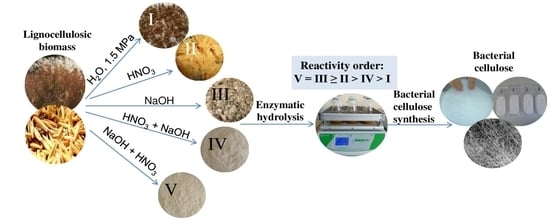
Pretreatments of Non-Woody Cellulosic Feedstocks for Bacterial Cellulose Synthesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstocks
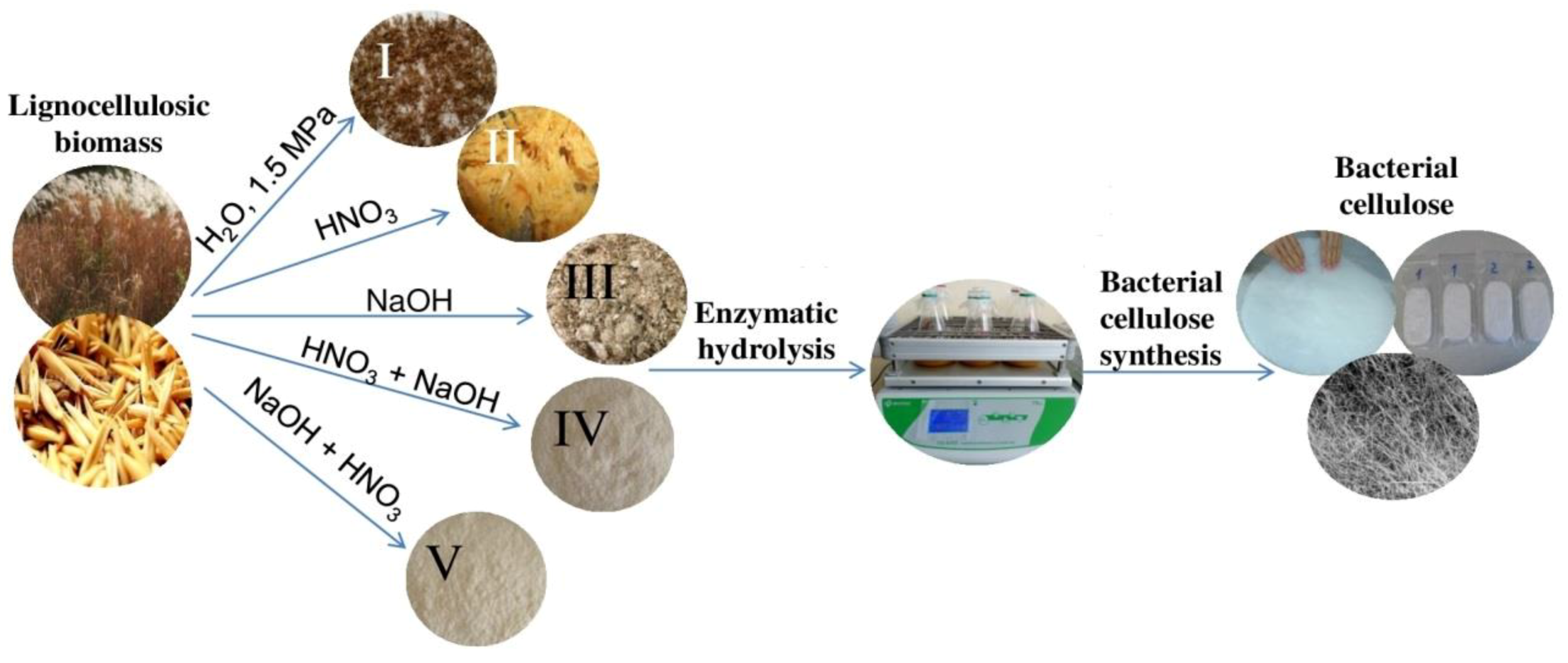
2.2. Pretreatments
2.2.1. Hydrothermobaric Treatment (HTBT)
2.2.2. Dilute Nitric-Acid Treatment (DNAT)
2.2.3. Alkaline Delignification (AD)
2.2.4. Nitric-Acid Pulping Method (NAPM)
2.2.5. Combined Pulping Method (CPM)
2.3. Analysis of Feedstocks and Pretreatment Products
2.4. Enzymes and Hydrolysis
2.5. Analysis of Hydrolyzates
2.6. Biosynthesis of BC in Nutrient Broths of Enzymatic Hydrolyzates of Pretreatment Products
3. Results and Discussion
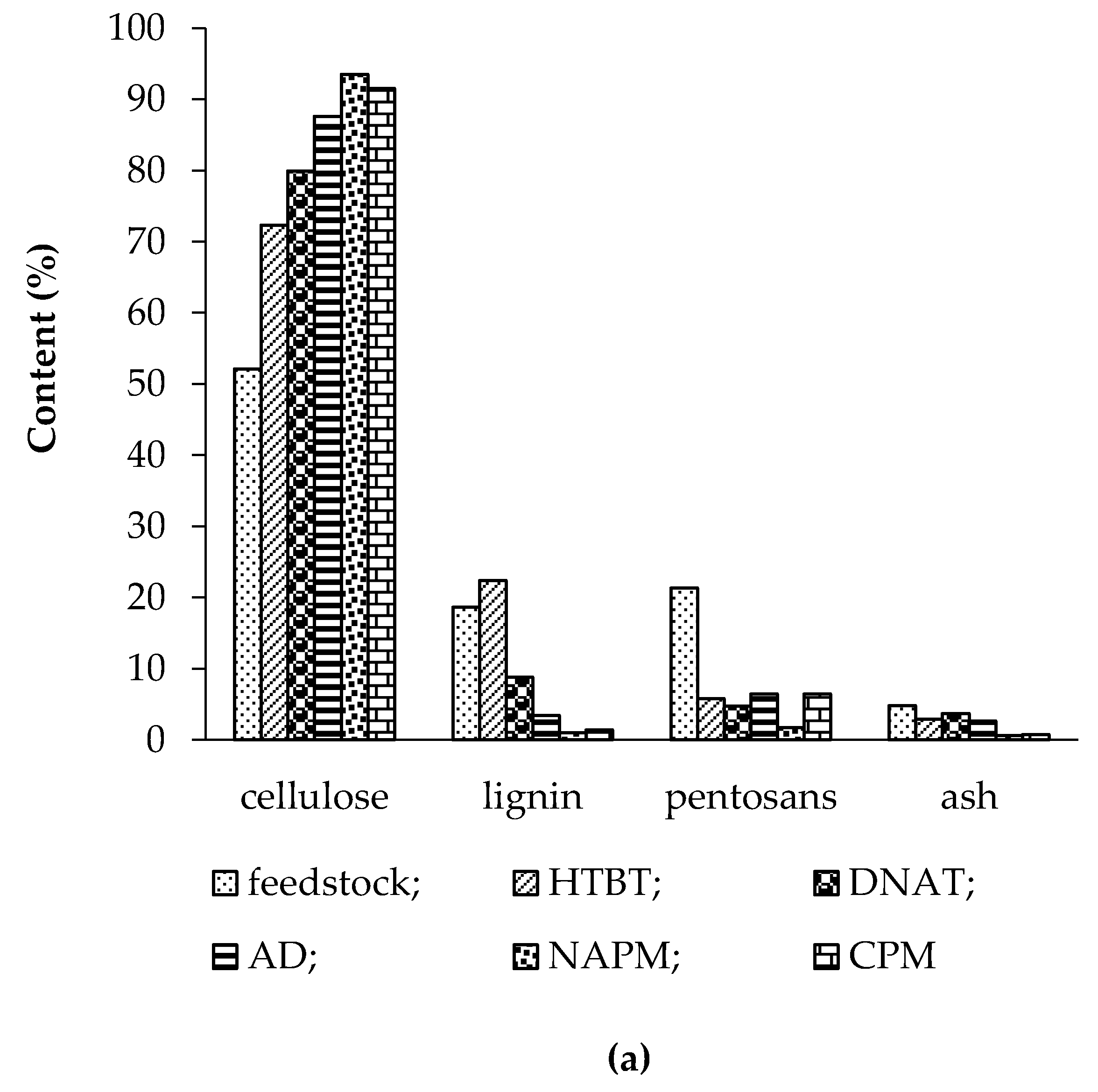
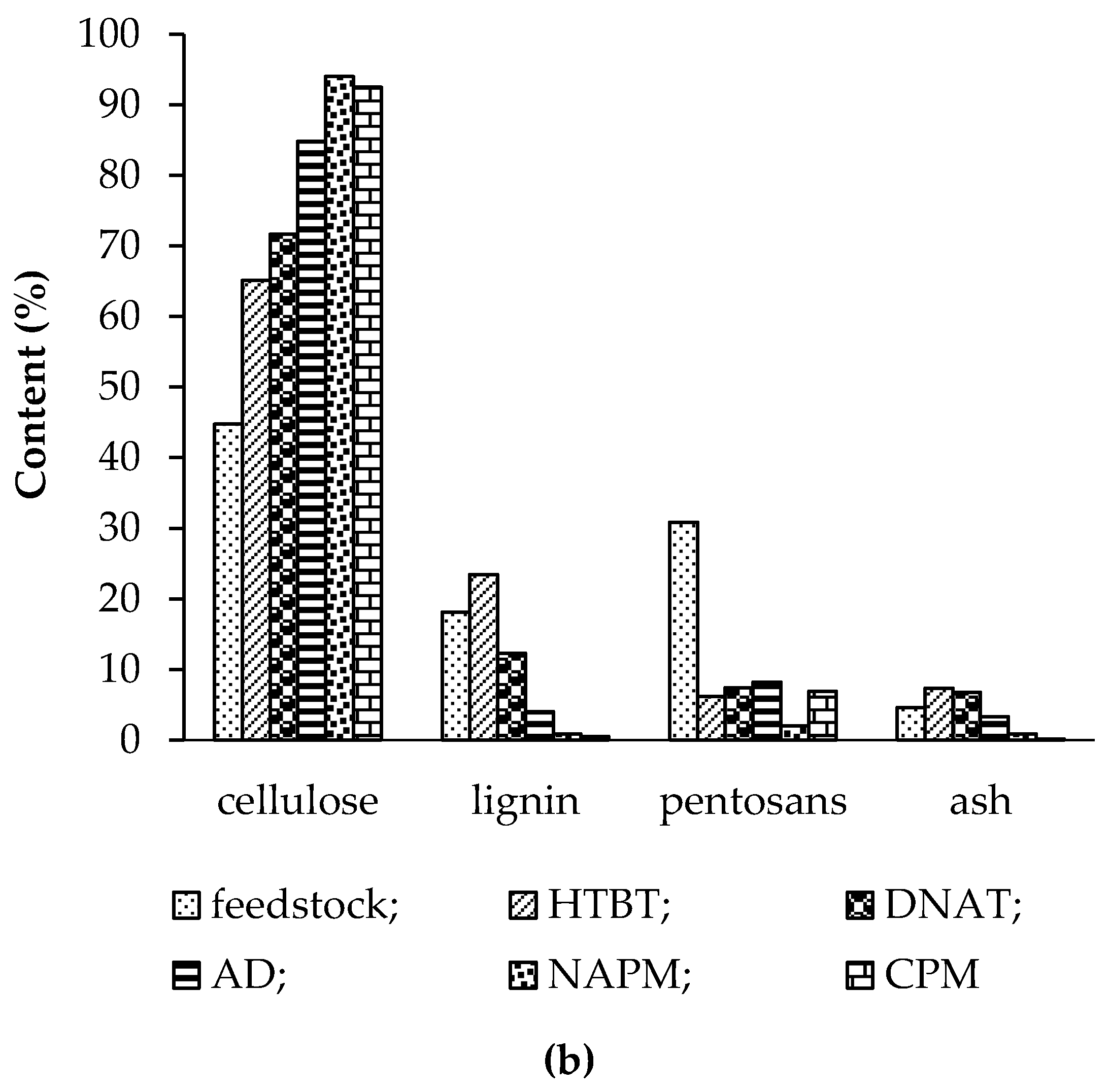
3.1. Effect of Pretreatment on Chemical Composition of Feedstocks
3.1.1. Feedstocks
3.1.2. Hydrothermobaric Treatment (HTBT)
3.1.3. Dilute Nitric-Acid Treatment (DNAT)
3.1.4. Alkaline Delignification (AD)
3.1.5. Nitric-Acid Pulping Method (NAPM)
3.1.6. Combined Pulping Method (CPM)
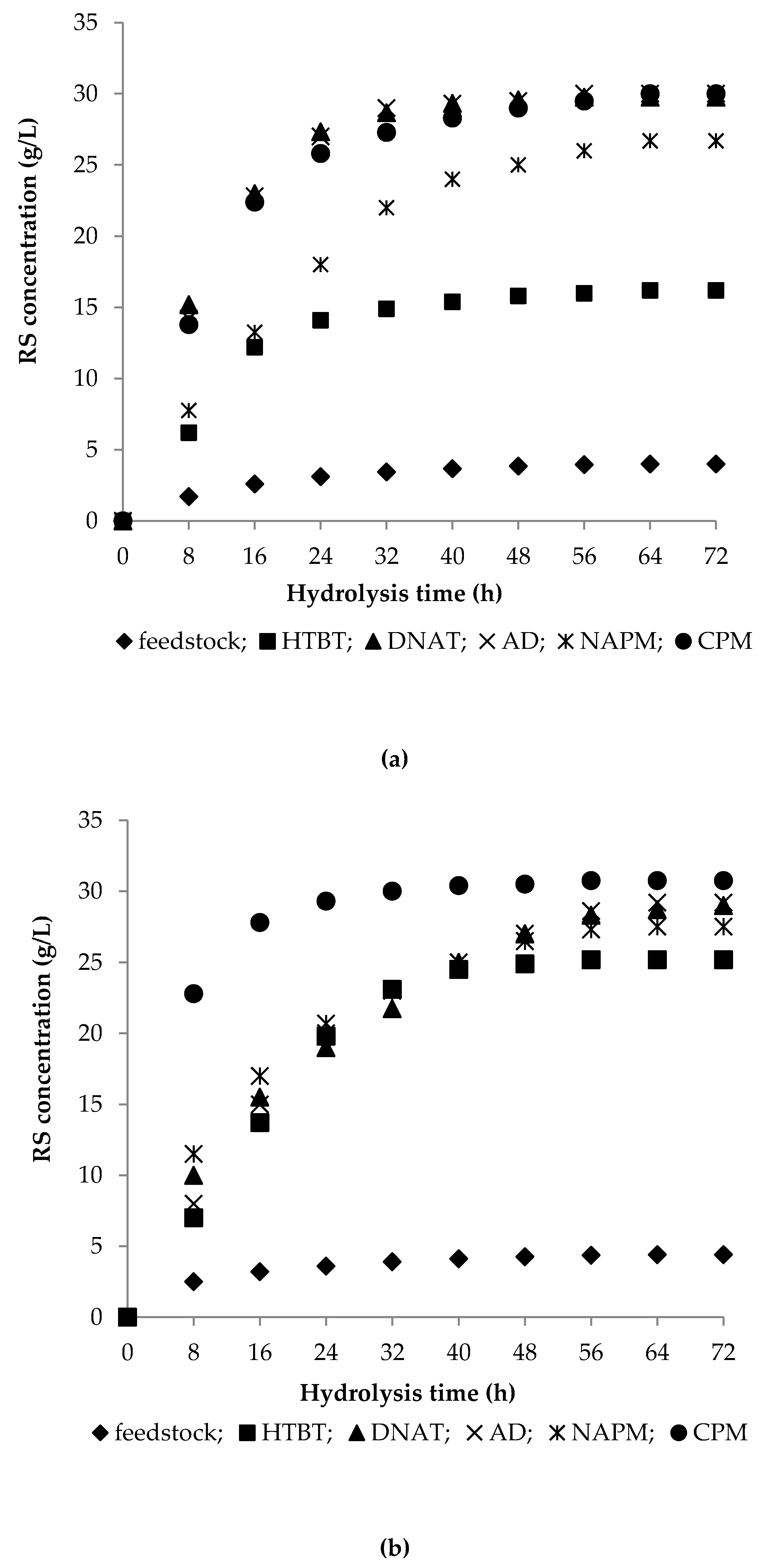
3.2. Enzymatic Hydrolysis of Pretreated Biomass
3.2.1. Enzymatic Hydrolysis of Feedstocks
3.2.2. Enzymatic Hydrolysis of HTBT Substrates
3.2.3. Enzymatic Hydrolysis of DNAT Substrates
3.2.4. Enzymatic Hydrolysis of AD Substrates
3.2.5. Enzymatic Hydrolysis of Celluloses
3.3. Biosynthesis of BC in Nutrient Broths Prepared from Enzymatic Hydrolyzates
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bergeron, C.; Carrier, D.J.; Ramaswamy, S. Biorefinery Co-Products. Phytochemicals, Primary Metabolites and Value-Added Biomass Processing; John Wiley & Sons: Chichester, England, 2012. [Google Scholar]
- Oh, Y.H.; Eom, I.Y.; Joo, J.C. Recent advances in development of biomass pretreatment technologies used in biorefinery for the production of bio-based fuels, chemicals and polymers. Korean, J. Chem. Eng. 2015, 32, 1945–1959. [Google Scholar] [CrossRef]
- Hu, F.; Ragauskas, A. Pretreatment and lignocellulosic chemistry. BioEnergy Res. 2012, 5, 1043–1066. [Google Scholar] [CrossRef]
- Sun, S.; Sun, S.; Cao, X.; Sun, R. The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 2016, 199, 49–58. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Barud, H.G.; da Silva, R.R.; da Silva Barud, H.; Tercjak, A.; Gutierrez, J.; Lustri, W.R.; de Oliveira, O.B., Jr.; Ribeiro, S.J.L. A multipurpose natural and renewable polymer in medical applications: Bacterial cellulose. Carbohydr. Polym. 2016, 153, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, J.B.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.L.; Han, S. A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process. Technol. 2017, 160, 196–206. [Google Scholar] [CrossRef]
- Reiniati, I.; Hrymak, A.N.; Margaritis, A. Kinetics of cell growth and crystalline nanocellulose production by Komagataeibacter xylinus. Biochem. Eng. J. 2017, 127, 21–31. [Google Scholar] [CrossRef]
- Pacheco, G.; Nogueiraa, C.R.; Bagliotti, M.A.; Trovatti, E.; Silva, M.C.C.; Machado, R.T.A.; Ribeiro, S.J.L.; Da Silva Filho, E.C.; Baruda, H.D. Development and characterization of bacterial cellulose produced by cashew tree residues as alternative carbon source. Ind. Crop. Prod. 2017, 107, 13–19. [Google Scholar] [CrossRef]
- Chen, L.; Hong, F.; Yang, X.X.; Han, S.F. Biotransformation of wheat straw to bacterial cellulose and its mechanism. Bioresour. Technol. 2013, 135, 464–468. [Google Scholar] [CrossRef]
- Gama, M. Bacterial Nanocellulose from Biotechnology to Bio-Economy; Elsevier: New York, NY, USA, 2016. [Google Scholar]
- Sakovich, G.V.; Skiba, E.A.; Budaeva, V.V.; Gladysheva, E.K.; Aleshina, L.A. Technological fundamentals of bacterial nanocellulose production from zero prime-cost feedstock. Dokl. Biochem. Biophys. 2017, 477, 357–359. [Google Scholar] [CrossRef]
- Mondal, S. Preparation, properties and applications of nanocellulosic materials. Carbohydr. Polym. 2017, 163, 301–316. [Google Scholar] [CrossRef]
- Ioelovich, M.; Morag, E. Study of enzymatic hydrolysis of mild pretreated lignocellulosic biomasses. BioResources 2012, 7, 1040–1052. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Podgorbunskikh, E.M.; Bychkov, A.L.; Lomovsky, O.I. Determination of Surface Accessibility of the Cellulose Substrate According to Enzyme Sorption. Polymers 2019, 11, 1201. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Miao, Y.L.; Jiang, X.J.; Xu, Z.D.; Ouyang, P.K. Enhancing the enzymatic hydrolysis of corn stover by an integrated wet-milling and alkali pretreatment. Appl. Biochem. Biotechnol. 2010, 160, 2449–2457. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.A.; Chen, Y.; Sharma-Shivappa, R.R.; Boyette, M.D.; Osborne, J. A comparison of chemical pretreatment methods for improving saccharification of cotton stalks. Bioresour. Technol. 2007, 98, 3000–3011. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Raj, T.; Vijayaraj, M.; Chopra, A.; Gupta, R.P.; Tuli, D.K.; Kumar, R. Structural features of dilute acid, steam exploded, and alkali pretreated mustard stalk and their impact on enzymatic hydrolysis. Carbohydr. Polym. 2015, 124, 265–273. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Lu, M.; Guo, X.; Zhang, H.; Han, L. Integrated chemical and multi-scale structural analyses for the processes of acid pretreatment and enzymatic hydrolysis of corn stover. Carbohydr. Polym. 2016, 141, 1–9. [Google Scholar] [CrossRef]
- Palamae, S.; Dechatiwongse, P.; Choorit, W.; Chisti, Y.; Prasertsan, P. Cellulose and hemicellulose recovery from oil palm empty fruit bunch (EFB) fibers and production of sugars from the fibers. Carbohydr. Polym. 2017, 155, 491–497. [Google Scholar] [CrossRef]
- Sun, R.C. Cereal Straw as a Resource for Sustainable Biomaterials and Biofuels; Elsevier: London, UK, 2010. [Google Scholar]
- Gismatulina, Y.A.; Budaeva, V.V. Chemical composition of five Miscanthus sinensis harvests and nitric-acid cellulose therefrom. Ind. Crop. Prod. 2017, 109, 227–232. [Google Scholar] [CrossRef]
- Potucek, F.; Gurung, B.; Hajkova, K. Soda pulping of rapeseed straw. Cellulose Chem. Technol. 2014, 48, 683–691. [Google Scholar]
- Jones, M.B.; Walsh, M. Miscanthus: For Energy and Fibre; Earthscan: London, UK, 2001. [Google Scholar]
- Shumny, V.K.; Veprev, S.G.; Nechiporenko, N.N.; Goryachkovskaya, T.N.; Slynko, N.M.; Kolchanov, N.A.; Peltek, S.E. A new form of Miscanthus (Chinese silver grass, Miscanthus sinensis–Andersson) as a promising source of cellulosic biomass. Adv. Biosci. Biotechnol. 2010, 1, 167–170. [Google Scholar] [CrossRef][Green Version]
- Chaud, L.C.S.; Silva, D.D.V.; Mattos, R.T.; Felipe, M.G.A. Evaluation of oat hull hemicellulosic hydrolysate fermentability employing Pichia stipites. Braz. Arch. Biol. Technol. 2012, 55, 771–777. [Google Scholar] [CrossRef]
- Yadav, M.P.; Hicks, K.B.; Johnston, D.B.; Hotchkiss, A.T.; Chau, H.K.; Hanah, K. Production of bio-based fiber gums from the waste streams resulting from the commercial processing of corn bran and oat hulls. Food Hydrocoll. 2016, 53, 125–133. [Google Scholar] [CrossRef]
- Vanderghem, C.; Brostaux, Y.; Jacquet, N.; Blecker, C.; Paquot, M. Optimization of formic/acetic acid delignification of Miscanthus×giganteus for enzymatic hydrolysis using response surface methodology. Ind. Crop. Prod. 2012, 35, 280–286. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, M.Y.; Jeong, S.J.; Jang, M.S.; Chung, I.M. Analysis of the biomass content of various Miscanthus genotypes for biofuel production in Korea. Ind. Crop. Prod. 2012, 38, 46–49. [Google Scholar] [CrossRef]
- Arnoult, S.; Brancourt-Hulmel, M. A Review on Miscanthus biomass production and composition for bioenergy use: Genotypic and environmental variability and implications for breeding. Bioenergy Res. 2015, 8, 502–526. [Google Scholar] [CrossRef]
- Morandi, F.; Perrin, A.; Østergård, H. Miscanthus as energy crop: Environmental assessment of a miscanthus biomass production case study in France. J. Clean. Prod. 2016, 137, 313–321. [Google Scholar] [CrossRef]
- Baker, P.W.; Charlton, A.; Hale, M.D.C. Increased delignification by white rot fungi after pressure refining Miscanthus. Bioresour. Technol. 2015, 189, 81–86. [Google Scholar] [CrossRef]
- Brosse, N.; Dufour, A.; Meng, X.; Sun, Q.; Ragauskas, A. Miscanthus: A fast-growing crop for biofuels and chemicals production. Biofuel. Bioprod. Bior. 2012, 6, 580–598. [Google Scholar] [CrossRef]
- Li, Y.; Zhuo, J.; Liu, P.; Chen, P.; Hu, H.; Wang, Y.; Zhou, S.; Tu, Y.; Peng, L.; Wang, Y. Distinct wall polymer deconstruction for high biomass digestibility under chemical pretreatment in Miscanthus and rice. Carbohydr. Polym. 2018, 192, 273–281. [Google Scholar] [CrossRef]
- Yu, G.; Afzal, W.; Yang, F.; Padmanabhan, S.; Liu, Z.; Xie, H.; Shafy, M.A.; Bella, A.T.; Prausnitz, J.M. Pretreatment of Miscanthus×giganteus using aqueous ammonia with hydrogen peroxide to increase enzymatic hydrolysis to sugars. J. Chem. Technol. Biotechnol. 2014, 89, 698–706. [Google Scholar] [CrossRef]
- Si, S.; Chen, Y.; Fan, C.; Hu, H.; Li, Y.; Huang, J.; Liao, H.; Hao, B.; Li, Q.; Peng, L.; et al. Lignin extraction distinctively enhances biomass enzymatic saccharification in hemicelluloses-rich Miscanthus species under various alkali and acid pretreatments. Bioresour. Technol. 2015, 183, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.I.S.; Jackson, J.J.; Montross, M.D. A molar basis comparison of calcium hydroxide, sodium hydroxide, and potassium hydroxide on the pretreatment of switchgrass and miscanthus under high solids conditions. Ind. Crop. Prod. 2016, 92, 165–173. [Google Scholar] [CrossRef]
- Pavlov, I.N.; Denisova, M.N.; Makarova, E.I.; Budaeva, V.V.; Sakovich, G.V. Versatile thermobaric setup and production of hydrotropic cellulose therein. Cell. Chem. Technol. 2015, 49, 847–852. [Google Scholar]
- Denisova, M.N.; Makarova, E.I.; Pavlov, I.N.; Budaeva, V.V.; Sakovich, G.V. Enzymatic hydrolysis of hydrotropic pulps at different substrate loadings. Appl. Biochem. Biotech. 2016, 178, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Kataria, R.; Mol, A.; Schulten, E.; Happel, A.; Mussatto, S.I. Bench scale steam explosion pretreatment of acid impregnated elephant grass biomass and its impacts on biomass composition, structure and hydrolysis. Ind. Crop. Prod. 2017, 106, 48–58. [Google Scholar] [CrossRef]
- Sun, Y. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Budaeva, V.V.; Makarova, E.I.; Skiba, E.A.; Sakovich, G.V. Enzymatic hydrolysis of the products of hydro-thermobaric processing of Miscanthus and oat hulls. Catal. Ind. 2013, 5, 335–341. [Google Scholar] [CrossRef]
- Torgashov, V.I.; Gert, E.V.; Zubets, O.V.; Kaputsky, F.N. A comparative study of isolation conditions, morphology, and properties of cellulose obtained from the stalks of cereals and oil-yielding plants. Russ. J. Bioorg. Chem. 2010, 36, 838–846. [Google Scholar] [CrossRef]
- Shishonok, M.V.; Shadrina, V.I. Recovery of wool from flax fibers using nitric acid. Russ. J. Appl. Chem. 2006, 79, 1542–1545. [Google Scholar] [CrossRef]
- Kiziltas, E.E.; Kiziltas, A.; Gardner, D.J. Synthesis of bacterial cellulose using hot water extracted wood sugars. Carbohydr. Polym. 2015, 124, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Vadanan, S.V.; Lim, S. Rational design of a scalable bioprocess platform for bacterial cellulose production. Carbohydr. Polym. 2019, 207, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial cellulose production, properties and applications with different culture methods – A review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Aydogdu, M.O.; Altun, E.; Ahmed, J.; Gunduz, O.; Edirisinghe, M. Fiber forming capability of binary and ternary compositions in the polymer system: Bacterial cellulose-polycaprolactone-polylactic acid. Polymers 2019, 11, 1148. [Google Scholar] [CrossRef] [PubMed]
- Abdelraof, M.; Hasanin, M.S.; El-Saied, H. Ecofriendly green conversion of potato peel wastes to high productivity bacterial cellulose. Carbohydr. Polym. 2019, 211, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Velásquez-Riaño, M.; Bojacá, V. Production of bacterial cellulose from alternative low-cost substrates. Cellulose 2017, 24, 2677–2698. [Google Scholar] [CrossRef]
- Kurschner, K.; Hoffer, A. Cellulose and cellulose derivative. Fresen. J. Anal. Chem. 1993, 92, 145–154. [Google Scholar] [CrossRef]
- Obolenskaya, A.V.; Yelnitskaya, Z.P.; Leonovich, A.A. Laboratornye raboty po khimii drevesiny i tsellyulozy; Ecology Publisher: Moscow, Russian, 1991. [Google Scholar]
- Technical Association of the Pulp and Paper Industry. TAPPI T 222 om-83 Standard: Acid-Insoluble Lignin in Wood and Pulp, Specifications of the Technical Association of the Pulp and Paper Industry. Atlanta, USA. 1988. Available online: https://standards.globalspec.com/std/10402544/tappi-t-222 (accessed on 7 October 2019).
- Technical Association of the Pulp and Paper Industry. TAPPI T 211 om-85 standard: Ash in wood, pulp, paper, and paperboard, specifications of the Technical Association of the Pulp and Paper Industry. Atlanta, USA. 1985. Available online: https://standards.globalspec.com/std/1524019/TAPPI%20T%20211 (accessed on 7 October 2019).
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Goh, W.N.; Rosma, A.; Kaur, B.; Fazilah, A.; Karim, A.A.; Rajeev, B. Fermentation of black tea broth (Kombucha): I. Effects of sucrose concentration and fermentation time on the yield of microbial cellulose. Int. Food Res. J. 2012, 19, 109–117. [Google Scholar]
- Yurkevich, D.I.; Kutyshenko, V.P. Medusomyces (Tea Fungus): A scientific history, composition, features of physiology and metabolism. Biophysics 2002, 47, 1116–1129. [Google Scholar]
- Gladysheva, E.K.; Skiba, E.A.; Zolotukhin, V.N.; Sakovich, G.V. Study of the Conditions for the Biosynthesis of Bacterial Cellulose by the Producer Medusomyces gisevii Sa-12. Appl. Biochem. Micro. 2018, 54, 179–187. [Google Scholar] [CrossRef]
- Kashcheyeva, E.I.; Gladysheva, E.K.; Skiba, E.A.; Budaeva, V.V. A study of properties and enzymatic hydrolysis of bacterial cellulose. Cellulose 2019, 26, 2255–2265. [Google Scholar] [CrossRef]
- Klemm, D.; Cranston, E.D.; Fischer, D.; Gama, M.; Kedzior, S.A.; Kralisch, D.; Kramer, F.; Kondo, T.; Lindström, T.; Nietzsche, S.; et al. Nanocellulose as a natural source for groundbreaking applications in materials science: Today’s state. Mater. Today 2018, 21, 720–748. [Google Scholar] [CrossRef]
- Campano, C.; Balea, A.; Blanco, A.; Negro, C. Enhancement of the fermentation process and properties of bacterial cellulose: A review. Cellulose 2016, 23, 57–91. [Google Scholar] [CrossRef]
| Enzyme | Enzymatic Activity |
|---|---|
| CelloLux-A (cellulose-standardized) | Cellulase: 2000 ± 10% CMCaseAU/ga Xylanase: 8000 ± 10% XAU/gb β-glucanase: 1500 ± 10% β-glAU/gc |
| BrewZyme BGX (hemicellulose-standardized) | Cellulase: 2100 ± 5% CMCaseAU/g Xylanase: 4200 ± 5% XAU/g β-glucanase: 530 ± 5% β-glAU/g |
| Sample | Reducing Sugars | Concentration (g/L) | |||
|---|---|---|---|---|---|
| Conc. (g/L) | Yield (%) | Glucose | Xylose | ||
| On Solid Weight Basis | On Hydrolyzables Basis | ||||
| Miscanthus | 4.1 ± 0.1 | 11.1 ± 0.3 | 14.5 ± 0.4 | 3.9 ± 0.0 | 0.10 ± 0.0 |
| Oat hulls | 4.5 ± 0.1 | 12.2 ± 0.3 | 15.8 ± 0.4 | 4.3 ± 0.0 | 0.10 ± 0.0 |
| HTBT Miscanthus | 16.2 ± 0.1 | 43.7 ± 1.4 | 58.5 ± 1.5 | 14.2 ± 0.1 | 1.5 ± 0.1 |
| HTBT oat hulls | 25.2 ± 0.1 | 68.0 ± 1.4 | 98.1 ± 1.5 | 22.9 ± 0.1 | 1.5 ± 0.1 |
| DNAT Miscanthus | 29.8 ± 0.1 | 80.5 ± 1.4 | 92.0 ± 1.5 | 27.9 ± 0.2 | 1.0 ± 0.1 |
| DNAT oat hulls | 29.5 ± 0.1 | 79.7 ± 1.4 | 98.4 ± 1.5 | 26.6 ± 0.2 | 2.0 ± 0.1 |
| AD Miscanthus | 29.6 ± 0.1 | 79.9 ± 1.4 | 85.0 ± 1.5 | 27.0 ± 0.2 | 1.7 ± 0.1 |
| AD oat hulls | 30.9 ± 0.1 | 83.4 ± 1.4 | 90.0 ± 1.5 | 28.0 ± 0.2 | 2.0 ± 0.1 |
| NAPM Miscanthus cellulose | 26.7 ± 0.1 | 72.1 ± 1.4 | 73.3 ± 1.5 | 25.7 ± 0.2 | 0.2 ± 0.0 |
| NAPM oat hull cellulose | 27.5 ± 0.1 | 74.3 ± 1.4 | 75.5 ± 1.5 | 26.4 ± 0.2 | 0.3 ± 0.0 |
| CPM Miscanthus cellulose | 30.0 ± 0.1 | 81.0 ± 1.4 | 82.7 ± 1.5 | 28.3 ± 0.2 | 0.8 ± 0.1 |
| CPM oat hull cellulose | 30.8 ± 0.1 | 83.2 ± 1.4 | 83.7 ± 1.5 | 29.0 ± 0.2 | 0.9 ± 0.1 |
| Sample | Glucose Concentration (g/L) |
|---|---|
| HTBT Miscanthus | 14.0 ± 0.1 |
| HTBT oat hulls | 22.2 ± 0.1 |
| DNAT Miscanthus | 26.9 ± 0.2 |
| DNAT oat hulls | 25.8 ± 0.2 |
| AD Miscanthus | 26.3 ± 0.2 |
| AD oat hulls | 27.1 ± 0.2 |
| NAPM Miscanthus cellulose | 25.0 ± 0.2 |
| NAPM oat hull cellulose | 25.8 ± 0.2 |
| CPM Miscanthus cellulose | 27.5 ± 0.2 |
| CPM oat hull cellulose | 28.1 ± 0.2 |
| Sample | BC Biosynthesis |
|---|---|
| HTBT Miscanthus | – |
| HTBT oat hulls | – |
| DNAT Miscanthus | + |
| DNAT oat hulls | + |
| AD Miscanthus | + |
| AD oat hulls | + |
| NAPM Miscanthus cellulose | + |
| NAPM oat hull cellulose | + |
| CPM Miscanthus cellulose | + |
| CPM oat hull cellulose | + |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashcheyeva, E.I.; Gismatulina, Y.A.; Budaeva, V.V. Pretreatments of Non-Woody Cellulosic Feedstocks for Bacterial Cellulose Synthesis. Polymers 2019, 11, 1645. https://doi.org/10.3390/polym11101645
Kashcheyeva EI, Gismatulina YA, Budaeva VV. Pretreatments of Non-Woody Cellulosic Feedstocks for Bacterial Cellulose Synthesis. Polymers. 2019; 11(10):1645. https://doi.org/10.3390/polym11101645
Chicago/Turabian StyleKashcheyeva, Ekaterina I., Yulia A. Gismatulina, and Vera V. Budaeva. 2019. "Pretreatments of Non-Woody Cellulosic Feedstocks for Bacterial Cellulose Synthesis" Polymers 11, no. 10: 1645. https://doi.org/10.3390/polym11101645
APA StyleKashcheyeva, E. I., Gismatulina, Y. A., & Budaeva, V. V. (2019). Pretreatments of Non-Woody Cellulosic Feedstocks for Bacterial Cellulose Synthesis. Polymers, 11(10), 1645. https://doi.org/10.3390/polym11101645