Amphiphilic Block Copolymer Micelles in Selective Solvents: The Effect of Solvent Selectivity on Micelle Formation
Abstract
:1. Introduction
2. Materials and Methods
3. Results
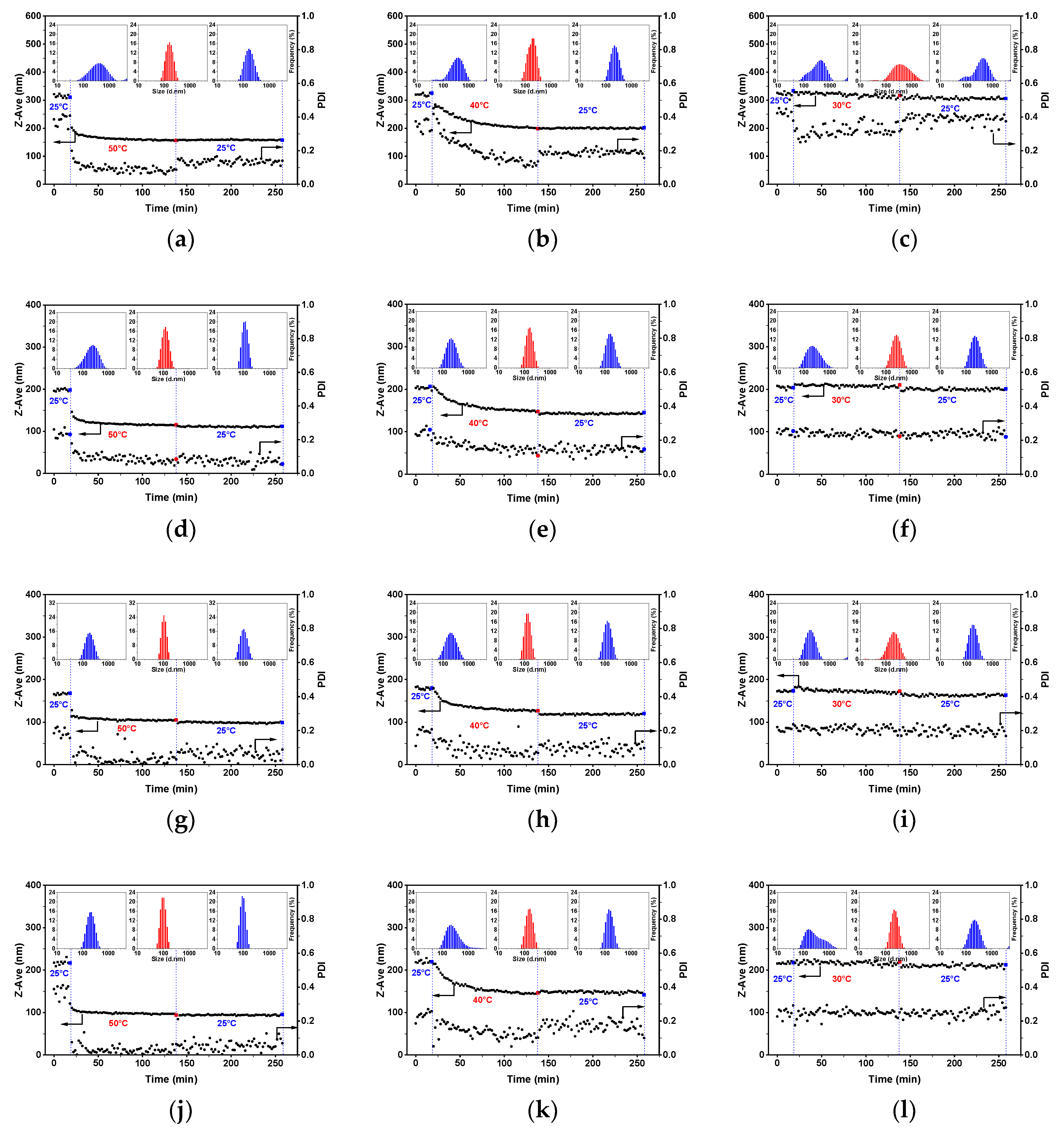
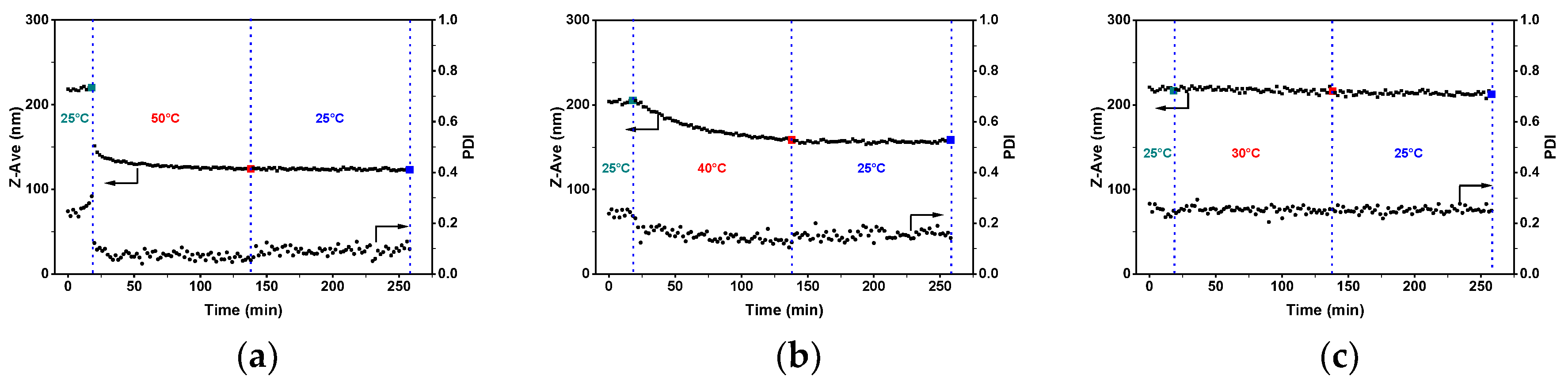
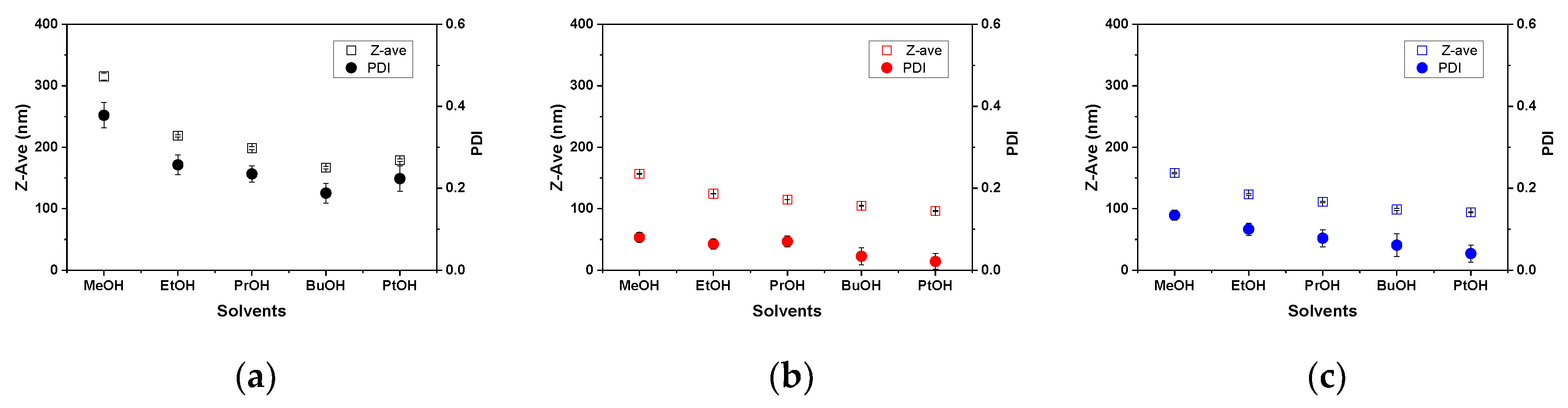
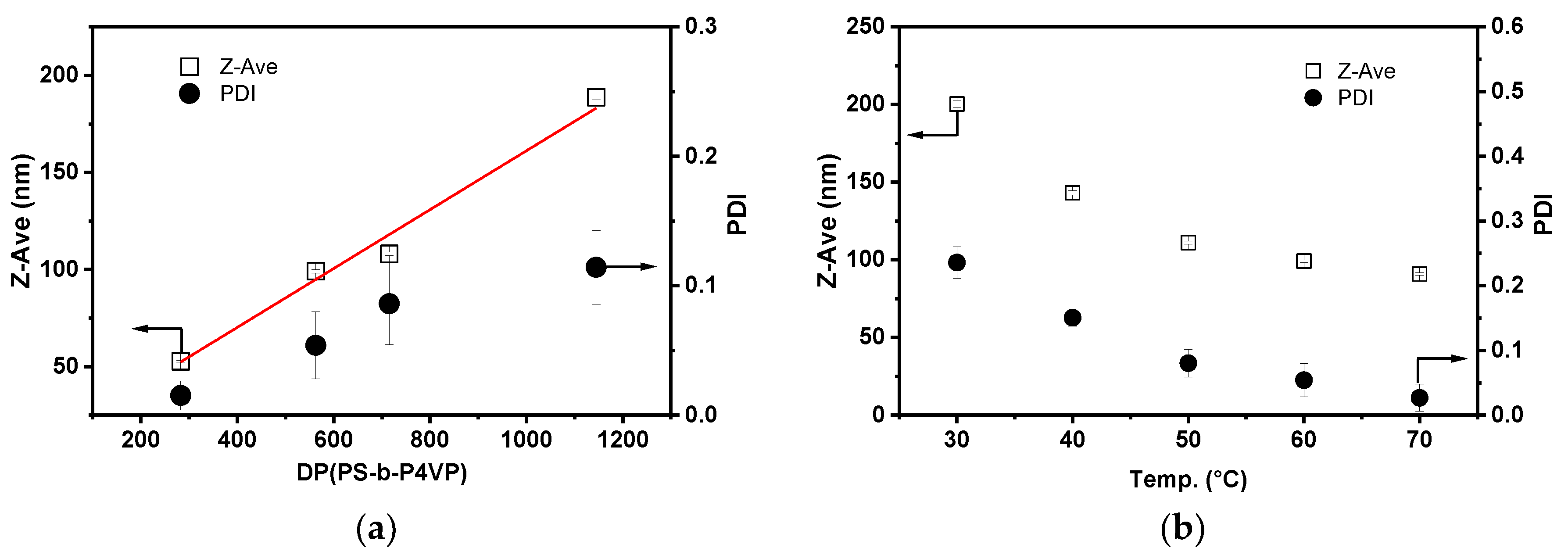
3.1. Micellization of PS-b-P4VP in Alcohol Solutions
3.2. Morphology of PS-b-P4VP(59) Aggregates in Different Alcohols
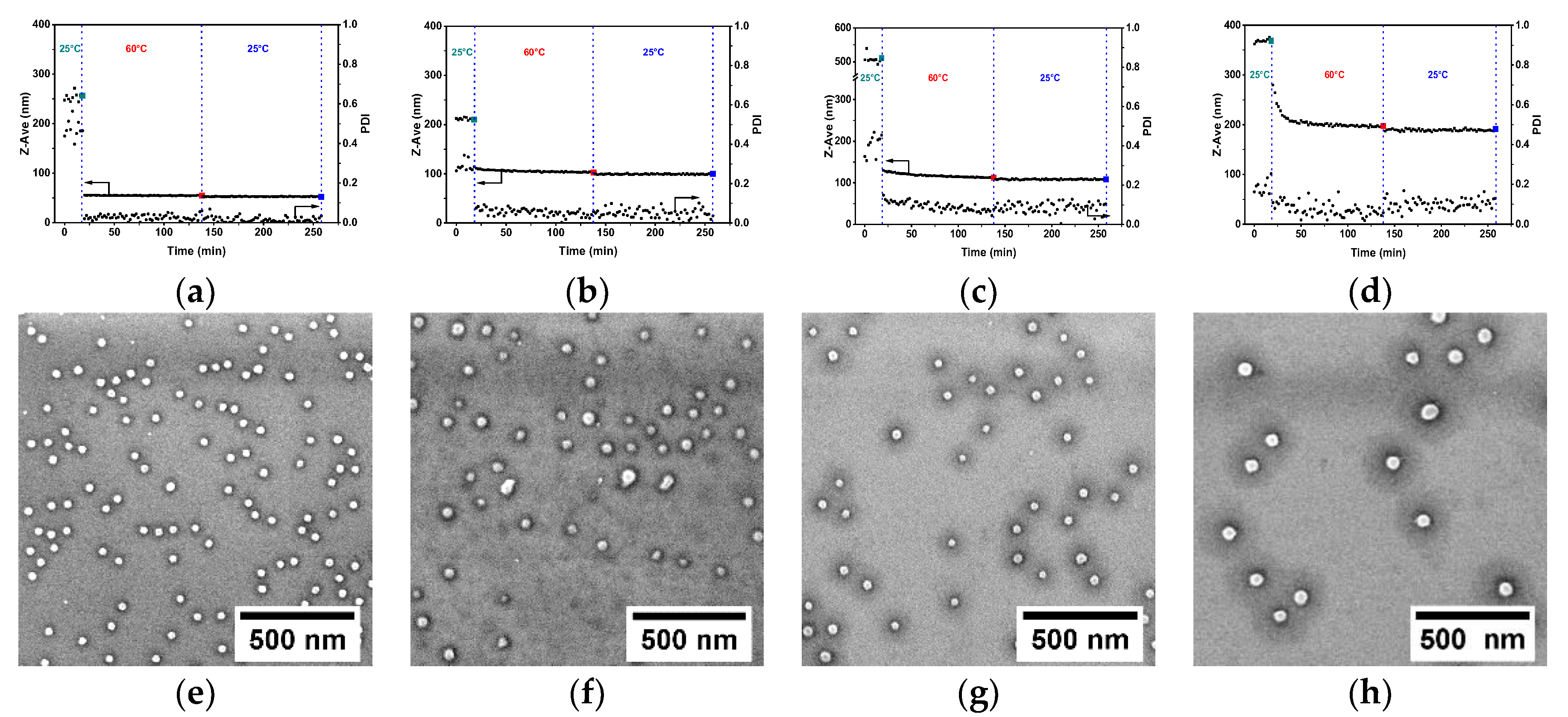
3.3. The Effect of PS-b-P4VP Molecular Weight
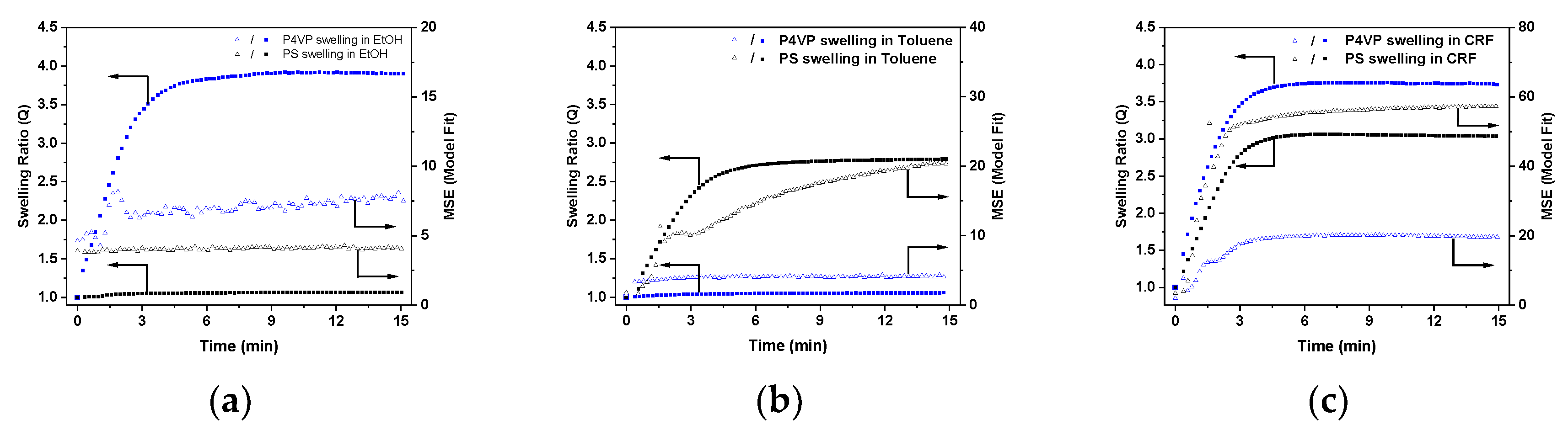
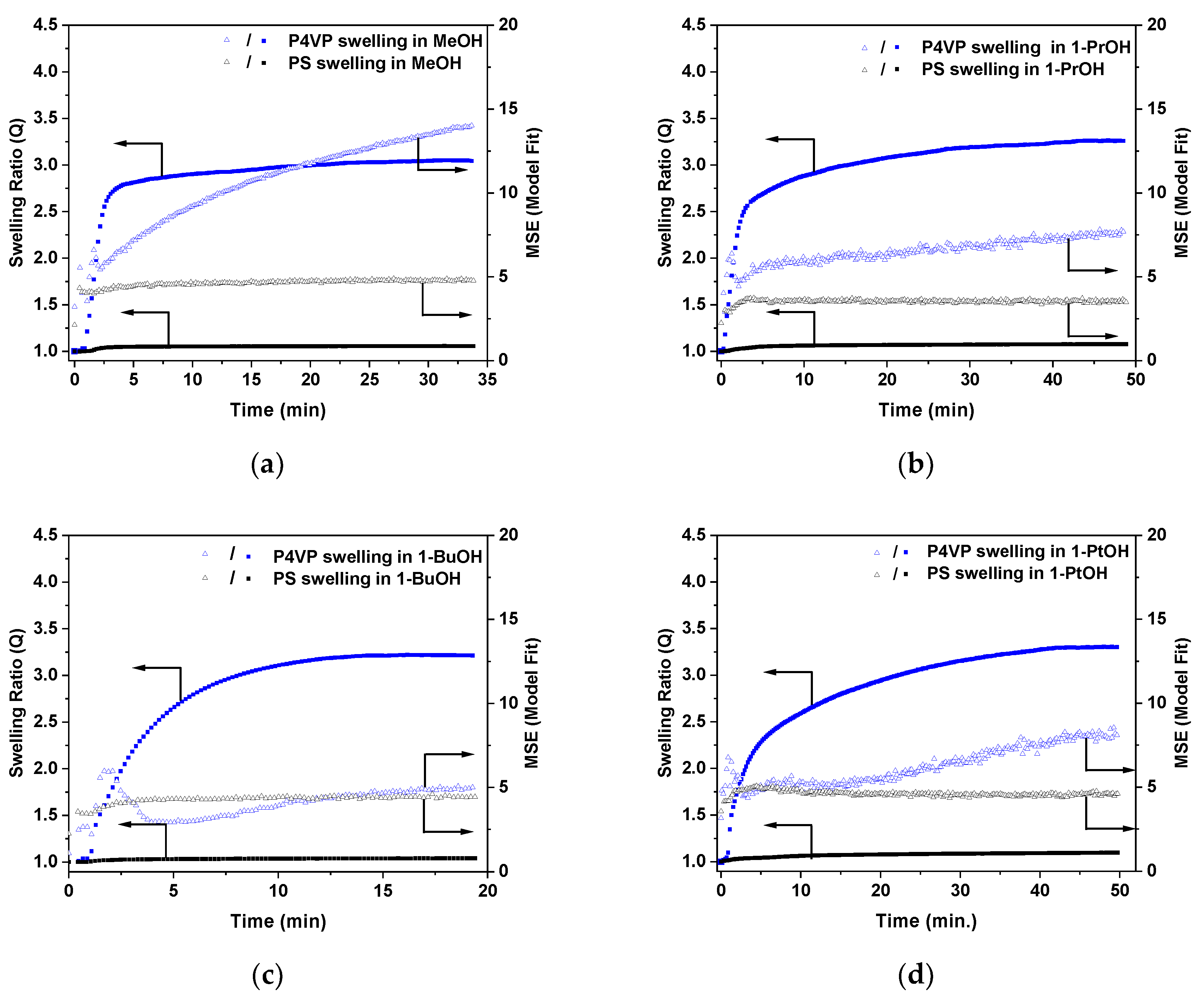
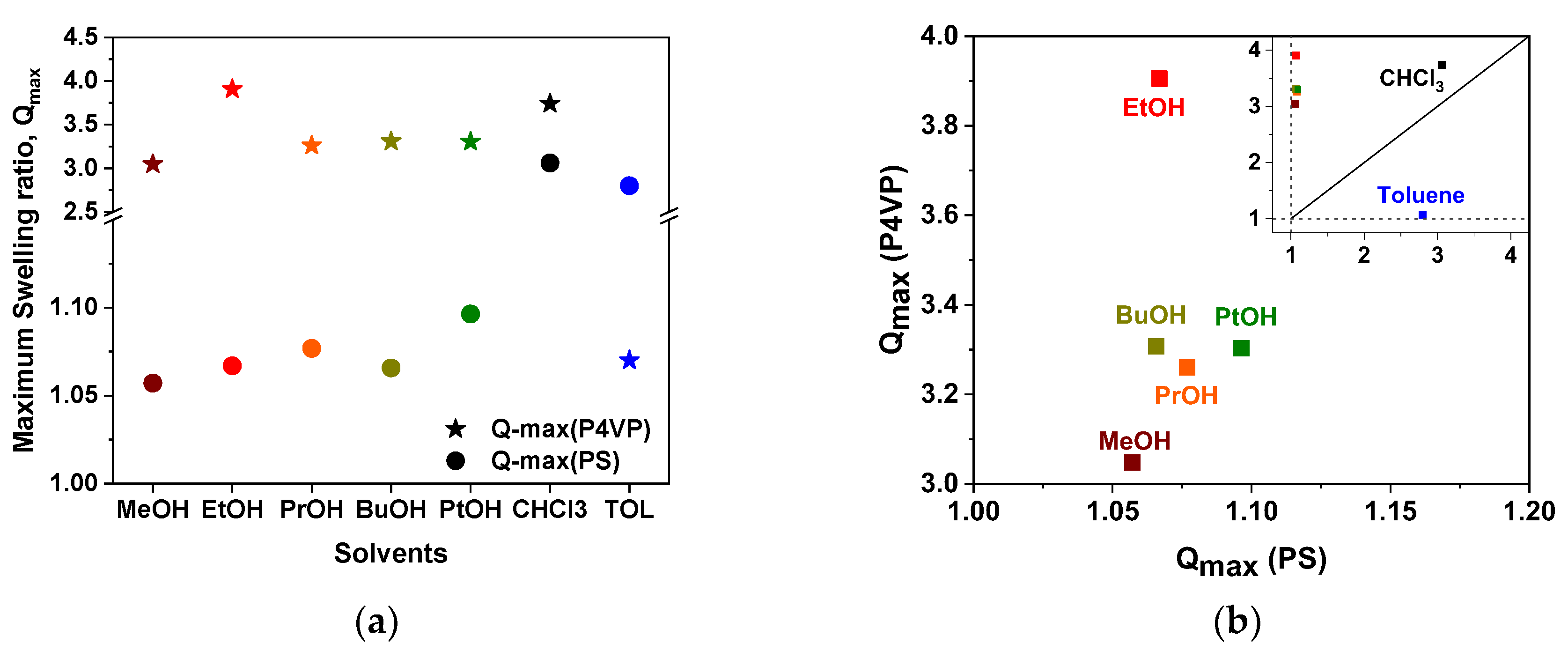
3.4. Swelling of Polymer Films in Solvent Vapors and Ellipsometry experiments
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Solvent | Z-ave a (nm) at 25 °C | PDI a at 25 °C | Z-ave b (nm) at 50 °C | PDI b at 50 °C | Z-ave c (nm) at 25 °C | PDI c at 25 °C |
|---|---|---|---|---|---|---|
| Methanol | 315.2 ± 5.6 | 0.378 ± 0.031 | 156.9 ± 0.8 | 0.080 ± 0.012 | 158.1 ± 1.1 | 0.134 ± 0.012 |
| Ethanol | 218.6 ± 1.6 | 0.257 ± 0.024 | 124.4 ± 0.6 | 0.064 ± 0.012 | 123.4 ± 1.1 | 0.100 ± 0.015 |
| 1-Propanol | 198.4 ± 2.6 | 0.235 ± 0.020 | 114.9 ± 0.1 | 0.070 ± 0.013 | 111.1 ± 1.0 | 0.078 ± 0.021 |
| 1-Butanol | 166.8 ± 2.0 | 0.188 ± 0.024 | 104.7 ± 0.7 | 0.034 ± 0.021 | 98.7 ± 1.6 | 0.061 ± 0.028 |
| 1-Pentanol | 178.9 ± 2.1 | 0.223 ± 0.031 | 96.3 ± 0.6 | 0.021 ± 0.019 | 94.0 ± 0.7 | 0.041 ± 0.021 |
| Block Copolymer | Z-ave a (nm) at 25 °C | PDI a at 25 °C | Z-ave b (nm) at 60 °C | PDI b at 60 °C | Z-ave c (nm) at 25 °C | PDI c at 25 °C |
|---|---|---|---|---|---|---|
| PS-b-P4VP(30) a | 253.9 ± 8.2 | 0.473 ± 0.046 | 54.6 ± 0.3 | 0.028 ± 0.017 | 52.7 ± 0.4 | 0.015 ± 0.011 |
| PS-b-P4VP(59) a | 211.4 ± 2.6 | 0.299 ± 0.0270 | 103.2 ± 0.5 | 0.044 ± 0.015 | 99.1 ± 0.9 | 0.054 ± 0.025 |
| PS-b-P4VP(75) a | 508.1 ± 12.0 | 0.405 ± 0.052 | 112.9 ± 0.6 | 0.068 ± 0.010 | 108.1 ± 1.0 | 0.086 ± 0.031 |
| PS-b-P4VP(120) a | 368.6 ± 3.5 | 0.185 ± 0.036 | 196.7 ± 1.4 | 0.062 ± 0.018 | 188.1 ± 1.2 | 0.121 ± 0.026 |
| Solvent | VP at 25 °C a (kPa) | Vm (cm3 mol−1) | δd b (J cm−3)1/2 | δp b (J cm−3)1/2 | δh b (J cm−3)1/2 | δt c (J cm−3)1/2 |
|---|---|---|---|---|---|---|
| Methanol | 16.7 | 40.7 | 15.1 | 12.3 | 22.3 | 29.6 |
| Ethanol | 8.7 | 58.5 | 15.8 | 8.8 | 19.4 | 26.1 |
| 1-Propanol | 3.3 | 74.8 | 16.0 | 6.8 | 17.4 | 24.4 |
| 1-Butanol | 1.3 | 91.5 | 16.0 | 5.7 | 15.8 | 23.3 |
| 1-Pentanol | 0.4 | 108.2 | 16.0 | 4.5 | 13.9 | 21.6 |
| Toluene | 3.9 | 106.8 | 18.0 | 1.4 | 2.0 | 18.3 |
| Chloroform | 25.5 | 80.7 | 17.8 | 3.1 | 5.7 | 19.0 |
| THF | 23.5 | 81.7 | 16.8 | 5.7 | 8.0 | 19.5 |
| Polymer | δH a (J cm−3)1/2 | δd b (J cm−3)1/2 | δp b (J cm−3)1/2 | δh2 b(J cm−3)1/2 | δt c (J cm−3)1/2 | R0 | Ref. |
|---|---|---|---|---|---|---|---|
| PS | 18.5 | - | - | - | - | - | [7] |
| P2VP | 21.3 | - | - | - | - | - | [29] |
| P4VP | 24.0 | - | - | - | - | - | [30] |
| PS | - | 18.5 | 4.5 | 2.9 | 19.3 | 8 | [25] |
| P4VP | - | 18.1 | 6.8 | 7.2 | 20.6 | ? | [14] |
| PS/Solvent | P4VP/Solvent | ||||||
|---|---|---|---|---|---|---|---|
| Solvent | χS-PS a | χS-P2VP a | χS-P4VP a | Ra b | RED b | Ra b | RED b |
| Methanol | 2.51 | 1.55 | 0.89 | 22.0 | 2.75 | 17.2 | ? |
| Ethanol | 1.89 | 1.00 | 0.49 | 17.9 | 2.24 | 13.2 | ? |
| 1-Propanol | 1.48 | 0.67 | 0.35 | 15.5 | 1.94 | 11.0 | ? |
| 1-Butanol | 1.14 | 0.47 | 0.37 | 13.9 | 1.73 | 9.6 | ? |
| 1-Pentanol | 0.77 | 0.35 | 0.38 | 12.1 | 1.51 | 8.3 | ? |
| Toluene | 0.34 | 0.66 | 1.44 | 3.4 | 0.42 | 7.5 | ? |
| Chloroform | 0.35 | 0.58 | 1.45 | 3.4 | 0.43 | 4.4 | ? |
| THF | 0.37 | 1.55 | 0.89 | 6.3 | 0.78 | 2.9 | ? |
References
- Jackson, E.A.; Hillmyer, M.A. Nanoporous membranes derived from block copolymers: From drug delivery to water filtration. ACS Nano 2010, 4, 3548−3553. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Goswami, A.; Asefa, T.; Guo, H.; Biradar, A.V.; Peng, D.L.; Zboril, R.; Varma, R.S. Core-shell nanoparticles: Synthesis and applications in catalysis and electrocatalysis. Chem. Soc. Rev. 2015, 44, 7540−7590. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.N.; Thakre, R.D.; More, J.C.; Sharma, P.K.; Agrawal, Y.K. Block copolymer nanostructures and their applications: A review. Polym. Plast. Technol. Eng. 2015, 54, 1077−1095. [Google Scholar] [CrossRef]
- Zhang, J.L.; Zhang, M.X.; Tang, K.J.; Verpoort, F.; Sun, T.L. Polymer-based stimuli-responsive recyclable catalytic systems for organic synthesis. Small 2014, 10, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Blanazs, A.; Armes, S.P.; Ryan, A.J. Self-assembled block copolymer aggregates: From micelles to vesicles and their biological applications. Macromol. Rapid Commun. 2009, 30, 267−277. [Google Scholar] [CrossRef]
- Nicolai, T.; Colombani, O.; Chassenieux, C. Dynamic polymeric micelles versus frozen nanoparticles formed by block copolymers. Soft Matter 2010, 6, 3111–3118. [Google Scholar] [CrossRef]
- Kennemur, J.G. Poly(vinylpyridine) segments in block copolymers: Synthesis, self-assembly, and versatility. Macromolecules 2019, 52, 1354–1370. [Google Scholar] [CrossRef]
- Antonietti, M.; Heinz, S.; Schmidt, M.; Rosenauer, C. Determination of the micelle architecture of polystyrene poly(4-vinylpyridine) block-copolymers in dilute solution. Macromolecules 1994, 27, 3276–3281. [Google Scholar] [CrossRef]
- Forster, S.; Zisenis, M.; Wenz, E.; Antonietti, M. Micellization of strongly segregated block copolymers. J. Chem. Phys. 1996, 104, 9956–9970. [Google Scholar] [CrossRef]
- Park, S.Y.; Chang, Y.J.; Farmer, B.L. Study of the ordered structures of poly(styrene-b-vinyl4pyridine) in a solution state by using small-angle X-ray scattering and generalized indirect Fourier transform. Langmuir 2006, 22, 11369–11375. [Google Scholar] [CrossRef]
- Park, S.Y.; Sul, W.H.; Chang, Y.J. A study on the selectivity of toluene/ethanol mixtures on the micellar and ordered structures of poly(styrene-b-4-vinylpyridine) using small-angle X-ray scattering, generalized indirect Fourier transform, and transmission electron microscopy. Macromolecules 2007, 40, 3757–3764. [Google Scholar] [CrossRef]
- Ali, N.; Sul, W.H.; Lee, D.Y.; Kim, D.H.; Park, S.Y. Structures of the cylindrical and vesicular micelles of an P4VP-longer asymmetric PS-b-P4VP. Macromol. Res. 2009, 17, 553–556. [Google Scholar] [CrossRef]
- Kim, T.H.; Huh, J.; Hwang, J.; Kim, H.C.; Kim, S.H.; Sohn, B.H.; Park, C. Ordered arrays of PS-b-P4VP Micelles by fusion and fission process upon solvent annealing. Macromolecules 2009, 42, 6688–6697. [Google Scholar] [CrossRef]
- Ghoshal, T.; Chaudhari, A.; Cummins, C.; Shaw, M.T.; Holmes, J.D.; Morris, M.A. Morphological evolution of lamellar forming polystyrene-block-poly(4-vinylpyridine) copolymers under solvent annealing. Soft Matter 2016, 12, 5429–5437. [Google Scholar] [CrossRef] [PubMed]
- Roland, S.; Gamys, C.G.; Grosrenaud, J.; Boisse, S.; Pellerin, C.; Prud’homme, R.E.; Bazuin, C.G. Solvent Influence on thickness, composition, and morphology variation with dip-coating rate in supramolecular PS-b-P4VP thin films. Macromolecules 2015, 48, 4823–4834. [Google Scholar] [CrossRef]
- Sanwaria, S.; Pal, J.; Srivastava, R.; Formanek, P.; Stamm, M.; Horechyy, A.; Nandan, B. Synthesis of hollow silica nanostructures using functional hairy polymer nanofibers as templates. RSC Adv. 2013, 3, 24009–24012. [Google Scholar] [CrossRef]
- Sanwaria, S.; Singh, S.; Horechyy, A.; Formanek, P.; Stamm, M.; Srivastava, R.; Nandan, B. Fabrication of titania nanostructures using core–shell polymer nanofibers from block copolymers as templates. Nano Struct. Nano Objects 2016, 6, 14–22. [Google Scholar] [CrossRef]
- Shajkumar, A.; Nandan, B.; Sanwaria, S.; Albrecht, V.; Libera, M.; Lee, M.H.; Auffermann, G.; Stamm, M.; Horechyy, A. Silica-supported Au@hollow-SiO2 particles with outstanding catalytic activity prepared via block copolymer template approach. J. Colloid Interface Sci. 2017, 491, 246–254. [Google Scholar] [CrossRef]
- Zha, W.B.; Han, C.D.; Lee, D.H.; Han, S.H.; Kim, J.K.; Kang, J.H.; Park, C. Origin of the difference in order-disorder transition temperature between polystyrene-block-poly(2-vinylpyridine) and polystyrene-block-poly(4-vinylpyridine) copolymers. Macromolecules 2007, 40, 2109–2119. [Google Scholar] [CrossRef]
- Rodriguez, A.; Canosa, J.; Dominguez, A.; Tojo, J. Viscosities of dimethyl carbonate with alcohols at several temperatures UNIFAC-VISCO interaction parameters (-OCOO-/alcohol). Fluid Phase Equilib. 2004, 216, 167–174. [Google Scholar] [CrossRef]
- Pal, J.; Sanwaria, S.; Srivastava, R.; Nandan, B.; Horechyy, A.; Stamm, M.; Chen, H.L. Hairy polymer nanofibers via self-assembly of block copolymers. J. Mater. Chem. 2012, 22, 25102–25107. [Google Scholar] [CrossRef]
- Malynych, S.; Luzinov, I.; Chumanov, G. Poly(vinyl pyridine) as a universal surface modifier for immobilization of nanoparticles. J. Phys. Chem. B 2002, 106, 1280–1285. [Google Scholar] [CrossRef]
- Emerson, J.A.; Toolan, D.T.W.; Howse, J.R.; Furst, E.M.; Epps, T.H. Determination of solvent-polymer and polymer-polymer Flory-Huggins interaction parameters for Poly(3-hexylthiophene) via solvent vapor swelling. Macromolecules 2013, 46, 6533–6540. [Google Scholar] [CrossRef]
- Vayer, M.; Vital, A.; Sinturel, C. New insights into polymer-solvent affinity in thin films. Eur. Polym. J. 2017, 93, 132–139. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters. A Users Handbook, 2rd ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2007; p. 544. [Google Scholar]
- Nandan, B.; Vyas, M.K.; Bohme, M.; Stamm, M. Composition-dependent morphological transitions and pathways in switching of fine structure in thin films of block copolymer supramolecular assemblies. Macromolecules 2010, 43, 2463–2473. [Google Scholar] [CrossRef]
- Nagarajan, R.; Ganesh, K. Block copolymer self-assembly in selective solvents—Spherical micelles with segregated cores. J. Chem. Phys. 1989, 90, 5843–5856. [Google Scholar] [CrossRef]
- Smallwood, I.M. Handbook of Organic Solvent Properties, 1st ed.; Butterworth Heinemann Books—Elsevier: London, UK, 1996; p. 306. [Google Scholar]
- Arichi, S.; Matsuura, H.; Tanimoto, Y.; Murata, H. Studies of Poly-2-vinylpyridine. 2. Solubilities in various solvents. Bull. Chem. Soc. Jpn. 1966, 39, 434–438. [Google Scholar] [CrossRef]
- Horechyy, A.; Nandan, B.; Zafeiropoulos, N.E.; Jehnichen, D.; Gobel, M.; Stamm, M.; Pospiech, D. Nanoparticle directed domain orientation in thin films of asymmetric block copolymers. Colloid Polym. Sci. 2014, 292, 2249–2260. [Google Scholar] [CrossRef]



| BCP or HP Assignments | Mn(PS) (g mol−1) | Mn(P4VP) (g mol−1) | Mn(PS-b-P4VP) (g mol−1) | PDI | DP | φ(PS) b |
|---|---|---|---|---|---|---|
| PS-b-P4VP(30) a | 10,400 | 19,200 | 29,600 | 1.27 | 282 | ≈0.36 |
| PS-b-P4VP(59)a | 18,500 | 40,500 | 59,000 | 1.10 | 563 | ≈0.33 |
| PS-b-P4VP(75)a | 24,000 | 51,000 | 75,000 | 1.10 | 716 | ≈0.33 |
| PS-b-P4VP(120)a | 38,000 | 82,000 | 120,000 | 1.28 | 1145 | ≈0.33 |
| PS | 51,000 | - | - | 1.05 | 490 | - |
| P4VP | - | 48,000 | - | 1.07 | 457 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, L.; Horechyy, A.; Bittrich, E.; Nandan, B.; Uhlmann, P.; Fery, A. Amphiphilic Block Copolymer Micelles in Selective Solvents: The Effect of Solvent Selectivity on Micelle Formation. Polymers 2019, 11, 1882. https://doi.org/10.3390/polym11111882
Kumar L, Horechyy A, Bittrich E, Nandan B, Uhlmann P, Fery A. Amphiphilic Block Copolymer Micelles in Selective Solvents: The Effect of Solvent Selectivity on Micelle Formation. Polymers. 2019; 11(11):1882. https://doi.org/10.3390/polym11111882
Chicago/Turabian StyleKumar, Labeesh, Andriy Horechyy, Eva Bittrich, Bhanu Nandan, Petra Uhlmann, and Andreas Fery. 2019. "Amphiphilic Block Copolymer Micelles in Selective Solvents: The Effect of Solvent Selectivity on Micelle Formation" Polymers 11, no. 11: 1882. https://doi.org/10.3390/polym11111882
APA StyleKumar, L., Horechyy, A., Bittrich, E., Nandan, B., Uhlmann, P., & Fery, A. (2019). Amphiphilic Block Copolymer Micelles in Selective Solvents: The Effect of Solvent Selectivity on Micelle Formation. Polymers, 11(11), 1882. https://doi.org/10.3390/polym11111882





