Thermomechanical Characterization and Modeling of Cold-Drawing of Poly(ethylene Terephthalate)
Abstract
:1. Introduction
2. Thermomechanical Model
3. Materials and Methods
3.1. Materials and Sample Preparation
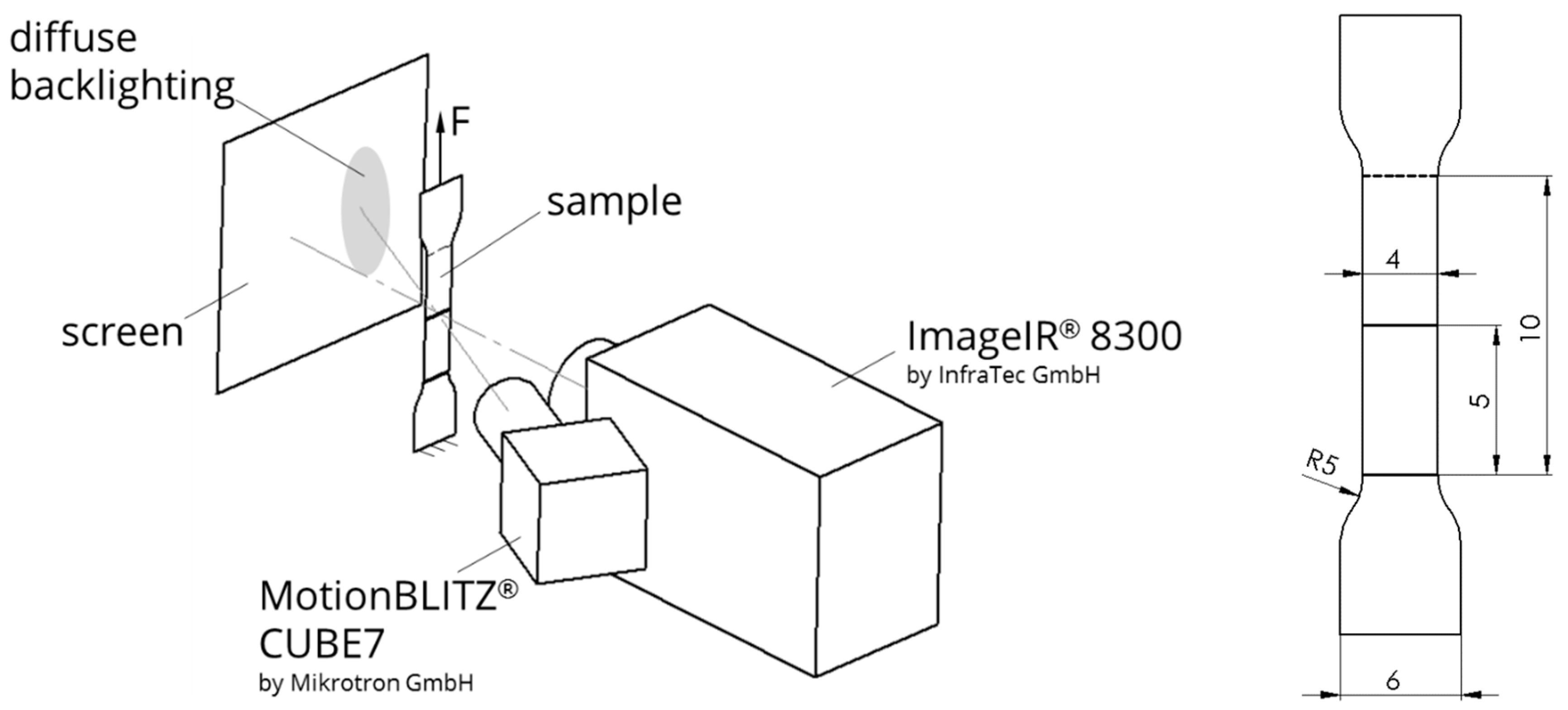
3.2. Mechanical and Thermographic Studies
3.3. Differential Scanning Calorimetry
3.4. Application of Thermomechanical Model for Cold-Drawing
4. Results
4.1. Experimental Overview
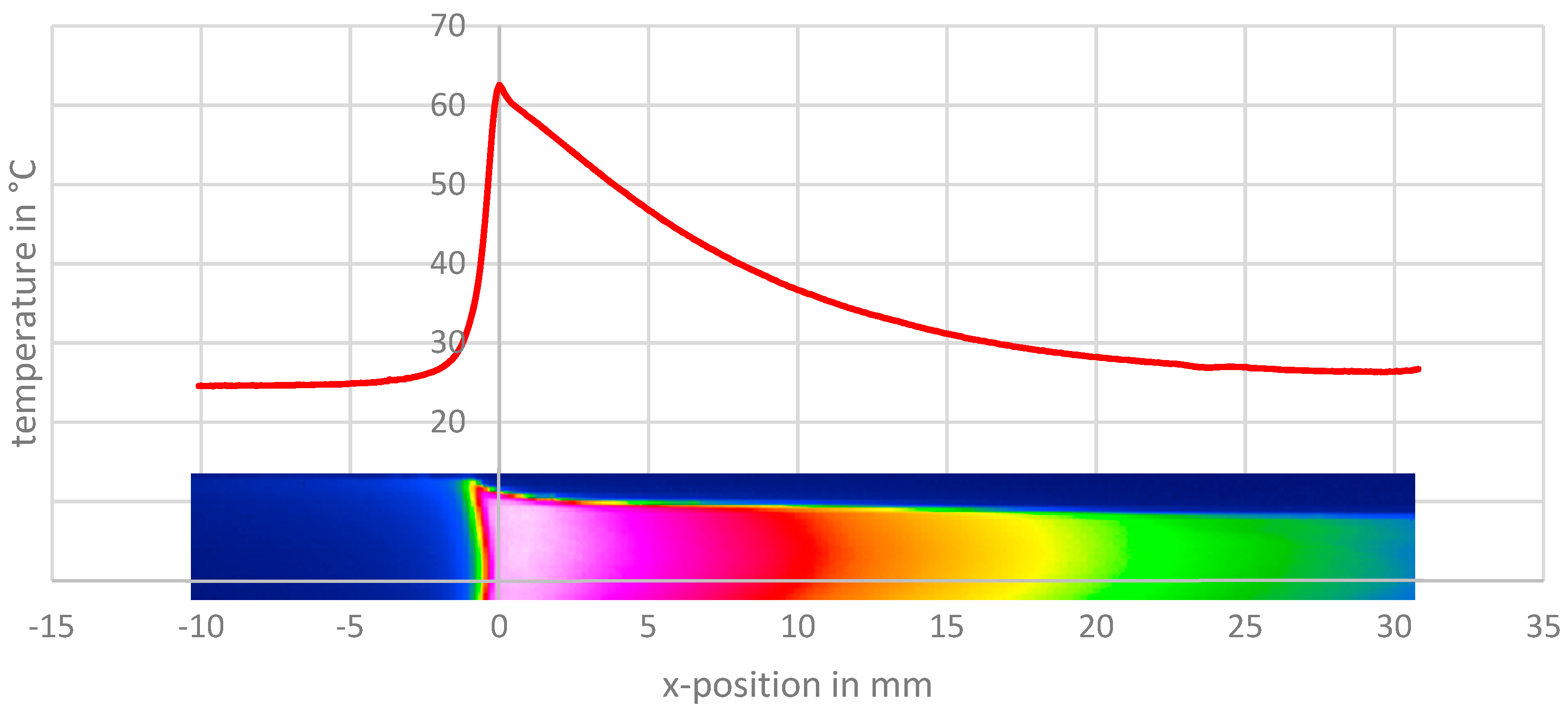
4.2. Neck Propagation/Cold-Drawing
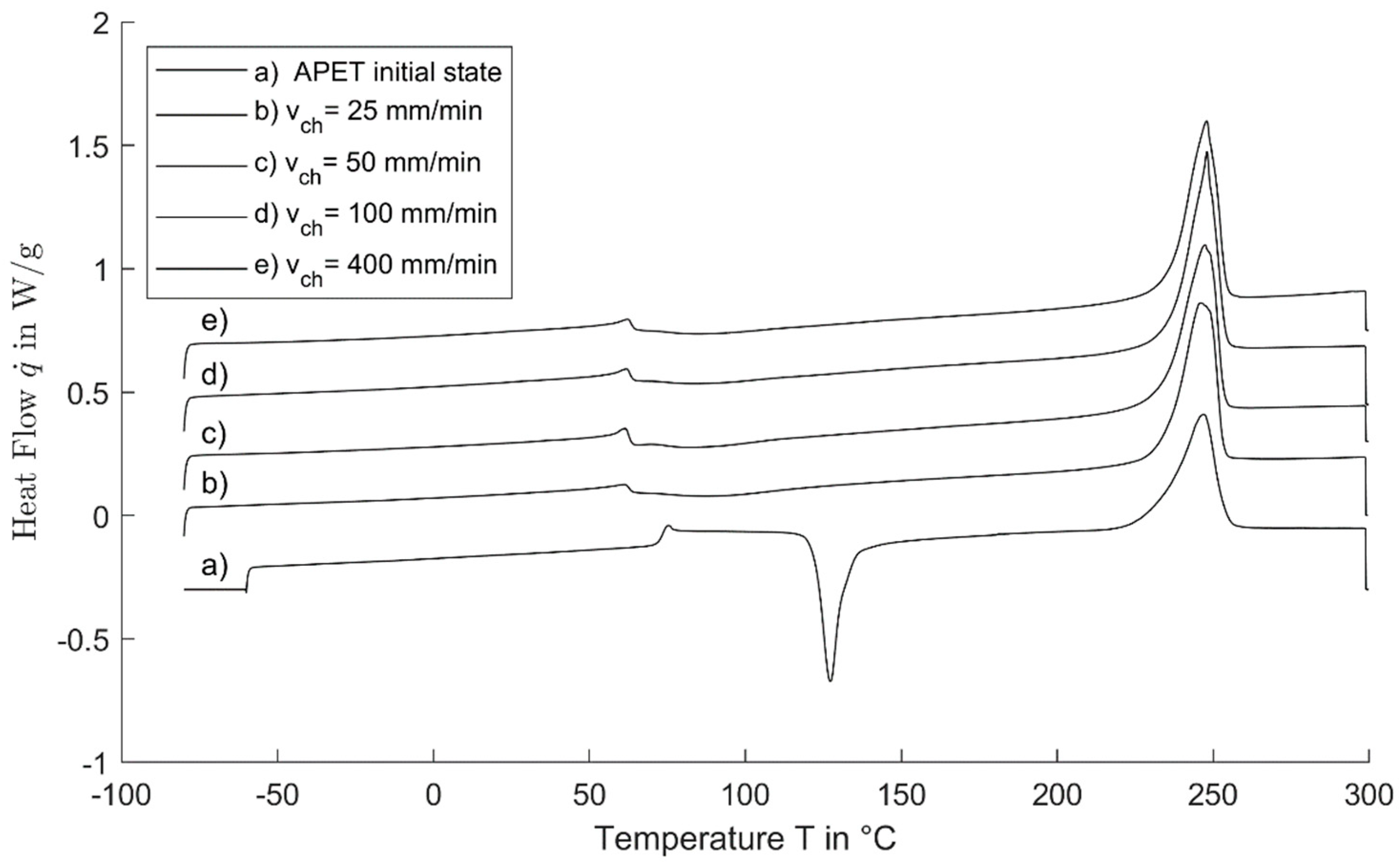
4.3. DSC Measurement
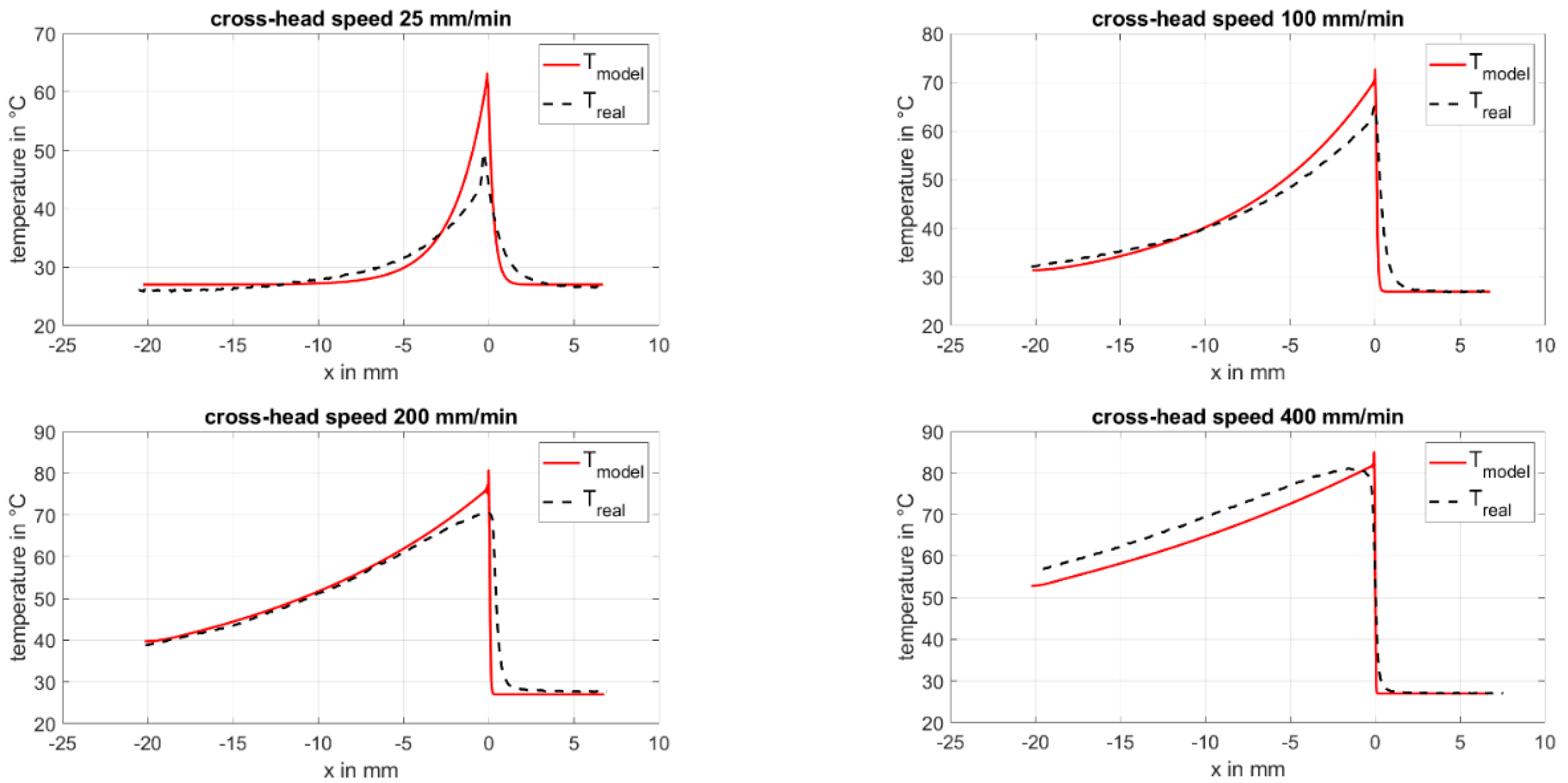
4.4. Temperature Model
5. Discussion
6. Conclusions
- Investigation of the drawing process of amorphous polyethylene terephthalate with IR thermography and optical strain measurement shows the typical trend for stress–strain curves for polymers with necking behavior, as well as for the local maximum temperature in the sample.
- The measured temperatures in the transition zone for cold-drawing exceed the strain-induced heating due to the dissipation of mechanical work through viscous friction. An additional heat source is strain-induced crystallization, which is dependent on the molecule orientation and mobility and seem to be time-dependent.
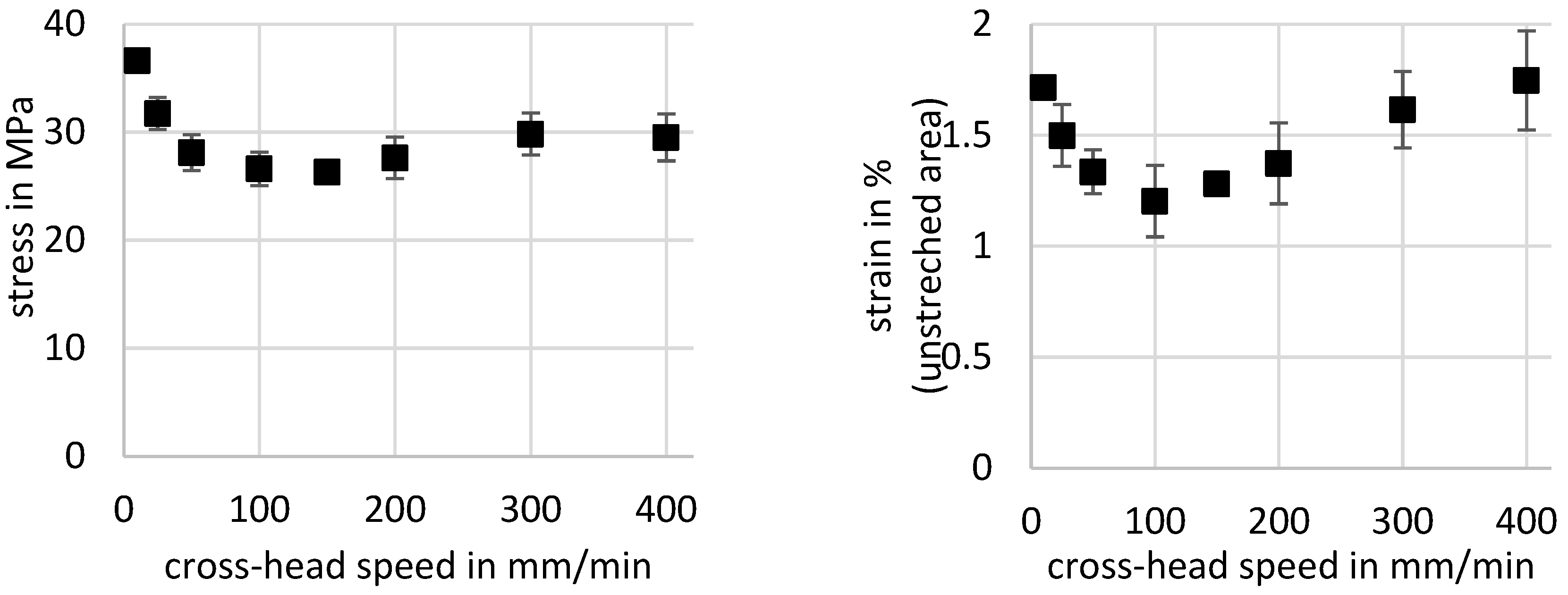
- The softening and hardening of the material with respect to the crosshead speed can be accounted to the superposition of the rising temperature in the transition zone, the higher orientation, and the increasing crystallinity of the material.
- For cold-drawn material, the glass transition is shifted to smaller temperatures. This shift of about 12 K in the case of PET is independent of the crosshead speed. The cause is yet unknown.
- Cold crystallization is not clearly differentiable for the cold-drawn material. If the enthalpy is integrated over a wide area from glass transition up to the melting, a dependency of the crosshead speed with the same trend as the increasing temperature and the draw ratio can be evaluated. This points out that some time-dependent molecular rearrangements and crystallization occurs.
- The melting enthalpy for cold-drawn material is greater than that for the amorphous material. This is accounted to the high orientation of the polymer chains, which results in the development of smectic areas in which crystallization is faster as well as possible at lower temperatures. The smectic areas enable the crystalline lamellae to grow in a time-delayed annealing process.
- The model for the temperature profile for cold-drawing, based on the first law of thermodynamics, gives good results for small crosshead speeds of 100 mm/min and lower. For higher crosshead speeds, there is a deviation in the peak obtained from the model with respect to the real data. For correction, the time-delayed annealing and crystallization processes should be considered.
Author Contributions
Funding
Conflicts of Interest
References
- Haward, R.N. Heating effects in the deformation of thermoplastics. Thermochim. Acta 1994, 247, 87–109. [Google Scholar] [CrossRef]
- Akihiko, T.; Chiyoko, T.; Masamichi, H.; Yu, H.; Hideki, M.; Chisato, N.; Toshitake, S.; Hideaki, I. Thermo-mechanical coupling and self-excited oscillation in the neck propagation of PET films. Polym. Mattice Spec. Issue 2002, 43, 947–951. [Google Scholar]
- Tatsuo, Y. Observation of polymer film drawing by use of thermography. An introductory investigation on the thermodynamics. J. Appl. Polym. Sci. 2006, 100, 2895–2900. [Google Scholar]
- Wang, M.; Liao, Y.; Chen, D. Study of the necking phenomenon in fiber drawing by infrared thermography. Polym. Test. 2010, 29, 674–678. [Google Scholar] [CrossRef]
- Sergey, B. Self-oscillatory neck propagation in polymers. J. Appl. Polym. Sci. 2011, 119, 654–661. [Google Scholar]
- Bazhenov, S.L.; Kechek’yan, A.S. SHeating of polymers during neck propagation. Polym. Sci. Ser. A 2013, 55, 404–414. [Google Scholar] [CrossRef]
- Bazhenov, S. Non-adiabatic heating of polymer films under drawing. Int. J. Mech. Mater. Eng. 2014, 9, 1. [Google Scholar] [CrossRef]
- Liao, Y.; Shan, D.; Liu, Z.; Wang, M. Thermal analysis of the necking phenomenon in fiber drawing. J. Therm. Anal. Calorim. 2015, 122, 323–327. [Google Scholar] [CrossRef]
- Guseva, M.A.; Gerasin, V.A.; Garishin, O.K.; Shadrin, V.V.; Plekhov, O.A.; Pawlak, A. Thermal effects under elastic and plastic deformation of polyethylene. Polym. Polym. Foams. 2015, 56, 416–427. [Google Scholar] [CrossRef]
- Egorov, E.A.; Žiženkov, V.V.; Bezladnov, S.N.; Sokolov, I.A.; Tomaševskij, E.E. Local exothermal effects during the fracture of poly(ethylene terephthalate) and the temperature profile of the heated zone. Acta Polym. 2003, 31, 541–545. [Google Scholar] [CrossRef]
- Cross, A.; Hall, M.; Haward, R.N. Thermal effects in the necking of thermoplastics. Nature 1975, 253, 340. [Google Scholar] [CrossRef]
- Guseva, M.A.; Gerasin, V.A.; Shklyaruk, B.F.; Dubinskiy, V.A. Relation between thermal effects and structural changes under deformation of thermoplastics. Polymer 2018, 144, 18–32. [Google Scholar] [CrossRef]
- Pakula, T.; Fischer, E.W. Instabilities of the deformation process in cold drawing of poly(ethylene terephthalate) and other polymers. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 1705–1726. [Google Scholar] [CrossRef]
- Chaari, F.; Chaouche, M.; Doucet, J. Crystallization of poly(ethylene terephthalate) under tensile strain: Crystalline development versus mechanical behaviour. Polymer 2003, 44, 473–479. [Google Scholar] [CrossRef]
- Baehr, H.D.; Stephan, K. Heat and Mass Transfer; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 978-3-642-20020-5. [Google Scholar]
- Sabine, S.; Wolfgang, G. Kunststoffprüfung; Carl Hanser Verlag: München, Germany, 2015; ISBN 978-3-446-44390-7. [Google Scholar]
- Bartolotta, A.; Di Marco, G.; Farsaci, F.; Lanza, M.; Pieruccini, M. DSC and DMTA study of annealed cold-drawn PET: A three phase model interpretation. Polymer 2003, 44, 5771–5777. [Google Scholar] [CrossRef]
- Asano, T.; Calleja, F.J.B.; Flores, A.; Tanigaki, M.; Mina, M.F.; Sawatari, C.; Itagaki, H.; Takahashi, H.; Hatta, I. Crystallization of oriented amorphous poly(ethylene terephthalate) as revealed by X-ray diffraction and microhardness. Polymer 1999, 40, 6475–6484. [Google Scholar] [CrossRef]



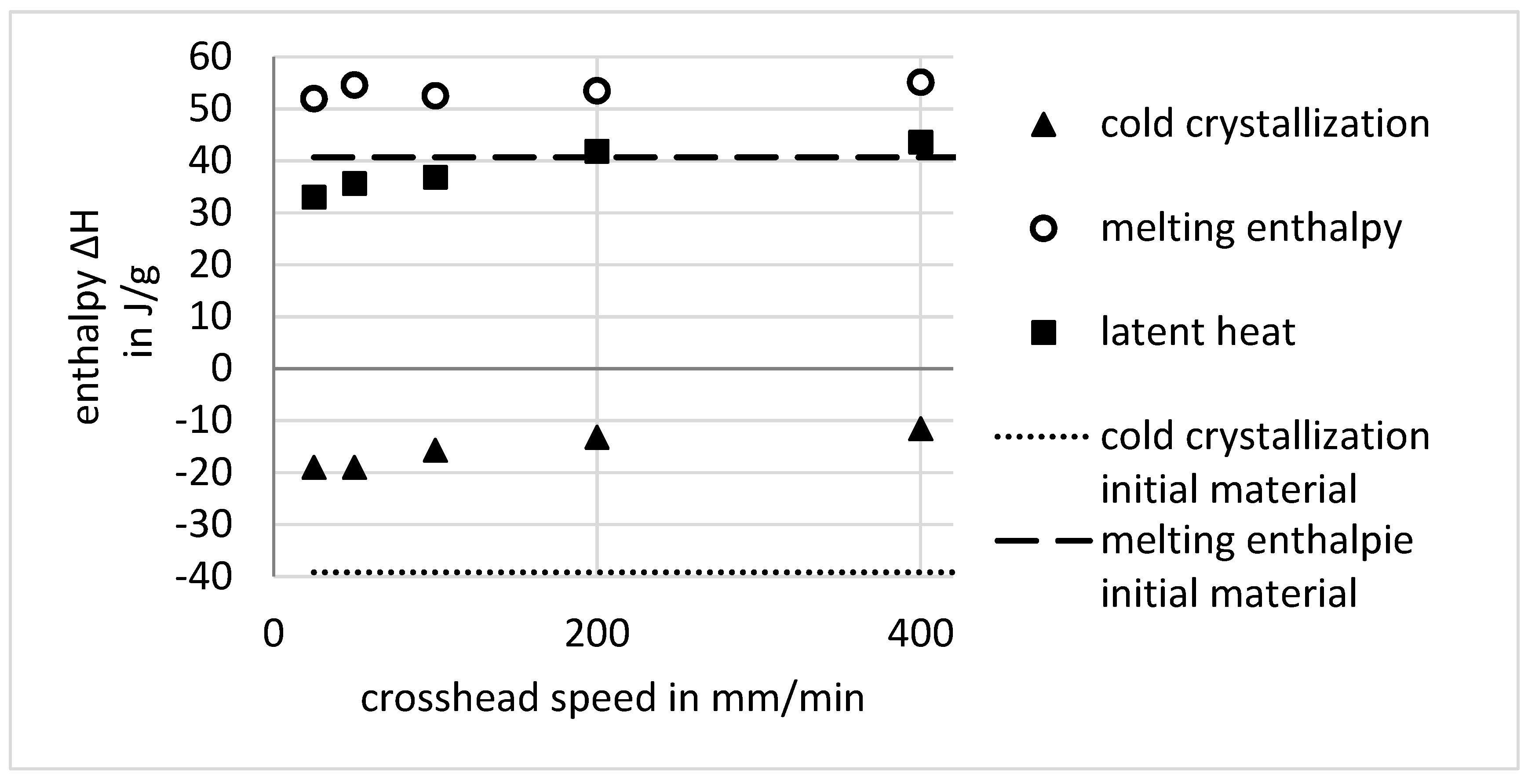
| Crosshead Speed | Integration Temperture | Integration Temperature | |||
|---|---|---|---|---|---|
| mm/min | °C | J/g | °C | J/g | J/g |
| 25 | 63.2–212 | −19 | 212–260 | 51.95 | 31.4 |
| 50 | 62.9–205 | −19 | 205–260 | 54.6 | 34.1 |
| 100 | 62.6–208.7 | −15.7 | 208.7–260 | 52.5 | 35.3 |
| 200 | 63.3–211.5 | −13.2 | 211.5–260 | 53.5 | 38.8 |
| 400 | 64–209 | −11.55 | 209–260 | 55.15 | 42.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oberer, J.; Schneider, K.; Majschak, J.-P. Thermomechanical Characterization and Modeling of Cold-Drawing of Poly(ethylene Terephthalate). Polymers 2019, 11, 1871. https://doi.org/10.3390/polym11111871
Oberer J, Schneider K, Majschak J-P. Thermomechanical Characterization and Modeling of Cold-Drawing of Poly(ethylene Terephthalate). Polymers. 2019; 11(11):1871. https://doi.org/10.3390/polym11111871
Chicago/Turabian StyleOberer, Jürgen, Konrad Schneider, and Jens-Peter Majschak. 2019. "Thermomechanical Characterization and Modeling of Cold-Drawing of Poly(ethylene Terephthalate)" Polymers 11, no. 11: 1871. https://doi.org/10.3390/polym11111871
APA StyleOberer, J., Schneider, K., & Majschak, J.-P. (2019). Thermomechanical Characterization and Modeling of Cold-Drawing of Poly(ethylene Terephthalate). Polymers, 11(11), 1871. https://doi.org/10.3390/polym11111871





