Characterization of Hydrothermal Deposition of Copper Oxide Nanoleaves on Never-Dried Bacterial Cellulose
Abstract
:1. Introduction
2. Methods
2.1. Materials
2.2. Bacterial Cellulose Production
2.3. Characterization of BC/Metal Oxide Nanocomposites
2.4. XRD Analysis
2.5. Copper(II)Oxide Composite Formation
2.6. Regenerated Cellulose Fibers
3. Results and Discussion
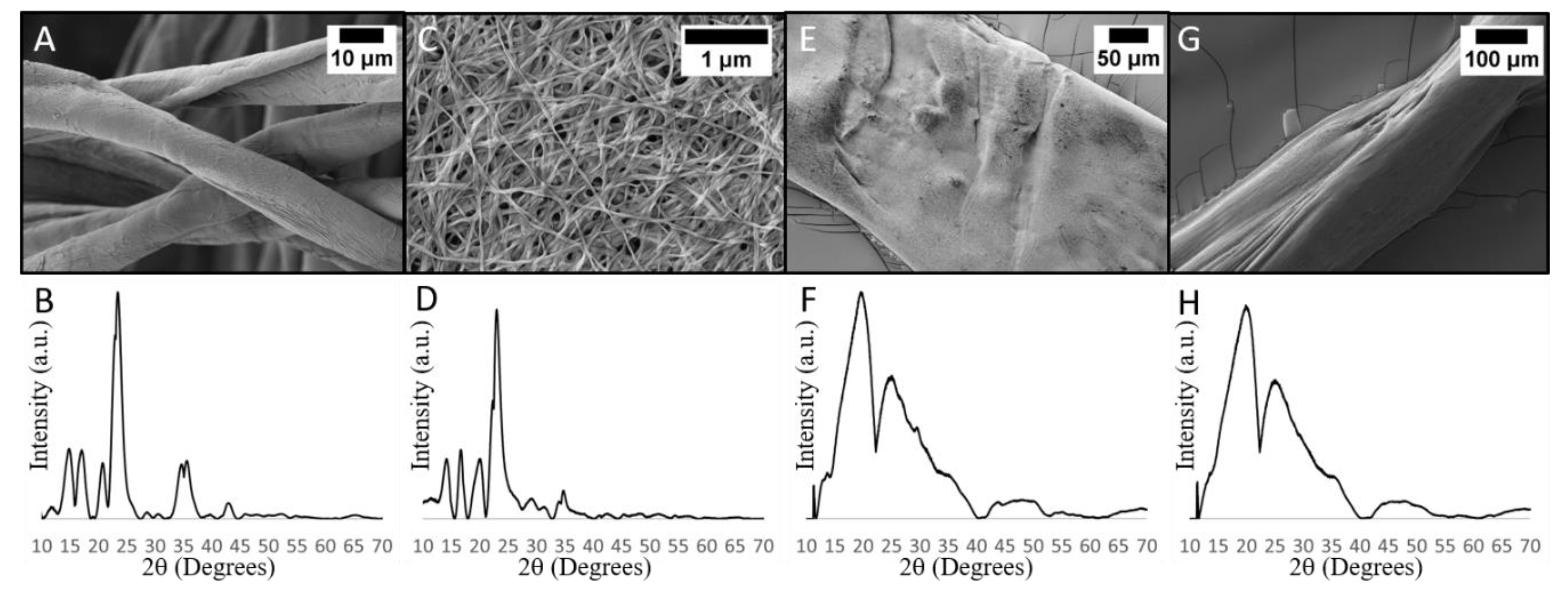
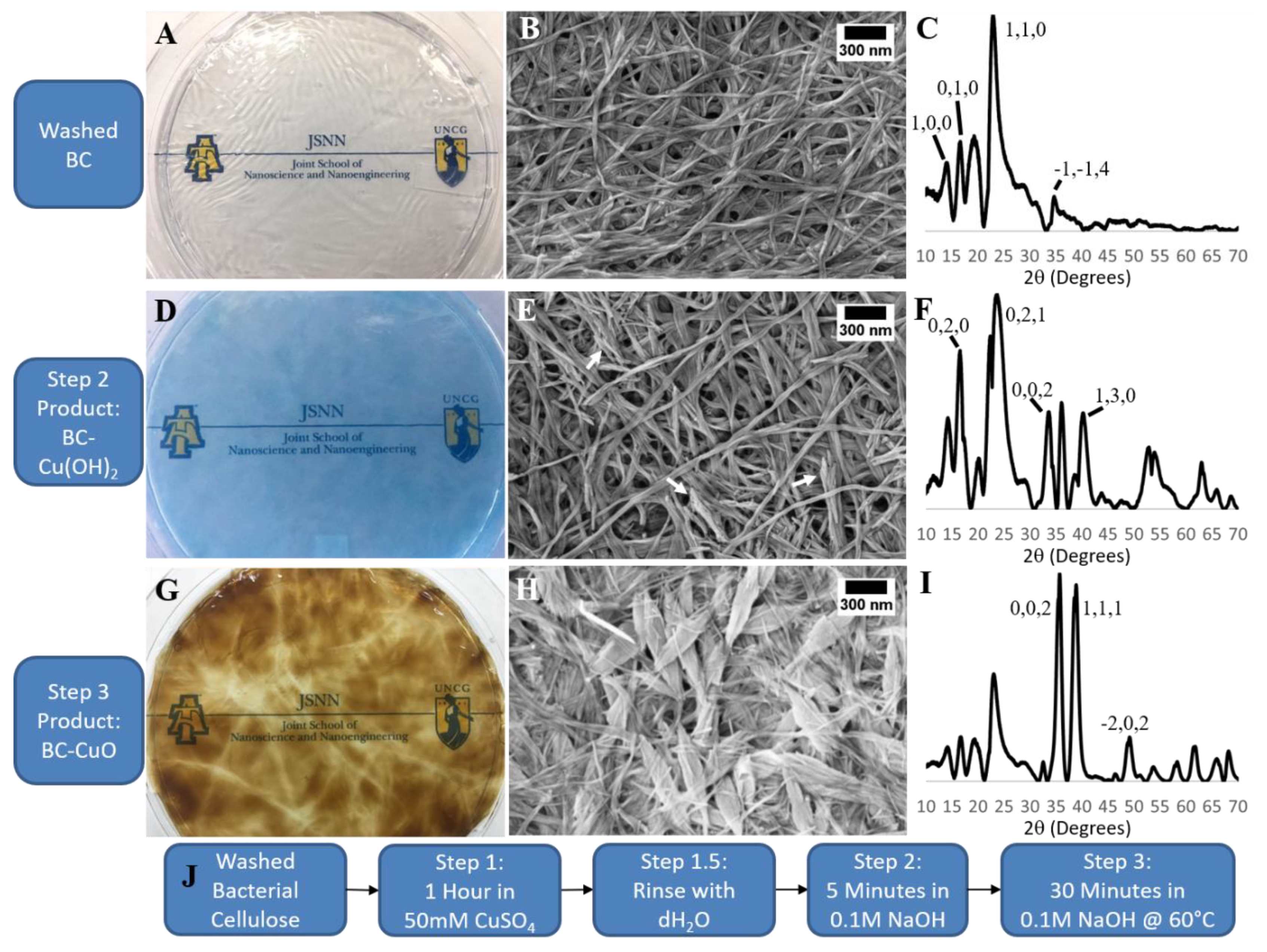
3.1. CuO Nanoleaf Synthesis in a BC Matrix
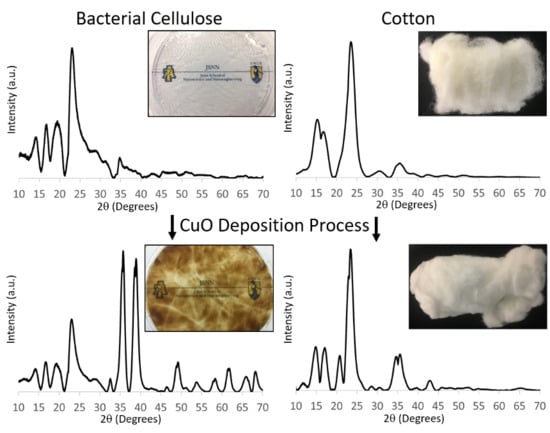
3.2. Comparison of Never-Dried BC and Other Cellulosic Materials after CuO Deposition Process
3.3. Bacterial Cellulose Fiber Organization as a Mechanism for CuSO4 Retention during Rinsing
3.4. When is Never-Dried BC Needed during BC–CuO Synthesis?
3.5. CuO Integration into the BC Matrix
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y. Key Advances in the Chemical Modification of Nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Buldum, G.; Mantalaris, A.; Bismarck, A. More than Meets the Eye in Bacterial Cellulose: Biosynthesis, Bioprocessing, and Applications in Advanced Fiber Composites. Macromol. Biosci. 2014, 14, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Mou, Z.; Xiao, H. Nanocellulose as a Sustainable Biomass Material: Structure, Properties, Present Status and Future Prospects in Biomedical Applications. Nanoscale 2017, 9, 14758–14781. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Scott Williams, W.S.; Cannon, R.E. Alternative Environmental Roles for Cellulose Produced by Acetobacter Xylinum. Appl. Environ. Microbiol. 1989, 55, 2448–2452. [Google Scholar]
- Fang, L.; Catchmark, J.M. Characterization of Water-Soluble Exopolysaccharides from Gluconacetobacter Xylinus and Their Impacts on Bacterial Cellulose Crystallization and Ribbon Assembly. Cellulose 2014, 21, 3965–3978. [Google Scholar] [CrossRef]
- Florea, M.; Hagemann, H.; Santosa, G.; Abbott, J.; Micklem, C.N.; Spencer-Milnes, X.; de Arroyo Garcia, L.; Paschou, D.; Lazenbatt, C.; Kong, D.; et al. Engineering Control of Bacterial Cellulose Production Using a Genetic Toolkit and a New Cellulose-Producing Strain. Proc. Natl. Acad. Sci. USA 2016, 113, E3431–E3440. [Google Scholar] [CrossRef]
- Wiegand, C.; Elsner, P.; Hipler, U.C.; Klemm, D. Protease and ROS Activities Influenced by a Composite of Bacterial Cellulose and Collagen Type I in Vitro. Cellulose 2006, 13, 689–696. [Google Scholar] [CrossRef]
- Ge, Y.; Chen, S.; Yang, J.; Wang, B.; Wang, H. Color-Tunable Luminescent CdTe Quantum Dots Membranes Based on Bacterial Cellulose (BC) and Application in Ion Detection. RSC Adv. 2015, 5, 55756–55761. [Google Scholar] [CrossRef]
- Peng, S.; Fan, L.; Wei, C.; Liu, X.; Zhang, H.; Xu, W.; Xu, J. Flexible Polypyrrole/Copper Sulfide/Bacterial Cellulose Nanofibrous Composite Membranes as Supercapacitor Electrodes. Carbohydr. Polym. 2017, 157, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Ji, P.; Wang, B.; Wang, H.; Chen, S. Color-Tunable Luminescent Macrofibers Based on CdTe QDs-Loaded Bacterial Cellulose Nanofibers for PH and Glucose Sensing. Sens. Actuators B 2018, 254, 110–119. [Google Scholar] [CrossRef]
- Foresti, M.L.; Vazquez, A.; Boury, B. Applications of Bacterial Cellulose as Precursor of Carbon and Composites with Metal Oxide, Metal Sulfide and Metal Nanoparticles: A Review of Recent Advances. Carbohydr. Polym. 2017, 157, 447–467. [Google Scholar] [CrossRef] [PubMed]
- Bin Hussein, M.Z.; Yahaya, A.H.; Ling, P.L.C.; Long, C.W. Acetobacter Xylenium as a Shape-Directing Agent for the Formation of Nano-, Micro-Sized Zinc Oxide. J. Mater. Sci. 2005, 40, 6325–6328. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, W.; Zhou, B.; Yin, N.; Wang, H.; Hu, W. Polyol Mediated Synthesis of ZnO Nanoparticles Templated by Bacterial Cellulose. Carbohydr. Polym. 2012, 92, 1953–1959. [Google Scholar] [CrossRef]
- Sun, D.; Yang, J.; Wang, X. Bacterial Cellulose/TiO2 Hybrid Nanofibers Prepared by the Surface Hydrolysis Method with Molecular Precision. Nanoscale 2010, 2, 287–292. [Google Scholar] [CrossRef]
- Wan, Y.; Yang, Z.; Xiong, G.; Luo, H. A General Strategy of Decorating 3D Carbon Nanofiber Aerogels Derived from Bacterial Cellulose with Nano-Fe3O4 for High-Performance Flexible and Binder-Free Lithium-Ion Battery Anodes. J. Mater. Chem. A 2015, 3, 15386–15393. [Google Scholar] [CrossRef]
- Araújo, I.M.S.; Silva, R.R.; Pacheco, G.; Lustri, W.R.; Tercjak, A.; Gutierrez, J.; Júnior, J.R.S.; Azevedo, F.H.C.; Figuêredo, G.S.; Vega, M.L.; et al. Hydrothermal Synthesis of Bacterial Cellulose—Copper Oxide Nanocomposites and Evaluation of Their Antimicrobial Activity. Carbohydr. Polym. 2018, 179, 341–349. [Google Scholar] [CrossRef]
- Pawar, S.M.; Pawar, B.S.; Inamdar, A.I.; Kim, J.; Jo, Y.; Cho, S.; Mali, S.S.; Hong, C.K.; Kwak, J.; Kim, H.; et al. In-Situ Synthesis of Cu(OH)2 and CuO Nanowire Electrocatalysts for Methanol Electro-Oxidation. Mater. Lett. 2017, 187, 60–63. [Google Scholar] [CrossRef]
- Shinde, S.K.; Fulari, V.J.; Kim, D.Y.; Maile, N.C.; Koli, R.R.; Dhaygude, H.D.; Ghodake, G.S. Chemical Synthesis of Flower-like Hybrid Cu(OH)2/CuO Electrode: Application of Polyvinyl Alcohol and Triton X-100 to Enhance Supercapacitor Performance. Colloids Surf. B Biointerfaces 2017, 156, 165–174. [Google Scholar] [CrossRef]
- Sang, X.; Zhang, J.; Wu, T.; Zhang, B.; Ma, X.; Peng, L.; Han, B.; Kang, X.; Liu, C.; Yang, G. Room-Temperature Synthesis of Mesoporous CuO and Its Catalytic Activity for Cyclohexene Oxidation. RSC Adv. 2015, 5, 67168–67174. [Google Scholar] [CrossRef]
- USGS Coastal and Marine Geology Program. Templates and Tables for Converting Degrees Two Theta to D-Spacings. Available online: https://pubs.usgs.gov/of/2001/of01-041/htmldocs/methods/2theta2d.htm (accessed on 2 April 2019).
- NileRed. How to Dissolve Cotton in Water. Available online: https://www.youtube.com/watch?v=tvoimHLHdnA&t (accessed on 2 April 2019).
- Breslau, A. A Simplified Preparation of Schewitzer’s Reagent. J. Chem. Educ. 1942, 19, 356. [Google Scholar] [CrossRef]
- Wilson, W.K.; Launer, H.F. Preparing Cuprammonium Solvent and Cellulose Solutions. Anal. Chem. 1950, 22, 455–458. [Google Scholar] [CrossRef]
- Burchard, W.; Habermann, N.; Klufers, P.; Serger, B.; Wilhelm, U. Cellulose in Schewizer’s Reagent: A Stable, Polymeric Metal Complex with High Chain Stiffness. Angew. Chem. Int. Ed. 1994, 33, 884–887. [Google Scholar] [CrossRef]
- Du, G.H.; Van Tendeloo, G. Cu(OH)2 Nanowires, CuO Nanowires and CuO Nanobelts. Chem. Phys. Lett. 2004, 393, 64–69. [Google Scholar] [CrossRef]
- Cudennec, Y.; Lecerf, A. The Transformation of Cu(OH)2 into CuO, Revisited. Solid State Sci. 2003, 5, 1471–1474. [Google Scholar] [CrossRef]
- Ibupoto, Z.H.; Khun, K.; Lu, J.; Willander, M.; Ibupoto, Z.H.; Khun, K.; Lu, J.; Willander, M. The Synthesis of CuO Nanoleaves, Structural Characterization, and Their Glucose Sensing Application. Appl. Phys. Lett. 2013, 102, 103701. [Google Scholar] [CrossRef]
- Zhou, N.; Yuan, M.; Li, D.; Yang, D. One-Pot Fast Synthesis of Leaf-Like CuO Nanostructures and CuO/Ag Microspheres with Photocatalytic Application. Nano 2017, 12, 1750035. [Google Scholar] [CrossRef]
- Li, Y.; Tan, B.; Wu, Y. Ammonia-Evaporation-Induced Synthetic Method for Metal (Cu, Zn, Cd, Ni) Hydroxide/Oxide Nanostructures. Chem. Mater. 2008, 20, 567–576. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, J.; Li, Y.; Ma, D.; Hou, S.; Li, L.; Hao, X.; Wang, Z. Room Temperature Synthesis of 2D CuO Nanoleaves in Aqueous Solution. Nanotechnology 2011, 22, 115604. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Shi, L.; Gao, M. Surfactant-Free Synthesis of CuO with Controllable Morphologies and Enhanced Photocatalytic Property. Nanoscale Res. Lett. 2016, 11, 125. [Google Scholar] [CrossRef] [PubMed]
- Cousins, S.K.; Brown, R.M. Cellulose-I Microfibril Assembly: Computational Molecular Mechanics Energy Analysis Favors Bonding by Vanderwaals Forces As the Initial Step in Crystallization. Polymer 1995, 36, 3885–3888. [Google Scholar] [CrossRef]
- Déléris, I.; Wallecan, J. Relationship between Processing History and Functionality Recovery after Rehydration of Dried Cellulose-Based Suspensions: A Critical Review. Adv. Colloid Interface Sci. 2017, 246, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pönni, R.; Vuorinen, T.; Kontturi, E. Proposed Nano-Scale Coalescence of Cellulose in Chemical Pulp Fibers during Technical Treatments. BioResources 2012, 7, 6077–6108. [Google Scholar] [CrossRef]
- Yamane, C. Structure Formation of Regenerated Cellulose from Its Solution and Resultant Features of High Wettability: A Review. Nord. Pulp Paper Res. J. 2015, 30, 78–91. [Google Scholar] [CrossRef]
- Torres, F.G.; Arroyo, J.J.; Troncoso, O.P. Bacterial Cellulose Nanocomposites: An All-Nano Type of Material. Mater. Sci. Eng. C 2019, 98, 1277–1293. [Google Scholar] [CrossRef]
- Bogdanova, O.I.; Chvalun, S.N. Polysaccharide-Based Natural and Synthetic Nanocomposites. Polym. Sci. Ser. A 2016, 58, 629–658. [Google Scholar] [CrossRef]
- Martínez-sanz, M.; Mikkelsen, D.; Flanagan, B.; Gidley, M.J.; Gilbert, E.P. Multi-Scale Model for the Hierarchical Architecture of Native Cellulose Hydrogels. Carbohydr. Polym. 2016, 147, 542–555. [Google Scholar] [CrossRef]
- Sun, J.; Guan, J.; Xiang, J.; Zhang, L.; Zhou, J.; Liu, S. Fiberlike Fe2O3 Macroporous Nanomaterials Fabricated by Calcinating Regenerate Cellulose Composite Fibers. Chem. Mater. 2008, 20, 3623–3628. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warren, W.R.; LaJeunesse, D.R. Characterization of Hydrothermal Deposition of Copper Oxide Nanoleaves on Never-Dried Bacterial Cellulose. Polymers 2019, 11, 1762. https://doi.org/10.3390/polym11111762
Warren WR, LaJeunesse DR. Characterization of Hydrothermal Deposition of Copper Oxide Nanoleaves on Never-Dried Bacterial Cellulose. Polymers. 2019; 11(11):1762. https://doi.org/10.3390/polym11111762
Chicago/Turabian StyleWarren, W. Ross, and Dennis R. LaJeunesse. 2019. "Characterization of Hydrothermal Deposition of Copper Oxide Nanoleaves on Never-Dried Bacterial Cellulose" Polymers 11, no. 11: 1762. https://doi.org/10.3390/polym11111762
APA StyleWarren, W. R., & LaJeunesse, D. R. (2019). Characterization of Hydrothermal Deposition of Copper Oxide Nanoleaves on Never-Dried Bacterial Cellulose. Polymers, 11(11), 1762. https://doi.org/10.3390/polym11111762