Gelatin Type A from Porcine Skin Used as Co-Initiator in a Radical Photo-Initiating System
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Precursor Formulations and Preparation of Hydrogels
2.3. Photorheology
2.4. Spectroscopic Characterization
2.5. Dynamic Mechanical Thermal Analysis
2.6. Swelling Degree and Gel Content
3. Results and Discussion
3.1. Photopolymerization Kinetics
3.2. Incorporation of Gelatin Within the Cross-Linked Network
3.3. Characterization of the Hydrogel
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Caló, E.; Vitaliy, V.; Khutoryanskiy, V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar]
- Nguyen, K.T.; West, J.L. Photopolymerizable hydorgels for tissue engineering applications. Biomaterials 2002, 23, 4307–4314. [Google Scholar] [CrossRef]
- Cavallo, A.; Madaghiele, M.; Masullo, U.; Lionetto, M.G.; Sannino, A. Photo-crosslinked poly(ethylene glycol) diacrylate (PEGDA) hydrogels from low molecular weight prepolymer: Swelling and permeation studies. J. Appl. Polym. Sci. 2017, 13, 44380. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Hubbell, J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol. 2005, 23, 47. [Google Scholar] [CrossRef] [PubMed]
- Ramires, P.A.; Miccoli, M.A.; Panzarini, E.; Dini, L.; Protopapa, C. In vitro and in vivo biocompatibility evaluation of a polyalkylimide hydrogel for soft tissue augmentation. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 72, 230. [Google Scholar] [CrossRef] [PubMed]
- Bryant, S.J.; Anseth, K.S. Controlling the spatial distribution of ECM components in degradable PEG hydrogels for tissue engineering cartilage. J. Biomed. Mater. Res. A 2003, 64, 70–79. [Google Scholar] [CrossRef]
- Zhu, J. Bioactive modification of poly (ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef]
- Beamish, J.A.; Zhu, J.; Kottke-Marchant, K.; Marchant, R.E. The effects of monoacrylated poly (ethylene glycol) on the properties of poly (ethylene glycol) diacrylate hydrogels used for tissue engineering. J. Biomed. Mater. Res. A 2010, 92, 441–450. [Google Scholar] [CrossRef]
- Malda, J.; Visser, J.; Melchels, F.P.; Jüngst, T.; Hennink, W.E.; Dhert, W.J.A.; Groll, J.; Hutmacher, D.W. 25th anniversary article: Engineering hydrogels for biofabrication. Adv. Mater. 2013, 25, 5011–5028. [Google Scholar] [CrossRef]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Jaipan, P.; Nguyen, A.; Narayan, R.J. Gelatin-based hydrogels for biomedical applications. MRS Commun. 2017, 7, 416–426. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Deiwick, A.; Vlierberghe, S.V.; Dubruel, P.; Moller, L.; Drager, G.; Chichkov, B. Laser fabrication of three-dimensional CAD scaffolds from photosensitive gelatin for applications in tissue engineering. Biomacromolecules 2011, 12, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-based hydrogels for organ 3D bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Yates, K.; Vogt, C.; Qian, Z.; Frost, M.C.; Zhao, F. Increasing mechanical strength of gelatin hydrogels by divalent metal ion removal. Sci. Rep. 2014, 4, 4706. [Google Scholar] [CrossRef]
- Lee, S.B.; Jeon, H.W.; Lee, Y.W.; Lee, Y.M.; Song, K.W.; Park, M.H.; Nam, Y.S.; Ahn, H.C. Bio-artificial skin composed of gelatin and (1→ 3),(1→ 6)-β-glucan. Biomaterials 2003, 24, 2503–2511. [Google Scholar] [CrossRef]
- Zheng, Y.; Liang, Y.; Zhang, D.; Sun, X.; Liang, L.; Li, J.; Liu, Y.N. Gelatin-based hydrogels blended with gellan as an injectable wound dressing. ACS Omega 2018, 3, 4766–4775. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef]
- Kuijpers, A.J.; Engbers, G.H.; Krijgsveld, J.; Zaat, S.A.; Dankert, J.; Feijen, J. Cross-linking and characterisation of gelatin matrices for biomedical applications. J. Biomater. Sci. Polym. 2000, 11, 225–243. [Google Scholar] [CrossRef]
- Singh, S.; Rajkumar, B.; Gupta, V.; Bhatt, A. Current photo-initiators in dental materials. Int. J. Appl. Dent. Sci. 2017, 3, 17–20. [Google Scholar]
- Alvim, H.H.; Alecio, A.C.; Vasconcellos, W.A.; Furlan, M.; Oliveira, J.E.; Saad, J.R. Analysis of camphorquinone in composite resins as a function of shade. Dent. Mater. 2007, 23, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Cook, W.D. Photopolymerization kinetics of dimethacrylates using the camphorquinone/amine initiator system. Polymer 1992, 33, 600–609. [Google Scholar] [CrossRef]
- Jakubiak, J.; Wrzyszczynski, A.; Linden, L.A.; Rebak, L.J.F. The role of amines in the camphorquinone photoinitiated polymerization of multifunctional monomer. J. Macromol. Sci. Part A Pure Appl. Chem. 2007, 44, 239–242. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Menzel, H. Crosslinking behavior of dextran modified with hydroxyethyl methacrylate upon irradiation with visible light—Effect of concentration, coinitiator type, and solvent. J. Appl. Polym. Sci. 2010, 117, 3128–3138. [Google Scholar] [CrossRef]
- Evans, T.R.; Leermakers, P.A. Emission spectra and excited-state geometry of. alpha.-diketones. J. Am. Chem. Soc. 1967, 89, 4380. [Google Scholar] [CrossRef]
- Luk, C.K.; Richardson, F.S. Circularly polarized luminescence spectrum of camphorquinone. J. Am. Chem. Soc. 1974, 96, 2006. [Google Scholar] [CrossRef]
- Jakubiak, J.; Allonas, X.; Fouassier, J.P.; Sionkowska, A.; Andrzejewska, E.; Linden, L.A.; Rabek, J.F. Camphorquinone–amines photoinitating systems for the initiation of free radical polymerization. Polymer 2003, 44, 5219–5226. [Google Scholar] [CrossRef]
- Stansbury, J.W. Curing dental resins and composites by photopolymerization. J. Esthet. Dent. 2000, 12, 300–308. [Google Scholar] [CrossRef]
- Laleveè, J.; Fouassier, J.P. Photopolymerisation Initiating System; Royal Society of Chemistry: London, UK, 2018. [Google Scholar]
- Hito, Y. Photochemistry for Biomedical Applications. From Device Fabrication to Diagnosis and Therapy; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Kamoun, E.A.; Winkel, A.; Eisenburger, M.; Menzel, H. Carboxylated camphorquinone as visible-light photoinitiator for biomedical application: Synthesis, characterization, and application. Arab. J. Chem. 2016, 9, 745–754. [Google Scholar] [CrossRef]
- Xing, Q.; Yates, K.; Bailey, A.; Vogt, C.; He, W.; Frost, M.C.; Zhao, F. Effects of local nitric oxide release on human mesenchymal stem cell attachment and proliferation on gelatin hydrogel surface. Surf. Innov. 2013, 1, 224–232. [Google Scholar] [CrossRef]
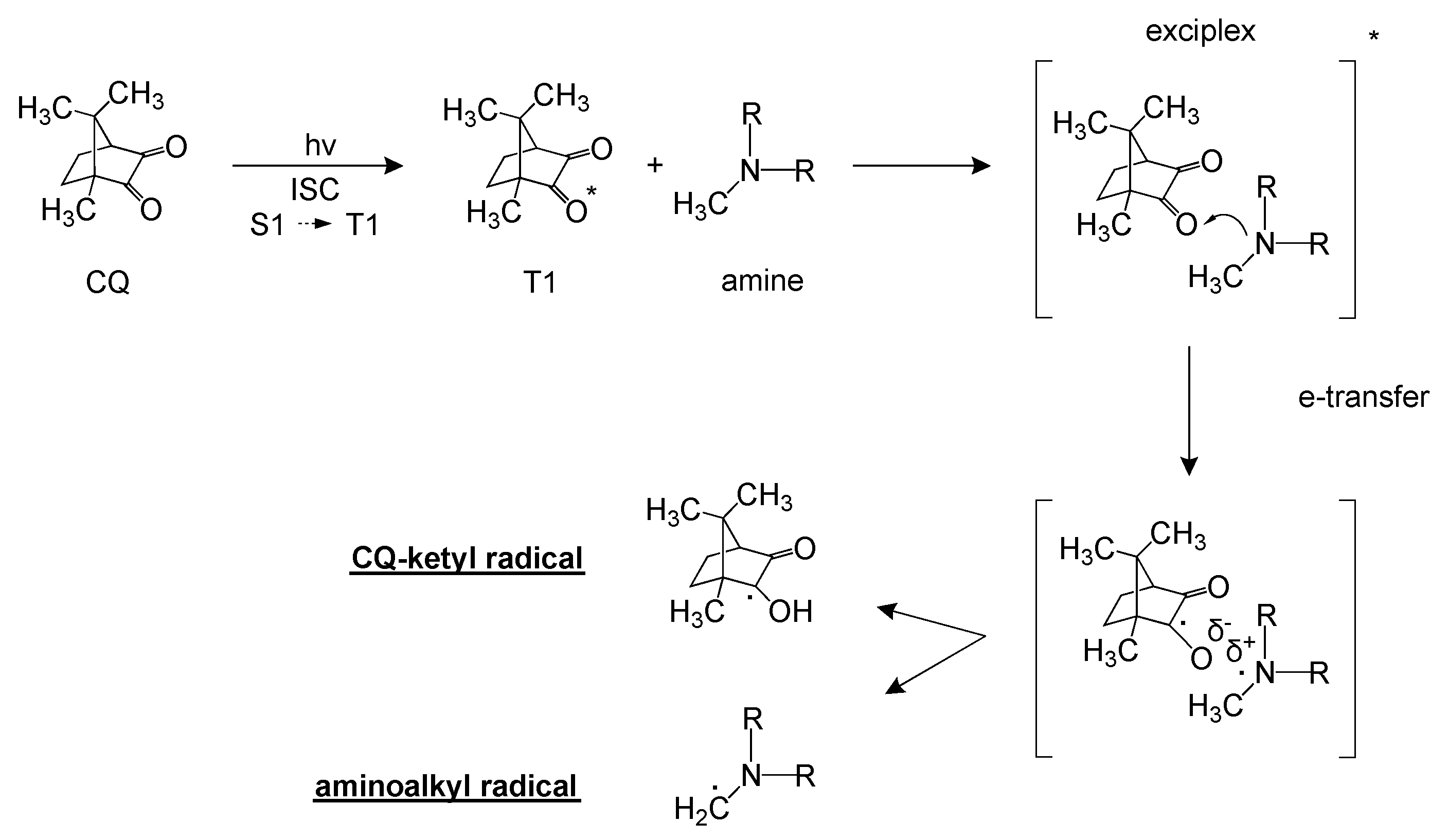
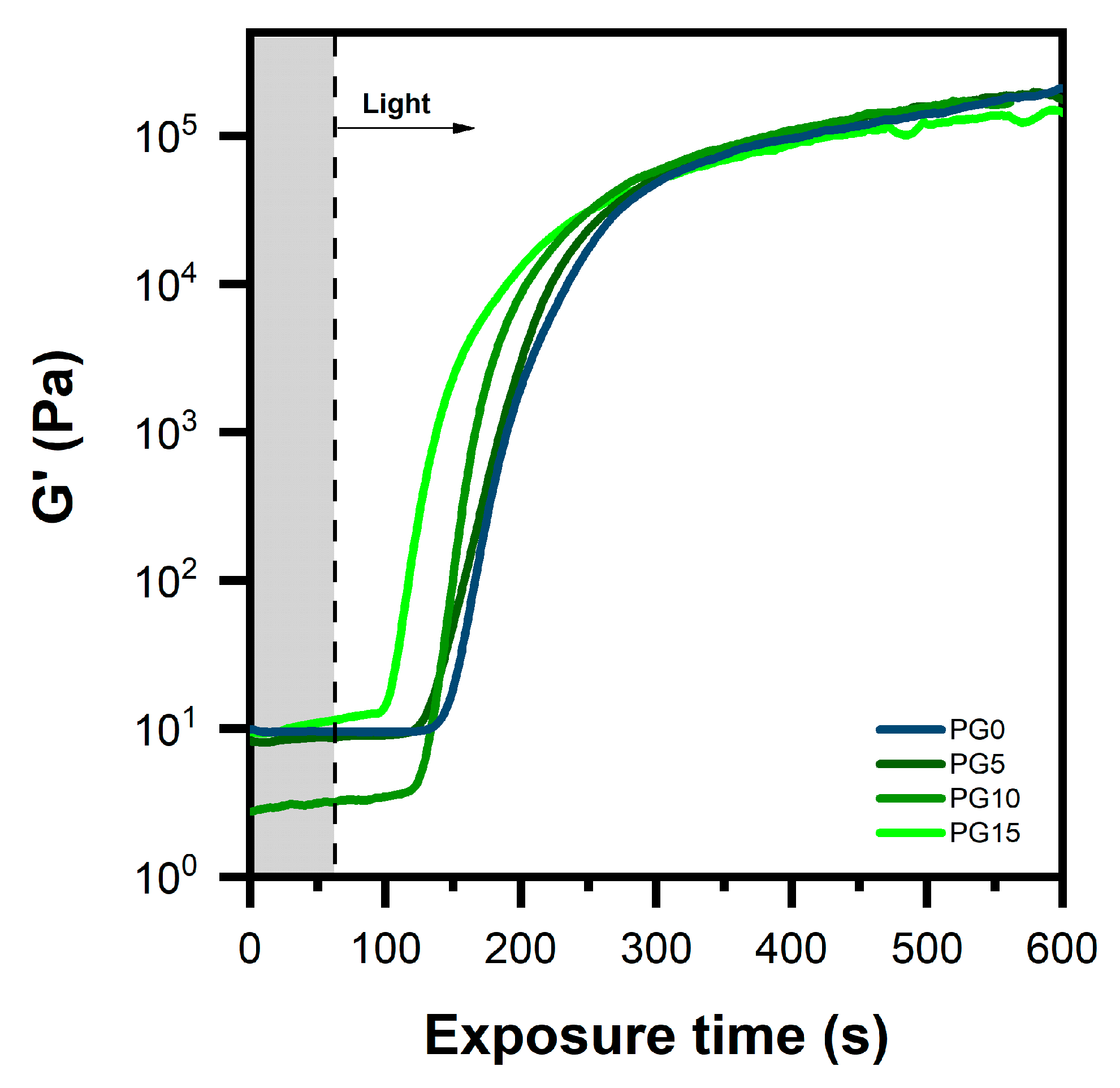


| Formulation | PEGDA 700 (wt%) | Gelatin Type A a (wt%) | CQa (wt%) |
|---|---|---|---|
| PG0 | 35 | 0 | 2 |
| PG5 | 35 | 5 | 2 |
| PG10 | 35 | 10 | 2 |
| PG15 | 35 | 15 | 2 |
| Formulation | Tg (°C) | Swelling Ratio (%) | Gel Content [%] |
|---|---|---|---|
| PG0 | −42.2 | 82 ± 9 | 90 ± 1.6 |
| PG5 | −37.5 | 60 ± 2 | 96 ± 0.6 |
| PG10 | −36.7 | 74 ± 8 | 95 ± 0.4 |
| PG15 | −35.6 | 69 ± 1 | 96 ± 0.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosola, A.; Chiappone, A.; Martinengo, C.; Grützmacher, H.; Sangermano, M. Gelatin Type A from Porcine Skin Used as Co-Initiator in a Radical Photo-Initiating System. Polymers 2019, 11, 1901. https://doi.org/10.3390/polym11111901
Cosola A, Chiappone A, Martinengo C, Grützmacher H, Sangermano M. Gelatin Type A from Porcine Skin Used as Co-Initiator in a Radical Photo-Initiating System. Polymers. 2019; 11(11):1901. https://doi.org/10.3390/polym11111901
Chicago/Turabian StyleCosola, Andrea, Annalisa Chiappone, Cinzia Martinengo, Hansjörg Grützmacher, and Marco Sangermano. 2019. "Gelatin Type A from Porcine Skin Used as Co-Initiator in a Radical Photo-Initiating System" Polymers 11, no. 11: 1901. https://doi.org/10.3390/polym11111901
APA StyleCosola, A., Chiappone, A., Martinengo, C., Grützmacher, H., & Sangermano, M. (2019). Gelatin Type A from Porcine Skin Used as Co-Initiator in a Radical Photo-Initiating System. Polymers, 11(11), 1901. https://doi.org/10.3390/polym11111901








