Chemically and Thermally Crosslinked PVA-Based Membranes: Effect on Swelling and Transport Behavior
Abstract
1. Introduction
2. Experimental
2.1. Materials
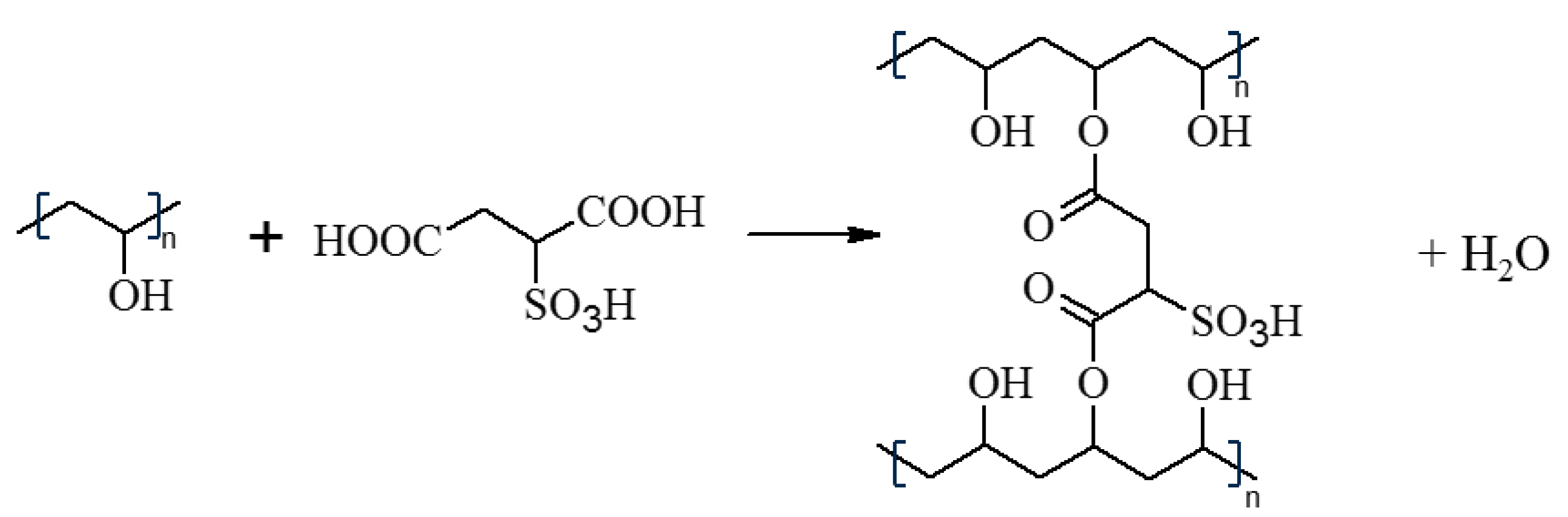
2.2. Membrane Preparation
2.3. Membrane Characterization
2.4. Swelling Measurements
2.5. Water Vapor Permeation Measurements
3. Results and Discussion
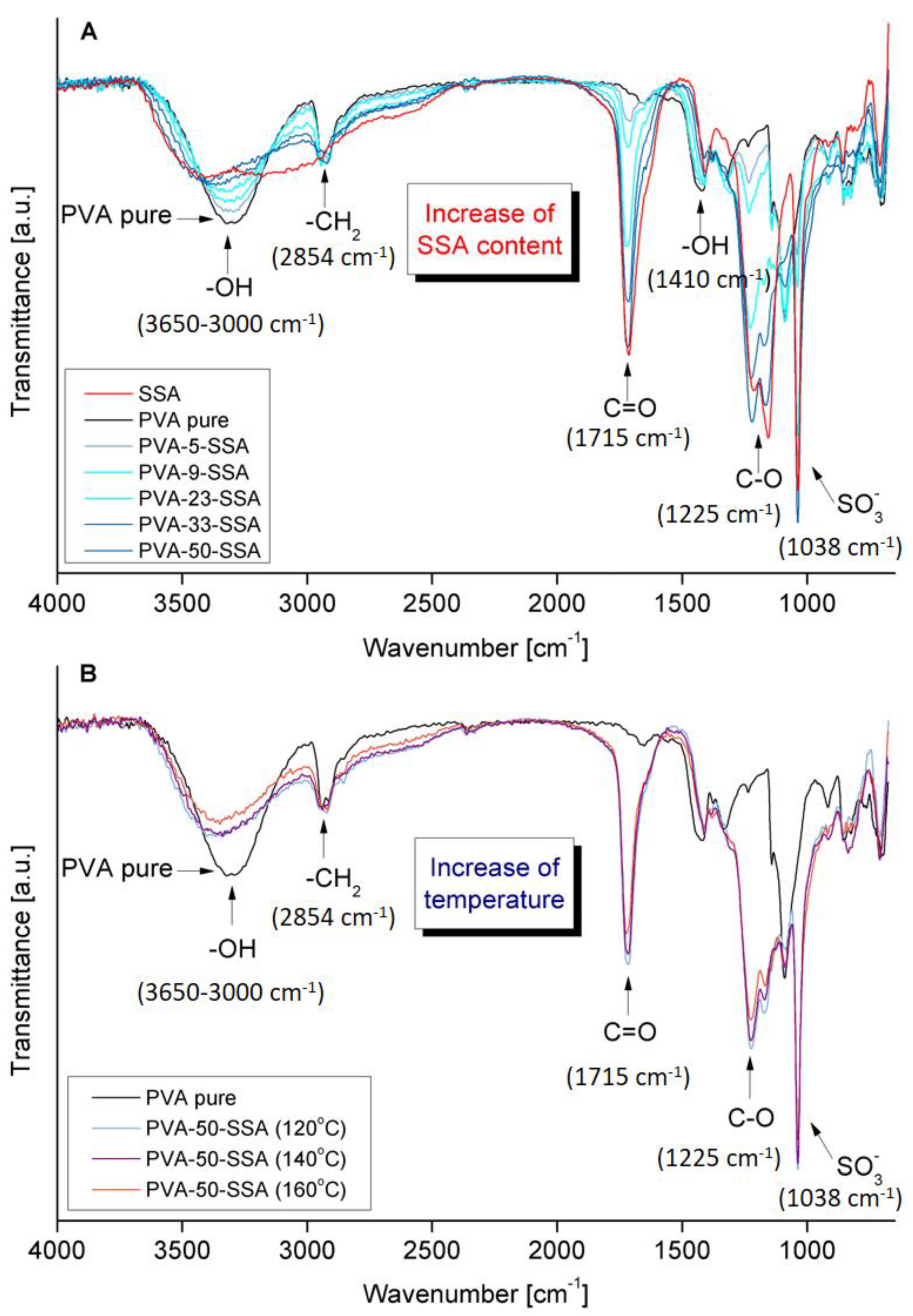
3.1. FTIR-ATR Analysis
3.2. Thermal Properties
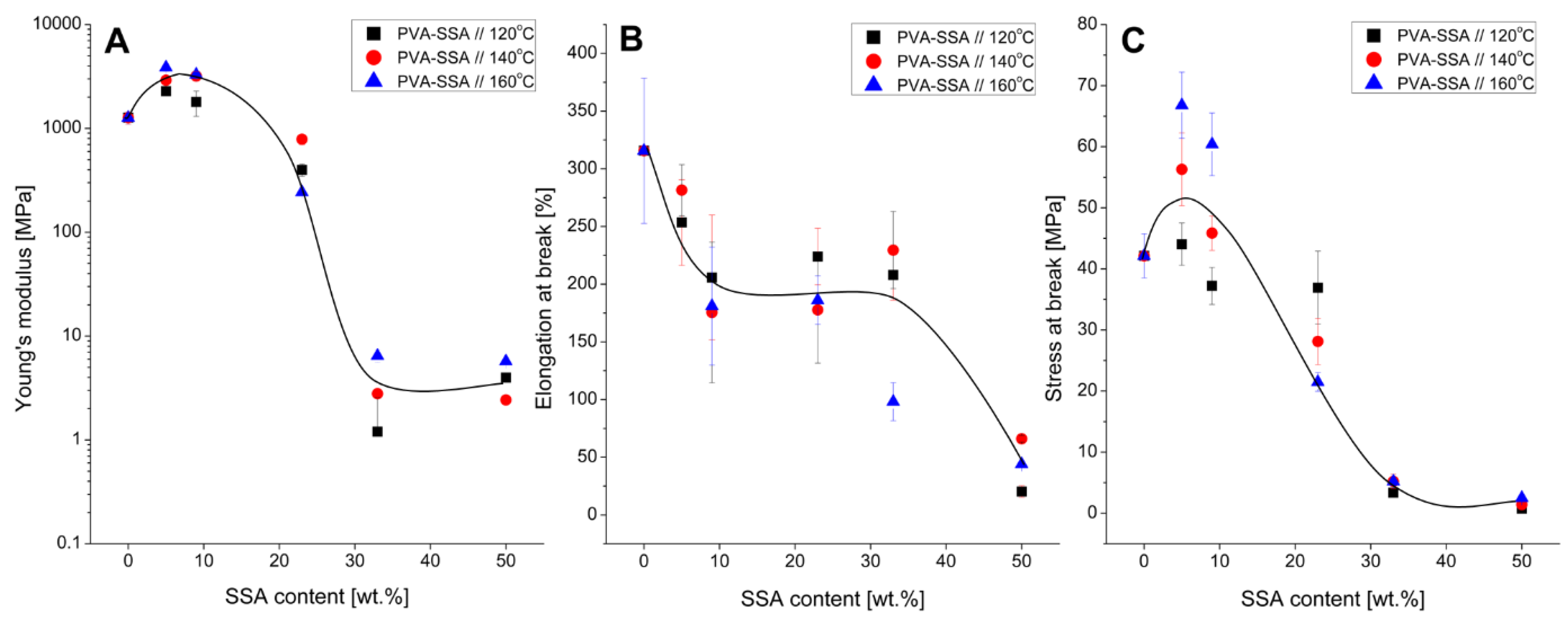
3.3. Mechanical Properties
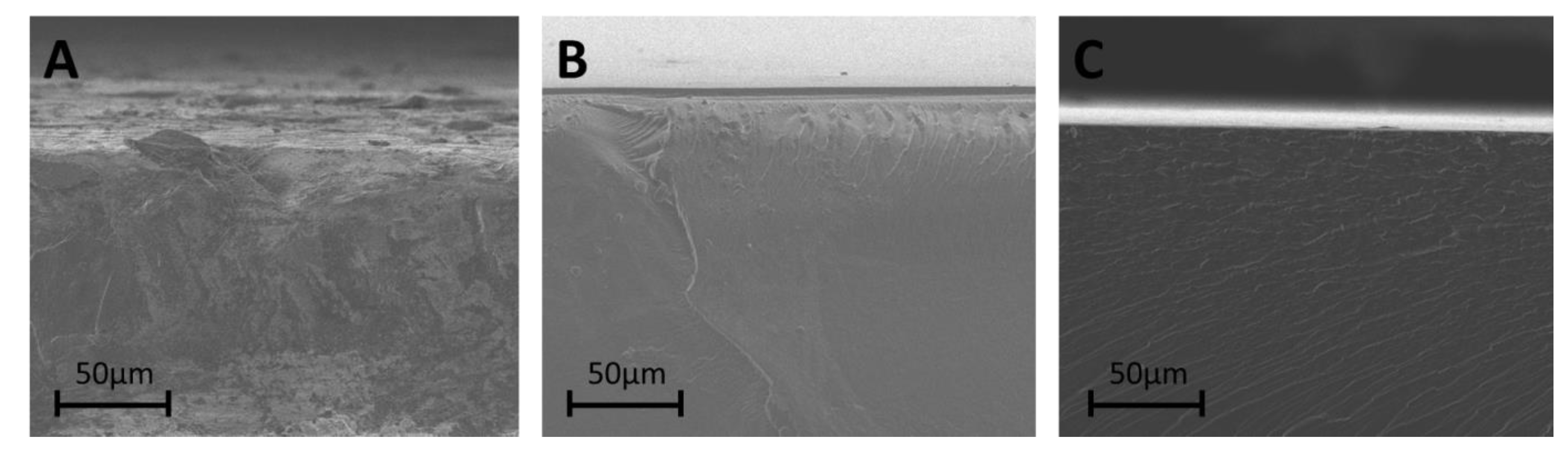
3.4. Membrane Morphology
3.5. Contact Angle Measurements
3.6. Water, Methanol and Propan-2-ol Uptake
3.7. Water Vapor Permeation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martínez-Felipe, A.; Moliner-Estopiñán, C.; Imrie, C.T.; Ribes-Greus, A. Characterization of crosslinked poly(vinyl alcohol)-based membranes with different hydrolysis degrees for their use as electrolytes in direct methanol fuel cells. J. Appl. Polym. Sci. 2012, 124, 1000–1011. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Park, H.B.; Lee, C.-S.; Jun, J.-H.; Kim, D.S.; Lee, Y.M. Crosslinked poly(vinyl alcohol) membranes containing sulfonic acid group: Proton and methanol transport through membranes. J. Membr. Sci. 2004, 238, 143–151. [Google Scholar] [CrossRef]
- Chan, L.W.; Hao, J.S.; Heng, P.W.S. Evaluation of permeability and mechanical properties of composite polyvinyl alcohol films. Chem. Pharm. Bull. 1999, 47, 1412–1416. [Google Scholar] [CrossRef]
- Mahdi Dadfar, S.M.; Kavoosi, G.; Ali Dadfar, S.M. Investigation of mechanical properties, antibacterial features, and water vapor permeability of polyvinyl alcohol thin films reinforced by glutaraldehyde and multiwalled carbon nanotube. Polym. Compos. 2014, 35, 1736–1743. [Google Scholar] [CrossRef]
- Guirguis, O.W.; Moselhey, M.T.H. Thermal and structural studies of poly (vinyl alcohol) and hydroxypropyl cellulose blends. Nat. Sci. 2012, 4, 57–67. [Google Scholar] [CrossRef]
- López-de-Dicastillo, C.; Jordá, M.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Development of active polyvinyl alcohol/β-cyclodextrin composites to scavenge undesirable food components. J. Agric. Food Chem. 2011, 59, 11026–11033. [Google Scholar] [CrossRef]
- Riyajan, S.-A.; Chaiponban, S.; Tanbumrung, K. Investigation of the preparation and physical properties of a novel semi-interpenetrating polymer network based on epoxised NR and PVA using maleic acid as the crosslinking agent. Chem. Eng. J. 2009, 153, 199–205. [Google Scholar] [CrossRef]
- Wang, Y.; Hsieh, Y.-L. Crosslinking of polyvinyl alcohol (PVA) fibrous membranes with glutaraldehyde and PEG diacylchloride. J. Appl. Polym. Sci. 2010, 116, 3249–3255. [Google Scholar] [CrossRef]
- Bano, S.; Mahmood, A.; Lee, K.-H. Vapor permeation separation of methanol-water mixtures: Effect of experimental conditions. Ind. Eng. Chem. Res. 2013, 52, 10450–10459. [Google Scholar] [CrossRef]
- Kim, K.-J.; Lee, S.-B.; Han, N.-W. Kinetics of crosslinking reaction of PVA membrane with glutaraldehyde. Korean J. Chem. Eng. 1994, 11, 41–47. [Google Scholar] [CrossRef]
- Figueiredo, K.C.S.; Alves, T.L.M.; Borges, C.P. Poly(vinyl alcohol) films crosslinked by glutaraldehyde under mild conditions. J. Appl. Polym. Sci. 2009, 111, 3074–3080. [Google Scholar] [CrossRef]
- Liu, L.; Kentish, S.E. Pervaporation performance of crosslinked PVA membranes in the vicinity of the glass transition temperature. J. Membr. Sci. 2018, 553, 63–69. [Google Scholar] [CrossRef]
- Ji, W.; Afsar, N.U.; Wu, B.; Sheng, F.; Shehzad, M.A.; Ge, L.; Xu, T. In-situ crosslinked SPPO/PVA composite membranes for alkali recovery via diffusion dialysis. J. Membr. Sci. 2019, 590, 117267. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, P.C.; Edgren, D. Crosslinking reaction of poly(vinyl alcohol) with glyoxal. J. Polym. Res. 2010, 17, 725–730. [Google Scholar] [CrossRef]
- Bolto, B.; Tran, T.; Hoang, M.; Xie, Z. Crosslinked poly(vinyl alcohol) membranes. Prog. Polym. Sci. 2009, 34, 969–981. [Google Scholar] [CrossRef]
- Birck, C.; Degoutin, S.; Tabary, N.; Miri, V.; Bacquet, M. New crosslinked cast films based on poly(vinyl alcohol): Preparation and physico-chemical properties. Express Polym. Lett. 2014, 8, 941–952. [Google Scholar] [CrossRef]
- Leone, G.; Consumi, M.; Pepi, S.; Pardini, A.; Bonechi, C.; Tamasi, G.; Donati, A.; Rossi, C.; Magnani, A. Poly-vinyl alcohol (PVA) crosslinked by trisodium trimetaphosphate (STMP) and sodium hexametaphosphate (SHMP): Effect of molecular weight, pH and phosphorylating agent on length of spacing arms, crosslinking density and water interaction. J. Mol. Struct. 2019, 127264. [Google Scholar] [CrossRef]
- Xu, S.; Shen, L.; Li, C.; Wang, Y. Properties and pervaporation performance of poly(vinyl alcohol) membranes crosslinked with various dianhydrides. J. Appl. Polym. Sci. 2018, 135, 46159. [Google Scholar] [CrossRef]
- Kudoh, Y.; Kojima, T.; Abe, M.; Oota, M.; Yamamoto, T. Proton conducting membranes consisting of poly(vinyl alcohol) and poly(styrene sulfonic acid): Crosslinking of poly(vinyl alcohol) with and without succinic acid. Solid State Ion. 2013, 253, 189–194. [Google Scholar] [CrossRef]
- Ajith, C.; Deshpande, A.P.; Varughese, S. Proton conductivity in crosslinked hydrophilic ionic polymer system: Competitive hydration, crosslink heterogeneity, and ineffective domains. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1087–1101. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Lin, Y.; Chen, C. Crosslinked poly(vinyl alcohol) membranes for separation of dimethyl carbonate/methanol mixtures by pervaporation. Chem. Eng. J. 2009, 146, 71–78. [Google Scholar] [CrossRef]
- Kumeta, K.; Nagashima, I.; Matsui, S.; Mizoguchi, K. Crosslinking reaction of poly(vinyl alcohol) with poly(acrylic acid) (PAA) by heat treatment: Effect of neutralization of PAA. J. Appl. Polym. Sci. 2003, 90, 2420–2427. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Yang, H.; Xu, Z.-L. Influence of post-treatments on the properties of porous poly(vinyl alcohol) membranes. J. Appl. Polym. Sci. 2008, 107, 1423–1429. [Google Scholar] [CrossRef]
- Rudawska, A.; Jacniacka, E. Analysis for determining surface free energy uncertainty by the Owne-Wendt method. Int. J. Adhes. Adhes. 2009, 129, 451–457. [Google Scholar] [CrossRef]
- Rynkowska, E.; Kujawa, J.; Chappey, C.; Fatyeyeva, K.; Karpenko-Jereb, L.; Kelterer, A.-M.; Marais, S.; Kujawski, W. Effect of the polar–nonpolar liquid mixtures on pervaporative behavior of perfluorinated sulfonic membranes in lithium form. J. Membr. Sci. 2016, 518, 313–327. [Google Scholar] [CrossRef]
- Marais, S.; Metayer, M.; Labbe, M. Water diffusion and permeability in unsaturated polyester resin films characterized by measurements performed with a water-specific permeameter: Analysis of the transient permeation. J. Appl. Polym. Sci. 1999, 74, 3380–3395. [Google Scholar] [CrossRef]
- Marais, S.; Nguyen, Q.T.; Devallencourt, C.; Metayer, M.; Nguyen, T.U.; Schaetzel, P. Permeation of water through polar and nonpolar polymers and copolymers: Determination of the concentration-dependent diffusion coefficient. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 1998–2008. [Google Scholar] [CrossRef]
- Tsai, C.-E.; Lin, C.-W.; Hwang, B.-J. A novel crosslinking strategy for preparing poly(vinyl alcohol)-based proton-conducting membranes with high sulfonation. J. Power Sources 2010, 195, 2166–2173. [Google Scholar] [CrossRef]
- Boroglu, M.S.; Celik, S.U.; Bozkurt, A.; Boz, I. Proton-conducting blend membranes of crosslinked poly(vinyl alcohol)–sulfosuccinic acid ester and poly(1-vinyl-1,2,4-triazole) for high temperature fuel cells. Polym. Eng. Sci. 2013, 53, 153–158. [Google Scholar] [CrossRef]
- Behera, K.; Chang, Y.-H.; Chiu, F.-C.; Yang, J.-C. Characterization of poly(lactic acid)s with reduced molecular weight fabricated through an autoclave process. Polym. Test. 2017, 60, 132–139. [Google Scholar]
- Morancho, J.M.; Salla, J.M.; Cadenato, A.; Fernández-Francos, X.; Colomer, P.; Calventus, Y.; Ramis, X.; Ruíz, R. Thermal analysis of enhanced poly(vinyl alcohol)-based proton-conducting membranes crosslinked with sulfonation agents for direct methanol fuel cells. J. Appl. Polym. Sci. 2012, 124, E57–E65. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, Y.; Ren, L.; Tong, J.; Liu, Z.; Xie, L. Preparation and characterization of surface crosslinked TPS/PVA blend films. Carbohydr. Polym. 2009, 76, 632–638. [Google Scholar] [CrossRef]
- Vashisth, P.; Pruthi, V. Synthesis and characterization of crosslinked gellan/PVA nanofibers for tissue engineering application. Mater. Sci. Eng. C 2016, 67, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Won, J.S.; Lee, J.E.; Jin, D.Y.; Lee, S.G. Mechanical properties and biodegradability of the kenaf/soy protein isolate-PVA biocomposites. Int. J. Polym. Sci. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Peppas, N.A. Effect of the morphology of hydrophilic polymeric matrices on the diffusion and release of water soluble drugs. J. Membr. Sci. 1981, 9, 211–227. [Google Scholar] [CrossRef]
- Rey, L.; Duchet, J.; Galy, J.; Sautereau, H.; Vouagner, D.; Carrion, L. Structural heterogeneities and mechanical properties of vinyl/dimethacrylate networks synthesized by thermal free radical polymerisation. Polymer 2002, 43, 4375–4384. [Google Scholar] [CrossRef]
- Chapman, P.D.; Oliveira, T.; Livingston, A.G.; Li, K. Membranes for the dehydration of solvents by pervaporation. J. Membr. Sci. 2008, 318, 5–37. [Google Scholar] [CrossRef]
- Kujawa, J.; Rozicka, A.; Cerneaux, S.; Kujawski, W. The influence of surface modification on the physicochemical properties of ceramic membranes. Colloids Surf. A Physicochem. Eng. Asp. 2014, 443, 567–575. [Google Scholar] [CrossRef]
- Gindl, M.; Sinn, G.; Gindl, W.; Reiterer, A.; Tschegg, S. A comparison of different methods to calculate the surface free energy of wood using contact angle measurements. Colloids Surf. A Physicochem. Eng. Asp. 2001, 181, 279–287. [Google Scholar] [CrossRef]
- Kwok, D.Y.; Neumann, A.W. Contact angle measurement and contact angle interpretation. Adv. Colloid Interface Sci. 1999, 81, 167–249. [Google Scholar] [CrossRef]
- Żenkiewicz, M. New method of analysis of the surface free energy of polymeric materials calculated with Owens-Wendt and Neumann methods. Polimery 2006, 51, 584–587. [Google Scholar] [CrossRef]
- Tavana, H.; Neumann, A.W. Recent progress in the determination of solid surface tensions from contact angles. Adv. Colloid Interface Sci. 2007, 132, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Hejda, F.; Solar, P.; Kousal, J. Surface free energy determination by contact angle measurements—A comparison of various approaches. In Proceedings of the 19th Annual Conference of Doctoral Students—WDS 2010, Prague, Czech Republic, 1–4 June 2010; pp. 25–30. [Google Scholar]
- Penkova, A.V.; Acquah, S.F.A.; Dmitrenko, M.E.; Chen, B.; Semenov, K.N.; Kroto, H.W. Transport properties of cross-linked fullerenol–PVA membranes. Carbon 2014, 76, 446–450. [Google Scholar] [CrossRef]
- Fowkes, F.M. Dispersion force contributions to surface and interfacial tensions, Contact angles, and heats of immersion. In Contact Angle, Wettability, and Adhesion; American Chemical Society: Washington, DC, USA, 1964; pp. 99–111. [Google Scholar]
- Yadav, M.; Chiu, F.-C. Cellulose nanocrystals reinforced κ-carrageenan based UV resistant transparent bionanocomposite films for sustainable packaging applications. Carbohydr. Polym. 2019, 211, 181–194. [Google Scholar] [CrossRef]
- Farid, O.; Mansour, F.; Habib, M.; Robinson, J.; Tarleton, S. Investigating the sorption influence of poly(vinyl alcohol) (PVA) at different crosslinking content. J. Environ. Chem. Eng. 2016, 4, 293–298. [Google Scholar] [CrossRef]
- Salt, Y.; Arçevik, E.; Ekinci, B. Sorption and pervaporation results of clinoptilolite filled poly(vinylalcohol) membrane prepared for dehydration of aqueous organic mixtures. Can. J. Chem. Eng. 2014, 92, 503–510. [Google Scholar] [CrossRef]
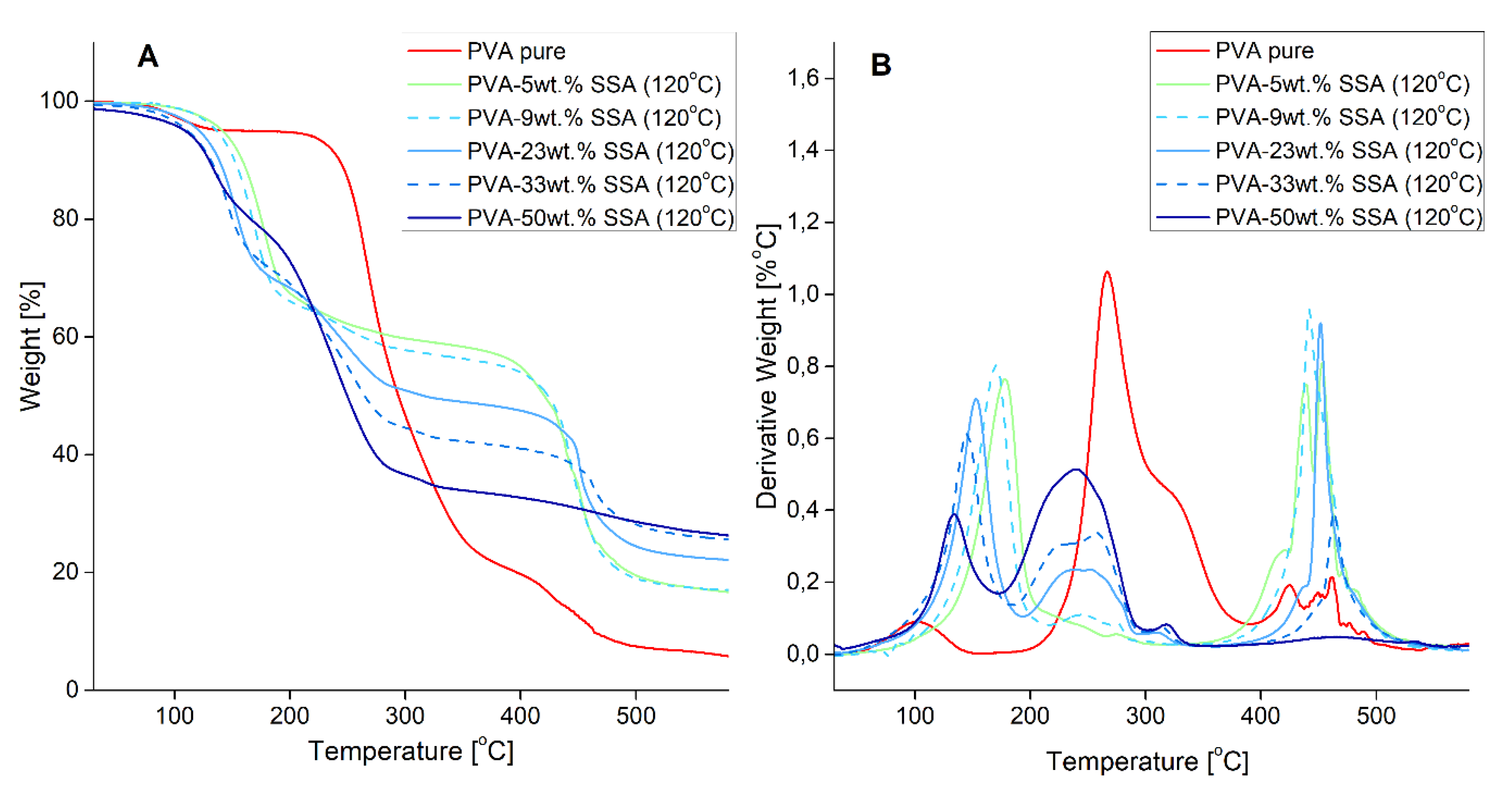
| Membrane | Degradation Temperature [°C] | |
|---|---|---|
| at 5% Weight Loss | at 10% Weight Loss | |
| PVA pure | 163 | 243 |
| PVA-5 wt.% SSA (120 °C) | 142 | 159 |
| PVA-5 wt.% SSA (140 °C) | 143 | 159 |
| PVA-5 wt.% SSA (160 °C) | 144 | 158 |
| PVA-9 wt.% SSA (120 °C) | 137 | 152 |
| PVA-9 wt.% SSA (140 °C) | 134 | 150 |
| PVA-9 wt.% SSA (160 °C) | 145 | 159 |
| PVA-23 wt.% SSA (120 °C) | 121 | 139 |
| PVA-23 wt.% SSA (140 °C) | 125 | 140 |
| PVA-23 wt.% SSA (160 °C) | 126 | 143 |
| PVA-33 wt.% SSA (120 °C) | 111 | 132 |
| PVA-33 wt.% SSA (140 °C) | 120 | 137 |
| PVA-33 wt.% SSA (160 °C) | 127 | 142 |
| PVA-50 wt.% SSA (120 °C) | 109 | 130 |
| PVA-50 wt.% SSA (140 °C) | 120 | 136 |
| PVA-50 wt.% SSA (160 °C) | 118 | 134 |
| Liquid | γ | γD | γP |
|---|---|---|---|
| mN·m−1 | mN·m−1 | mN·m−1 | |
| Water | 72.8 | 21.8 | 51.0 |
| Glycerol | 63.4 | 37.0 | 26.4 |
| Diiodomethane | 50.8 | 50.8 | 0.0 |
| Membrane | Crosslinking Temperature [°C] | Water Contact Angle [°] | γD [mJ·m−2] | γP [mJ·m−2] | SFE [mJ·m−2] |
|---|---|---|---|---|---|
| PVA-5 wt.% SSA | 120 | 86 | 33.2 | 1.3 | 34.5 |
| PVA-9 wt.% SSA | 91 | 34.7 | 0.8 | 35.5 | |
| PVA-23 wt.% SSA | 96 | 29.4 | 0.7 | 30.1 | |
| PVA-33 wt.% SSA | 92 | 31.0 | 0.8 | 31.8 | |
| PVA-5 wt.% SSA | 140 | 75 | 33.1 | 2.8 | 35.9 |
| PVA-9 wt.% SSA | 84 | 32.6 | 1.5 | 34.1 | |
| PVA-23 wt.% SSA | 98 | 31.2 | 0.3 | 31.5 | |
| PVA-33 wt.% SSA | 89 | 29.2 | 1.3 | 30.5 | |
| PVA-5 wt.% SSA | 160 | 80 | 33.6 | 1.8 | 35.4 |
| PVA-9 wt.% SSA | 89 | 33.5 | 0.9 | 34.3 | |
| PVA-23 wt.% SSA | 87 | 29.0 | 2.3 | 31.3 | |
| PVA-33 wt.% SSA | 91 | 30.5 | 2.7 | 33.1 |
| Membrane | Crosslinking Temperature [°C] | SDM [mol Solvent/g Dry Membrane] | ||
|---|---|---|---|---|
| Water | Methanol | Propan-2-ol | ||
| PVA-5 wt.% SSA | 120 | 179.3 | 0.9 | 0.01 |
| PVA-9 wt.% SSA | 402.9 | 4.2 | 0.01 | |
| PVA-23 wt.% SSA | 224.6 | 4.1 | 0.04 | |
| PVA-33 wt.% SSA | 166.4 | 3.4 | 0.01 | |
| PVA-50 wt.% SSA | 89.4 | - | - | |
| PVA-5 wt.% SSA | 140 | 149.1 | 0.9 | 0.03 |
| PVA-9 wt.% SSA | 362.4 | 1.7 | 0.12 | |
| PVA-23 wt.% SSA | 237.6 | 6.0 | 0.01 | |
| PVA-33 wt.% SSA | 149.4 | 11.9 | 0.05 | |
| PVA-50 wt.% SSA | 122.2 | - | 3.67 | |
| PVA-5 wt.% SSA | 160 | 174.0 | 0.4 | 0.05 |
| PVA-9 wt.% SSA | 369.6 | 2.0 | 0.04 | |
| PVA-23 wt.% SSA | 264.0 | 6.9 | 0.01 | |
| PVA-33 wt.% SSA | 75.4 | 11.3 | 0.03 | |
| PVA-50 wt.% SSA | - | - | 4.18 | |
| Membrane | Water Activity [–] | P [Barrer] * | P·10−4 [g·mm·m−2·kPa−1·h−1] | P·10−4 [g·m·m−2·atm−1·d−1] | Reference |
|---|---|---|---|---|---|
| PVA-23 wt.% SSA 140 °C | 0.24 | 17 | 4 | 9 | This work |
| 0.41 | 18 | 4 | 10 | This work | |
| 0.59 | 2742 | 596 | 1451 | This work | |
| 0.68 | 9312 | 2022 | 4928 | This work | |
| 0.76 | 13759 | 2981 | 7264 | This work | |
| 0.85 | 32762 | 7113 | 17335 | This work | |
| 0.91 | 63865 | 13819 | 33675 | This work | |
| PVA-4-GA | 0.60 | - | 1.5·10−5 | - | [4] |
| PVOH*.CD | 0.70 | - | - | 8.75 | [6] |
| (PVA.CD)* | 0.70 | - | - | 3.75 | [6] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rynkowska, E.; Fatyeyeva, K.; Marais, S.; Kujawa, J.; Kujawski, W. Chemically and Thermally Crosslinked PVA-Based Membranes: Effect on Swelling and Transport Behavior. Polymers 2019, 11, 1799. https://doi.org/10.3390/polym11111799
Rynkowska E, Fatyeyeva K, Marais S, Kujawa J, Kujawski W. Chemically and Thermally Crosslinked PVA-Based Membranes: Effect on Swelling and Transport Behavior. Polymers. 2019; 11(11):1799. https://doi.org/10.3390/polym11111799
Chicago/Turabian StyleRynkowska, Edyta, Kateryna Fatyeyeva, Stéphane Marais, Joanna Kujawa, and Wojciech Kujawski. 2019. "Chemically and Thermally Crosslinked PVA-Based Membranes: Effect on Swelling and Transport Behavior" Polymers 11, no. 11: 1799. https://doi.org/10.3390/polym11111799
APA StyleRynkowska, E., Fatyeyeva, K., Marais, S., Kujawa, J., & Kujawski, W. (2019). Chemically and Thermally Crosslinked PVA-Based Membranes: Effect on Swelling and Transport Behavior. Polymers, 11(11), 1799. https://doi.org/10.3390/polym11111799







