Stabilization of Silver Nanoparticles by Cationic Aminoethyl Methacrylate Copolymers in Aqueous Media—Effects of Component Ratios and Molar Masses of Copolymers
Abstract
1. Introduction
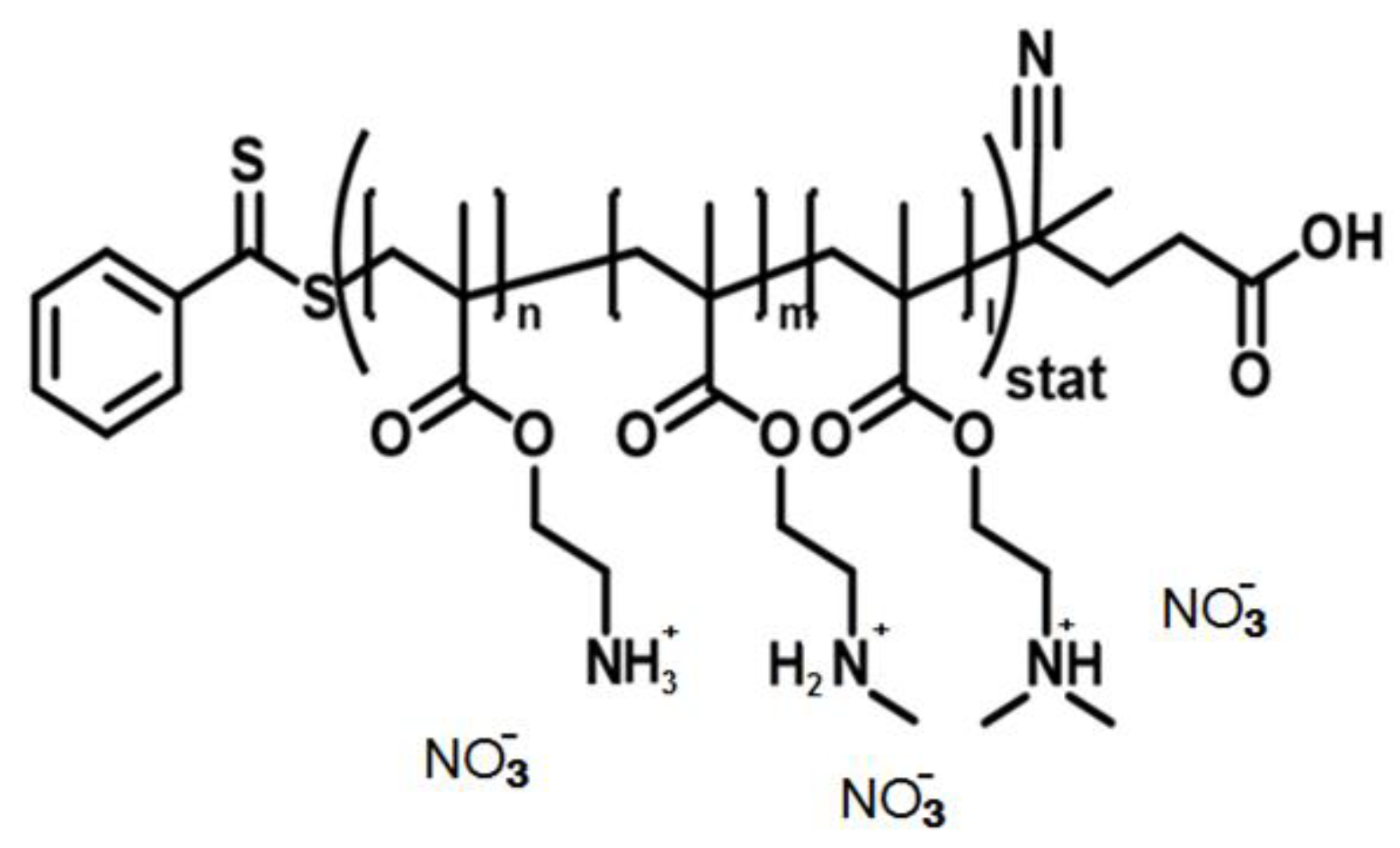
2. Materials, Methods and Synthetic Procedure for Stabilized Nanoparticles
2.1. Methods
2.2. Materials
2.3. Synthetic Procedure for Nanoparticles
3. Results and Discussion
3.1. The Effect of the Fraction of Reducing Agent
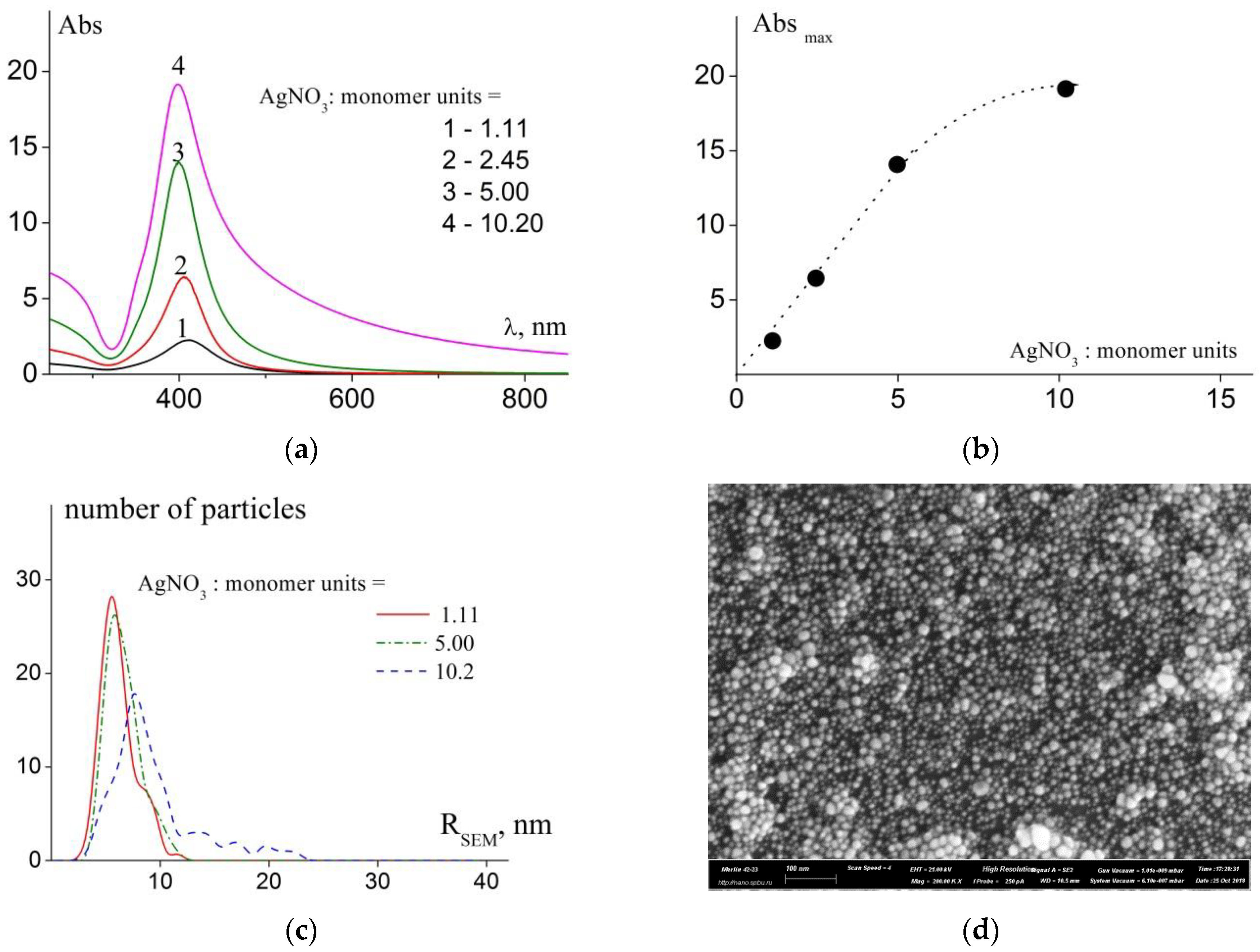
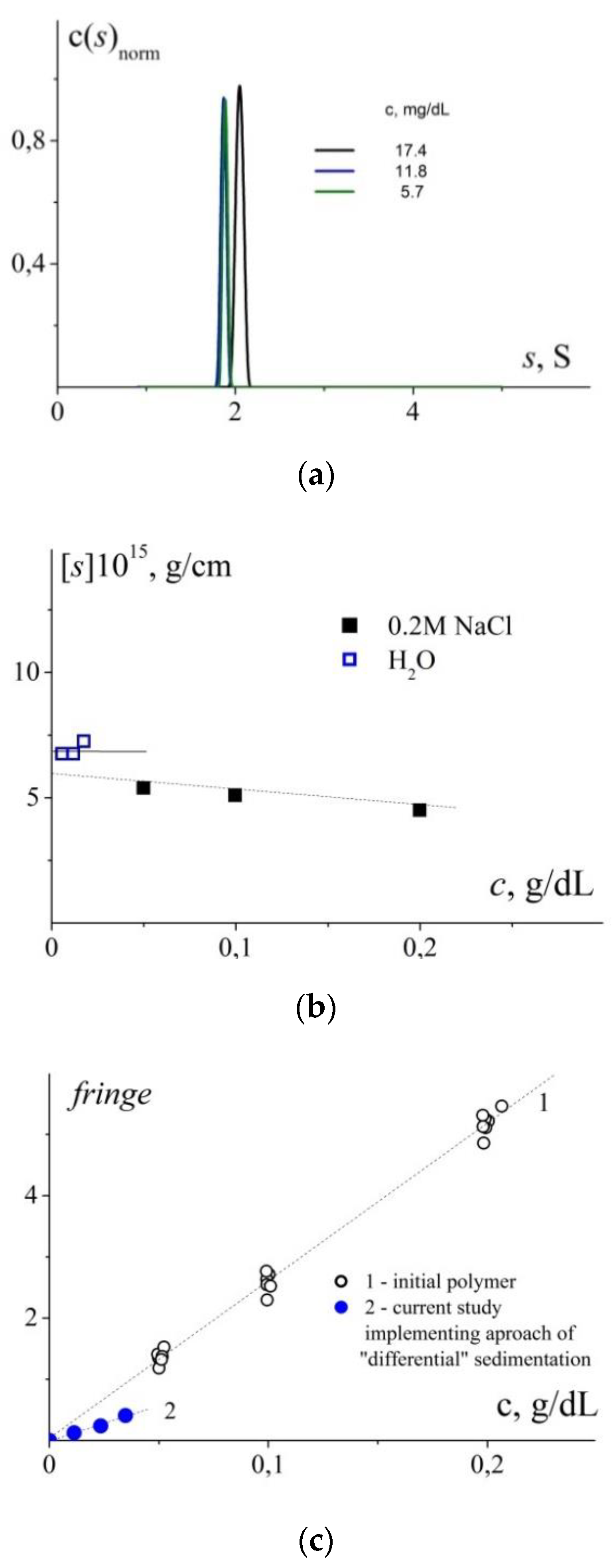
3.2. Holding Capacity of the Copolymers
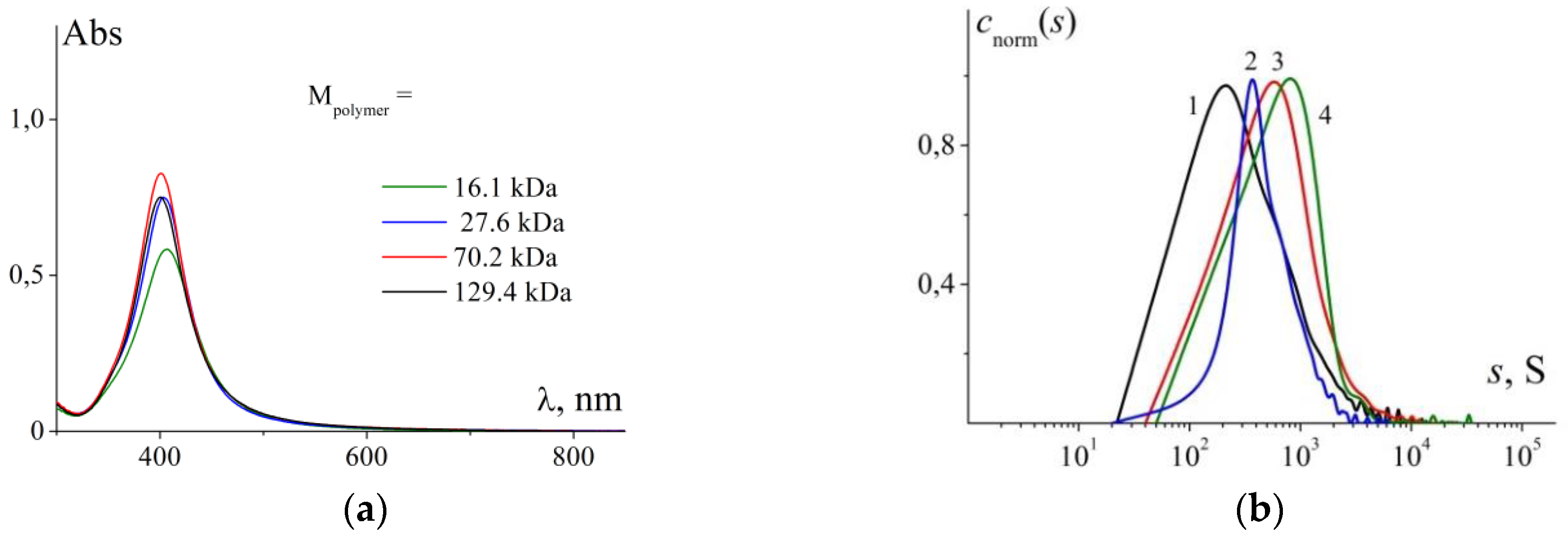
3.3. The Effect of Molar Mass
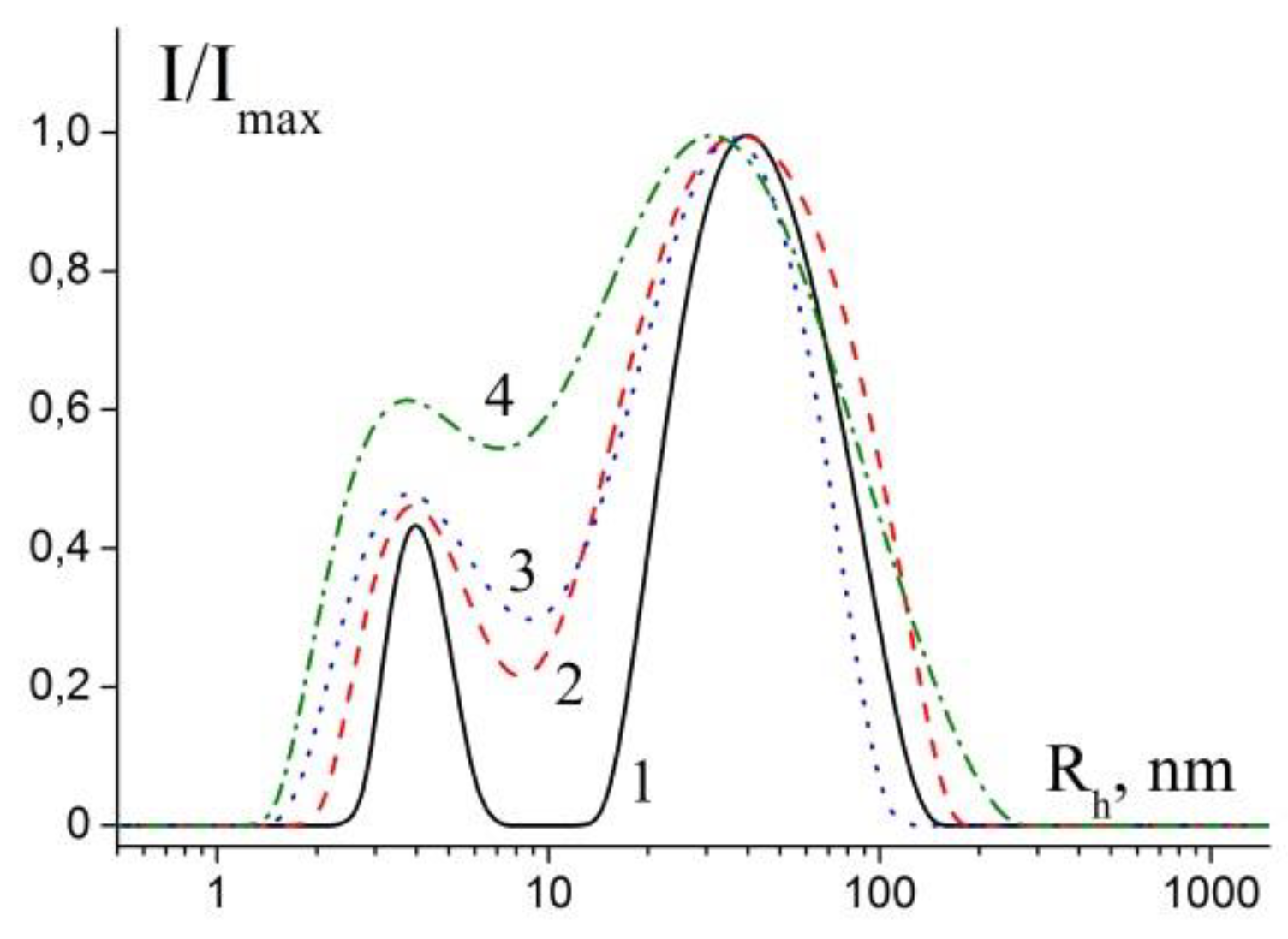
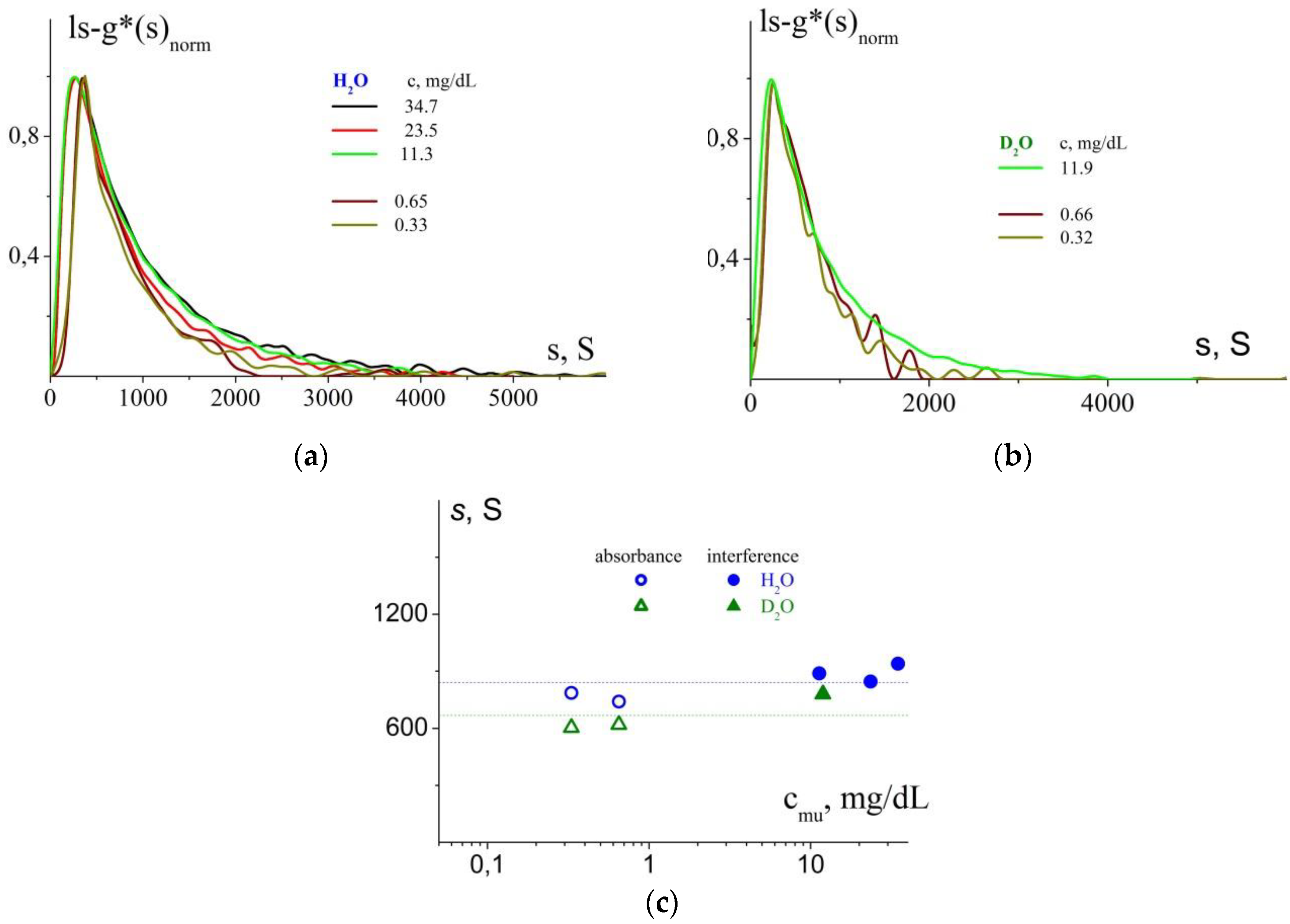
3.4. Analysis of Hydrodynamic Sizes of Stabilized NPs by AUC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kosmella, S.; Koetz, J. Polyelectrolytes and Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-3-540-46381-8. [Google Scholar]
- Capek, I. Noble Metal Nanoparticles; Nanostructure Science and Technology; Springer: Tokyo, Japan, 2017; ISBN 978-4-431-56554-3. [Google Scholar]
- Abdullaeva, Z. Synthesis of Nanoparticles and Nanomaterials; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-54074-0. [Google Scholar]
- Taghavizadeh Yazdi, M.E.; Hamidi, A.; Amiri, M.S.; Kazemi Oskuee, R.; Hosseini, H.A.; Hashemzadeh, A.; Darroudi, M. Eco-friendly and plant-based synthesis of silver nanoparticles using Allium giganteum and investigation of its bactericidal, cytotoxicity and photocatalytic effects. Mater. Technol. 2019, 34, 490–497. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Iqbal, H.M.N.; Hu, H.; Wang, W.; Zhang, X. Macromolecular agents with antimicrobial potentialities: A drive to combat antimicrobial resistance. Int. J. Biol. Macromol. 2017, 103, 554–574. [Google Scholar] [CrossRef]
- Haes, A.J.; Hall, W.P.; Chang, L.; Klein, W.L.; Van Duyne, R.P. A Localized Surface Plasmon Resonance Biosensor: First Steps toward an Assay for Alzheimer’s Disease. Nano Lett. 2004, 4, 1029–1034. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Chen, Z.; Zhou, L.; Xu, H.; Zhu, W. Fabrication of flower-like Bi2WO6 superstructures as high performance visible-light driven photocatalysts. J. Mater. Chem. 2007, 17, 2526–2532. [Google Scholar] [CrossRef]
- Kosiorek, A.; Kandulski, W.; Chudzinski, P.; Kempa, K.; Giersig, M. Shadow Nanosphere Lithography: Simulation and Experiment. Nano Lett. 2004, 4, 1359–1363. [Google Scholar] [CrossRef]
- Yan, F.; Goedel, W.A. A Simple and Effective Method for the Preparation of Porous Membranes with Three-Dimensionally Arranged Pores. Adv. Mater. 2004, 16, 911–915. [Google Scholar] [CrossRef]
- Wu, M.-H.; Whitesides, G.M. Fabrication of arrays of two-dimensional micropatterns using microspheres as lenses for projection photolithography. Appl. Phys. Lett. 2001, 78, 2273–2275. [Google Scholar] [CrossRef][Green Version]
- Baruwati, B.; Polshettiwar, V.; Varma, R.S. Glutathione promoted expeditious green synthesis of silver nanoparticles in water using microwaves. Green Chem. 2009, 11, 926–930. [Google Scholar] [CrossRef]
- Hasell, T.; Yang, J.; Wang, W.; Brown, P.D.; Howdle, S.M. A facile synthetic route to aqueous dispersions of silver nanoparticles. Mater. Lett. 2007, 61, 4906–4910. [Google Scholar] [CrossRef]
- Mostafavi, M.; Dey, G.R.; François, L.; Belloni, J. Transient and Stable Silver Clusters Induced by Radiolysis in Methanol. J. Phys. Chem. A 2002, 106, 10184–10194. [Google Scholar] [CrossRef]
- Zheng, M.; Gu, M.; Jin, Y.; Jin, G. Optical properties of silver-dispersed PVP thin film. Mater. Res. Bull. 2001, 36, 853–859. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, S.; Wang, C.; Li, X.; Zhu, Y.; Chen, Z. A novel ultraviolet irradiation photoreduction technique for the preparation of single-crystal Ag nanorods and Ag dendrites. Adv. Mater. 1999, 11, 850–852. [Google Scholar] [CrossRef]
- Cai, J.; Kimura, S.; Wada, M.; Kuga, S. Nanoporous Cellulose as Metal Nanoparticles Support. Biomacromolecules 2009, 10, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yuan, Q.; Yang, X. Preparation and characterization of metal–chitosan nanocomposites. Colloids Surf. B Biointerfaces 2004, 39, 31–37. [Google Scholar] [CrossRef]
- You, J.; Xiang, M.; Hu, H.; Cai, J.; Zhou, J.; Zhang, Y. Aqueous synthesis of silver nanoparticles stabilized by cationic cellulose and their catalytic and antibacterial activities. RSC Adv. 2013, 3, 19319–19329. [Google Scholar] [CrossRef]
- Klimov, D.I.; Zezina, E.A.; Lipik, V.C.; Abramchuk, S.S.; Yaroslavov, A.A.; Feldman, V.I.; Sybachin, A.V.; Spiridonov, V.V.; Zezin, A.A. Radiation-induced preparation of metal nanostructures in coatings of interpolyelectrolyte complexes. Radiat. Phys. Chem. 2019, 162, 23–30. [Google Scholar] [CrossRef]
- Mayer, A.B.R.; Hausner, S.H.; Mark, J.E. Colloidal Silver Nanoparticles Generated in the Presence of Protective Cationic Polyelectrolytes. Polym. J. 2000, 32, 15–22. [Google Scholar] [CrossRef]
- Mayer, A. Poly(2-hydroxyalkyl methacrylates) as stabilizers for colloidal noble metal nanoparticles. Polymer 2000, 41, 1627–1631. [Google Scholar] [CrossRef]
- Samal, S.K.; Dash, M.; Van Vlierberghe, S.; Kaplan, D.L.; Chiellini, E.; van Blitterswijk, C.; Moroni, L.; Dubruel, P. Cationic polymers and their therapeutic potential. Chem. Soc. Rev. 2012, 41, 7147–7194. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Astafyeva, I.V.; Chikindas, M.L.; Rosenblat, G.F.; Kiselev, V.I.; Severin, E.S.; Kabanov, V.A. DNA interpolyelectrolyte complexes as a tool for efficient cell transformation. Biopolymers 1991, 31, 1437–1443. [Google Scholar] [CrossRef]
- Ahmed, M.; Narain, R. Progress of RAFT based polymers in gene delivery. Prog. Polym. Sci. 2013, 38, 767–790. [Google Scholar] [CrossRef]
- Perevyazko, I.; Trützschler, A.-K.; Gubarev, A.; Lebedeva, E.; Traeger, A.; Tsvetkov, N.; Schubert, U.S. Absolute characteristics and conformation of cationic polymers by hydrodynamic approaches: Poly(AEMA-co-MAEMA-co-DMAEMA) copolymers. Eur. Polym. J. 2017, 97, 347–355. [Google Scholar] [CrossRef]
- Lowe, A.B.; McCormick, C.L. Polyelectrolytes and Polyzwitterions: Synthesis, Properties and Applications; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2006; ISBN 978-0-8412-3958-6. [Google Scholar]
- Senchukova, A.S.; Mikhailova, M.E.; Lezov, A.A.; Lebedeva, E.V.; Podseval’nikova, A.N.; Tsvetkov, N.V. Stabilization of Silver Nanoparticles in Water with a Cationic Copolymer Based on Poly(Aminoethyl Methacrylate). Colloid J. 2019, 81, 272–276. [Google Scholar] [CrossRef]
- Cummins, H.Z.; Pike, E.R. Photon Correlation and Light Beating Spectroscopy; Springer: Boston, MA, USA, 1974; ISBN 978-1-4615-8908-2. [Google Scholar]
- Schärtl, W. Light Scattering from Polymer Solutions and Nanoparticle Dispersions; Springer Laboratory Manuals in Polymer Science; Springer: Berlin, Germany; New York, NY, USA, 2007; ISBN 978-3-540-71950-2. [Google Scholar]
- Schuck, P. Size-Distribution Analysis of Macromolecules by Sedimentation Velocity Ultracentrifugation and Lamm Equation Modeling. Biophys. J. 2000, 78, 1606–1619. [Google Scholar] [CrossRef]
- Provencher, S.W. CONTIN: A general purpose constrained regularization program for inverting noisy linear algebraic and integral equations. Comput. Phys. Commun. 1982, 27, 229–242. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.K.; Aveyard, J.; Fernig, D.G. Determination of Size and Concentration of Gold Nanoparticles from UV-Vis Spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef]
- Liu, X.; Atwater, M.; Wang, J.; Huo, Q. Extinction coefficient of gold nanoparticles with different sizes and different capping ligands. Colloids Surf. B Biointerfaces 2007, 58, 3–7. [Google Scholar] [CrossRef]
- Millero, F.J.; Dexter, R.; Hoff, E. Density and viscosity of deuterium oxide solutions from 5-70.deg. J. Chem. Eng. Data 1971, 16, 85–87. [Google Scholar] [CrossRef]
- Scott, D.; Harding, S.E.; Rowe, A. Analytical Ultracentrifugation Techniques and Methods; Royal Society of Chemistry: London, UK, 2005; ISBN 978-1-84755-261-7. [Google Scholar]
- Schuck, P. Basic Principles of Analytical Ultracentrifugation; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2016; ISBN 978-1-4987-5115-5. [Google Scholar]
- Pavlov, G.; Finet, S.; Tatarenko, K.; Korneeva, E.; Ebel, C. Conformation of heparin studied with macromolecular hydrodynamic methods and X-ray scattering. Eur. Biophys. J. 2003, 32, 437–449. [Google Scholar] [CrossRef]
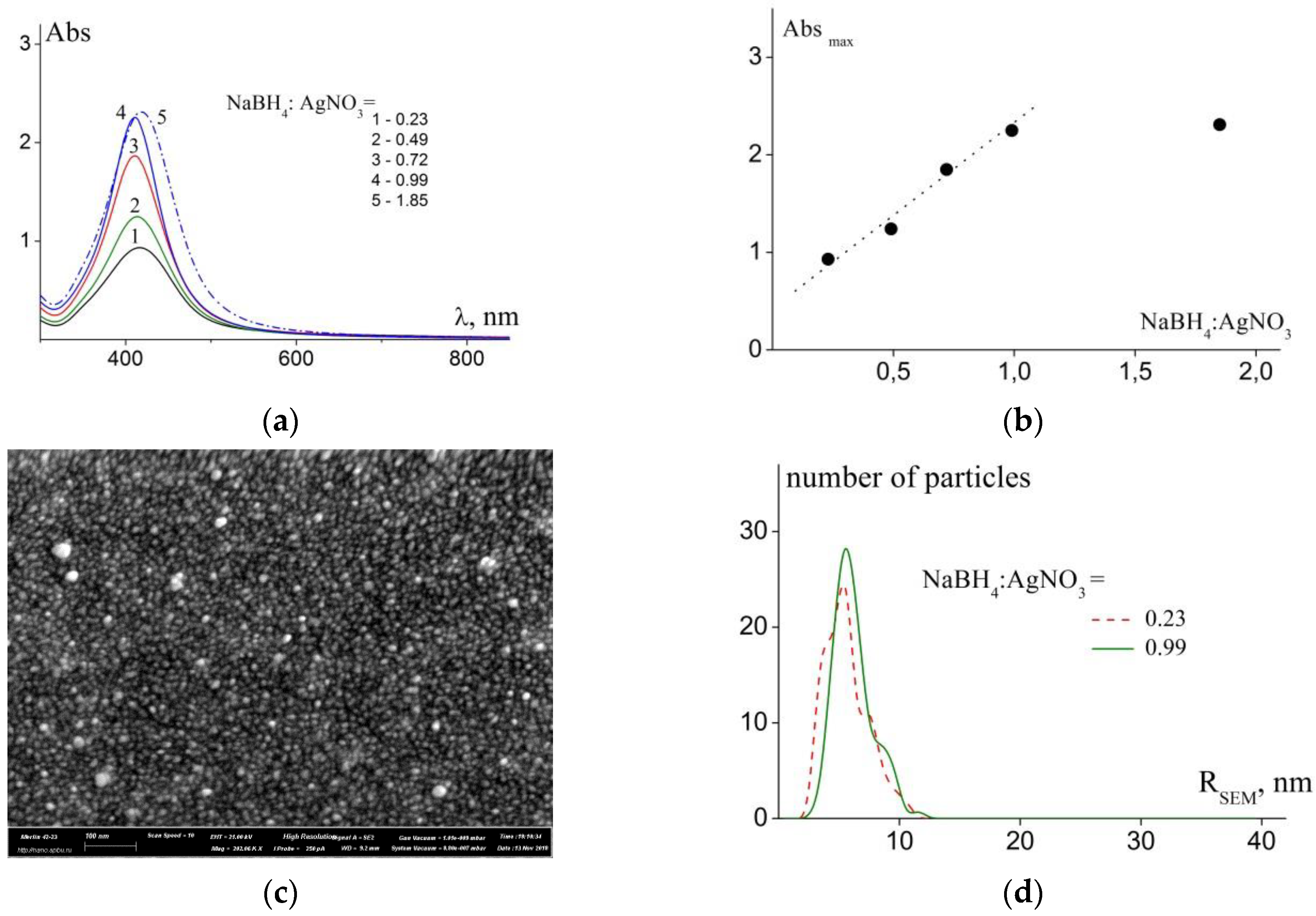
| NaBH4: AgNO3 | D01, 10−7 cm2/s | Rh1, nm | D02, 10−8 cm2/s | Rh2, nm | <Rh>c (PDI), nm | RSEM (X), nm | λmax, nm | fwhm, nm |
|---|---|---|---|---|---|---|---|---|
| 1.85 | 1.7 | 14.2 | 3.6 | 67 | 18 (2.3) | - | 418 | 95 |
| 0.99 | 3.58 | 6.8 | 4.74 | 52 | 19 (2.4) | 5.6 (2.8) | 411 | 72 |
| 0.72 | 3.6 | 7.6 | 6.3 | 43 | 16 (2.5) | - | 411 | 81 |
| 0.49 | 2.8 | 11.1 | 5.6 | 60 | 14 (2.7) | - | 415 | 91 |
| 0.23 | 3.3 | 7.8 | 3.8 | 75 | 12 (2.4) | 5.3 (3.4) | 416 | 100 |
| AgNO3:monomer units | D01, 10−7 cm2/s | Rh1, nm | D02, 10−8 cm2/s | Rh2, nm | <Rh>c (PDI), nm | RSEM (X), nm | λmax, nm | fwhm, nm |
|---|---|---|---|---|---|---|---|---|
| 1.11 | 3.58 | 6.8 | 4.74 | 52 | 19 (2.4) | 5.6 (2.8) | 411 | 72 |
| 2.45 | 5.60 | 4.4 | 11.2 | 22 | 12 (2.2) | - | 405 | 57 |
| 5.00 | 4.9 | 5.3 | 9.0 | 27 | 7 (2.6) | 5.8 (3.4) | 402 | 57 |
| 10.2 | 1.43 | 17.1 | - | - | 10 (2.0) | 7.7 (4.2) | 398 | 91 |
| Mpolymer, kDa | D01, 10−7 cm2/s | Rh1, nm | D02, 10−8 cm2/s | Rh2, nm | Rh *, nm | λmax, nm | fwhm, nm |
|---|---|---|---|---|---|---|---|
| 16.1 | 4.9 | 5.0 | 10.0 | 24 | 2.7 | 407 | 61 |
| 27.6 | 4.9 | 5.3 | 9.0 | 27 | 3.5 | 402 | 57 |
| 70.2 | 5.4 | 4.5 | 10.6 | 23 | 5.9 | 401 | 53 |
| 129.4 | 2.4 | 10.1 | 5.3 | 46 | 8.7 | 400 | 55 |
| Mpolymer, kDa | s, S | Rsf., nm | <Rh>c (PDI), nm | Rh1/Rh2 [DLS], nm |
|---|---|---|---|---|
| 16.1 | 1400 | 19 (12–29) * | 17 (2.7) | 5.0/24 |
| 27.6 | 840 | 15 (9–22) * | 7 (2.6) | 5.3/27 |
| 70.2 | 1900 | 22 (14–34) * | 11 (2.1) | 4.5/23 |
| 129.4 | 1100 | 17 (11–26) * | 13 (2.1) | 10.1/26 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhailova, M.E.; Senchukova, A.S.; Lezov, A.A.; Gubarev, A.S.; Trützschler, A.-K.; Schubert, U.S.; Tsvetkov, N.V. Stabilization of Silver Nanoparticles by Cationic Aminoethyl Methacrylate Copolymers in Aqueous Media—Effects of Component Ratios and Molar Masses of Copolymers. Polymers 2019, 11, 1647. https://doi.org/10.3390/polym11101647
Mikhailova ME, Senchukova AS, Lezov AA, Gubarev AS, Trützschler A-K, Schubert US, Tsvetkov NV. Stabilization of Silver Nanoparticles by Cationic Aminoethyl Methacrylate Copolymers in Aqueous Media—Effects of Component Ratios and Molar Masses of Copolymers. Polymers. 2019; 11(10):1647. https://doi.org/10.3390/polym11101647
Chicago/Turabian StyleMikhailova, Mariya E., Anna S. Senchukova, Alexey A. Lezov, Alexander S. Gubarev, Anne -K. Trützschler, Ulrich S. Schubert, and Nikolay V. Tsvetkov. 2019. "Stabilization of Silver Nanoparticles by Cationic Aminoethyl Methacrylate Copolymers in Aqueous Media—Effects of Component Ratios and Molar Masses of Copolymers" Polymers 11, no. 10: 1647. https://doi.org/10.3390/polym11101647
APA StyleMikhailova, M. E., Senchukova, A. S., Lezov, A. A., Gubarev, A. S., Trützschler, A.-K., Schubert, U. S., & Tsvetkov, N. V. (2019). Stabilization of Silver Nanoparticles by Cationic Aminoethyl Methacrylate Copolymers in Aqueous Media—Effects of Component Ratios and Molar Masses of Copolymers. Polymers, 11(10), 1647. https://doi.org/10.3390/polym11101647









