fu, X.-L.; Shao, H.-Q.; Zhou, Q.-X. A novel membrane biofouling mitigation strategy of D-amino acid supported by polydopamine and halloysite nanotube. J. Membr. Sci. 2019, 579, 131–140. [Google Scholar] [CrossRef]
- Li, X.-P.; Shan, H.-T.; Min, C.; Li, B.-A. Mussel-inspired modification of PTFE membranes in a miscible THF-Tris buffer mixture for oil-in-water emulsions separation. J. Membr. Sci. 2018, 555, 237–249. [Google Scholar] [CrossRef]
- Jin, J.-B.; Du, X.-L.; Yu, J.; Qin, S.-H.; He, M.; Zhang, K.-Z.; Yang, J.-K. Synthesis of Negatively Charged Polyol-Functional PSF Membranes with Good Hydrophilic and Efficient Boron Removal Properties. Polymers 2019, 11, 780. [Google Scholar] [CrossRef] [PubMed]
- Klosterman, L.; Riley, J.K.; Bettinger, C.J. Control of Heterogeneous Nucleation and Growth Kinetics of Dopamine Melanin by Altering Substrate Chemistry. Langmuir 2015, 31, 3451–3458. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, X.; Tao, L.; Li, K.; Xue, Z.; Feng, L.; Wei, Y. Mussel-inspired chemistry and Michael addition reaction for efficient oil/water separation. ACS Appl. Mater. Interfaces. 2013, 54, 4438–4442. [Google Scholar] [CrossRef]
- Jiang, Y.; Shi, K.-X.; Tang, H.; Wang, Y.-L. Enhanced wettability and wear resistance on TiO2/PDA thin films prepared by sol-gel dip coating. Surf. Coat. Technol. 2019, 375, 334–340. [Google Scholar] [CrossRef]
- Zhang, S.-F.; Wu, L.-L.; Deng, F.-R.; Zhao, D.-P.; Zhang, C.; Zhang, C.-C. Hydrophilic modification of PVDF porous membrane via a simple dip-coating method in plant tannin solution. RSC Adv. 2016, 6, 71287–72194. [Google Scholar] [CrossRef]
- Virgen-Ortíz, J.J.; Dos Santos, J.C.S.; Berenguer-Murcia, A.; Barbosa, O.; Rodrigues, R.C.; Fernandez-Lafuente, R. Polyethylenimine: A very useful ionic polymer in the design of immobilized enzyme biocatalysts. J. Mater. Chem. B 2017, 36, 7461–7490. [Google Scholar] [CrossRef]
- Vicennati, P.; Giuliano, A.; Ortaggi, G.; Masotti, A. Polyethylenimine in Medicinal Chemistry. Curr. Med. Chem. 2008, 15, 2826–2839. [Google Scholar] [CrossRef]
- Lim, M.Y.; Choi, Y.S.; Kim, J.; Kim, K.; Shin, H.; Kim, J.J.; Shin, D.M. Cross-linked graphene oxide membrane having high ion selectivity and antibacterial activity prepared using tannic acid functionalized graphene oxide and polyethyleneimine. J. Membr. Sci. 2017, 521, 1–9. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, S.-J.; Du, Y.; Yang, H.-C.; Xu, Z.-K. Co-deposition Kinetics of Polydopamine/Polyethyleneimine Coatings: Effects of Solution Composition and Substrate Surface. Langmuir 2018, 34, 13123–13131. [Google Scholar] [CrossRef]
- Bacelo, H.A.M.; Santos, S.C.; Botelho, R.C.M.S. Tannin-based biosorbents for environmental applications—A review. Chem. Eng. J. 2016, 303, 575–587. [Google Scholar] [CrossRef]
- Guo, J.-L.; Ping, Y.; Ejima, H.; Alt, K.; Meissner, M.; Richardson, J.J.; Yan, Y. Engineering Multifunctional Capsules through the Assembly of Metal–Phenolic Networks. Angew. Chem. Int. Ed. 2014, 53, 1–7. [Google Scholar]
- You, F.; Xu, Y.; Yang, X.; Zhang, Y.; Shao, L. Bio-inspired Ni2+-polyphenol hydrophilic network to achieve unconventional high-flux nanofiltration membranes for environmental remediation. Chem. Commun. 2017, 53, 6128–6131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, G.; Wang, M.; Wang, D.-F.; Cai, D.-Q.; Wu, Z.-Y. Efficient removal of hexavalent chromium from water and soil using magnetic ceramsite coated by functionalized nano carbon spheres. Chem. Eng. J. 2018, 334, 400–409. [Google Scholar] [CrossRef]
- Cui, T.-C. The research situation of silane coupling agent. Peak Data Sci. 2017, 4, 214–217. [Google Scholar]
- Ying Kei Lung, C.; Matinlinna, J.P. Aspects of silane coupling agents and surface conditioning in dentistry: An overview. Dent. Mater. 2017, 28, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q. Application of silane coupling agents in modified organic anti–corrosive coatings. Guangzhou Chem. Ind. 2017, 45, 16–18. [Google Scholar]
- Yang, Y.-F.; Wan, L.; Xu, Z.-K. Surface hydrophilization for polypropylene microporous membranes: A facile interfacial crosslinking approach. J. Membr. Sci. 2009, 326, 372–381. [Google Scholar] [CrossRef]
- Liu, C.; Wu, L.-L.; Zhang, C.-C.; Chen, W.-Y.; Luo, S. Surface hydrophilic modification of PVDF membranes by trace amounts of tannin and polyethyleneimine. Appl. Surf. Sci. 2018, 457, 695–704. [Google Scholar] [CrossRef]
- Li, M.; Wu, L.-L.; Zhang, C.-C.; Chen, W.-Y.; Liu, C. Hydrophilic and antifouling modification of PVDF membranes by one-step assembly of tannic acid and polyvinylpyrrolidone. Appl. Surf. Sci. 2019, 483, 967–978. [Google Scholar] [CrossRef]
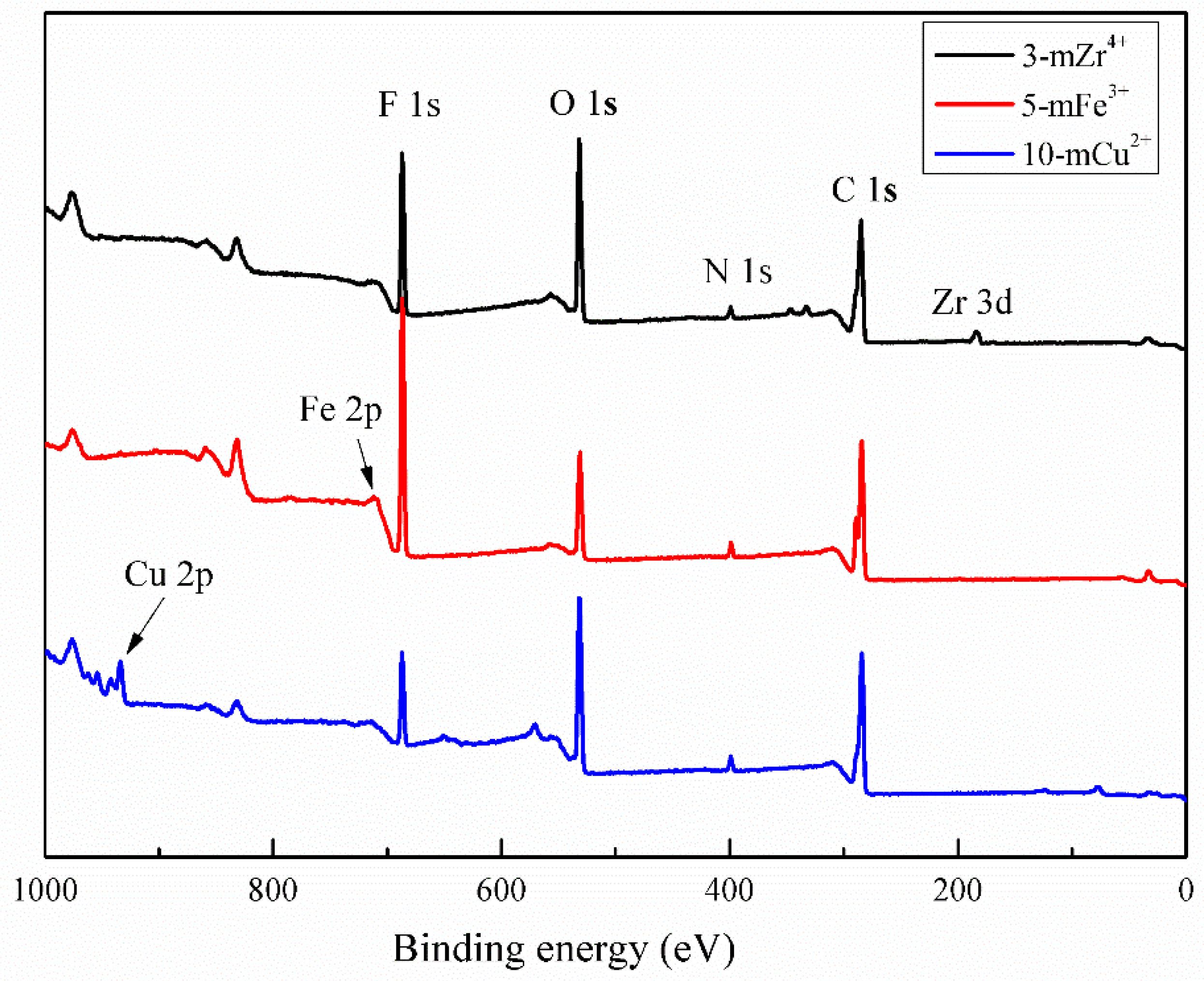
| Modified Membranes | Composition (at.%) | ||||||
|---|---|---|---|---|---|---|---|
| C | O | N | Zr | Fe | Cu | O/N | |
| 3-mZr4+ | 68.11 | 28.19 | 3.04 | 0.65 | 0 | 0 | 9.27 |
| 5-mFe3+ | 75.78 | 18.37 | 3.42 | 0 | 2.43 | 0 | 5.37 |
| 10-mCu2+ | 66.06 | 27.61 | 3.68 | 0 | 0 | 2.53 | 7.50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Lin, Q.; Liu, C.; Chen, W. A Stable Anti-Fouling Coating on PVDF Membrane Constructed of Polyphenol Tannic Acid, Polyethyleneimine and Metal Ion. Polymers 2019, 11, 1975. https://doi.org/10.3390/polym11121975
Wu L, Lin Q, Liu C, Chen W. A Stable Anti-Fouling Coating on PVDF Membrane Constructed of Polyphenol Tannic Acid, Polyethyleneimine and Metal Ion. Polymers. 2019; 11(12):1975. https://doi.org/10.3390/polym11121975
Chicago/Turabian StyleWu, Lili, Qiuhu Lin, Cong Liu, and Wanyu Chen. 2019. "A Stable Anti-Fouling Coating on PVDF Membrane Constructed of Polyphenol Tannic Acid, Polyethyleneimine and Metal Ion" Polymers 11, no. 12: 1975. https://doi.org/10.3390/polym11121975
APA StyleWu, L., Lin, Q., Liu, C., & Chen, W. (2019). A Stable Anti-Fouling Coating on PVDF Membrane Constructed of Polyphenol Tannic Acid, Polyethyleneimine and Metal Ion. Polymers, 11(12), 1975. https://doi.org/10.3390/polym11121975