The Design and Manufacture of a Multilayer Low-Temperature Protective Composite Fabric Based on Active Heating Materials and Passive Insulating Materials
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of MicroPCMs
2.3. Preparation of the Thermal Insulation Layer
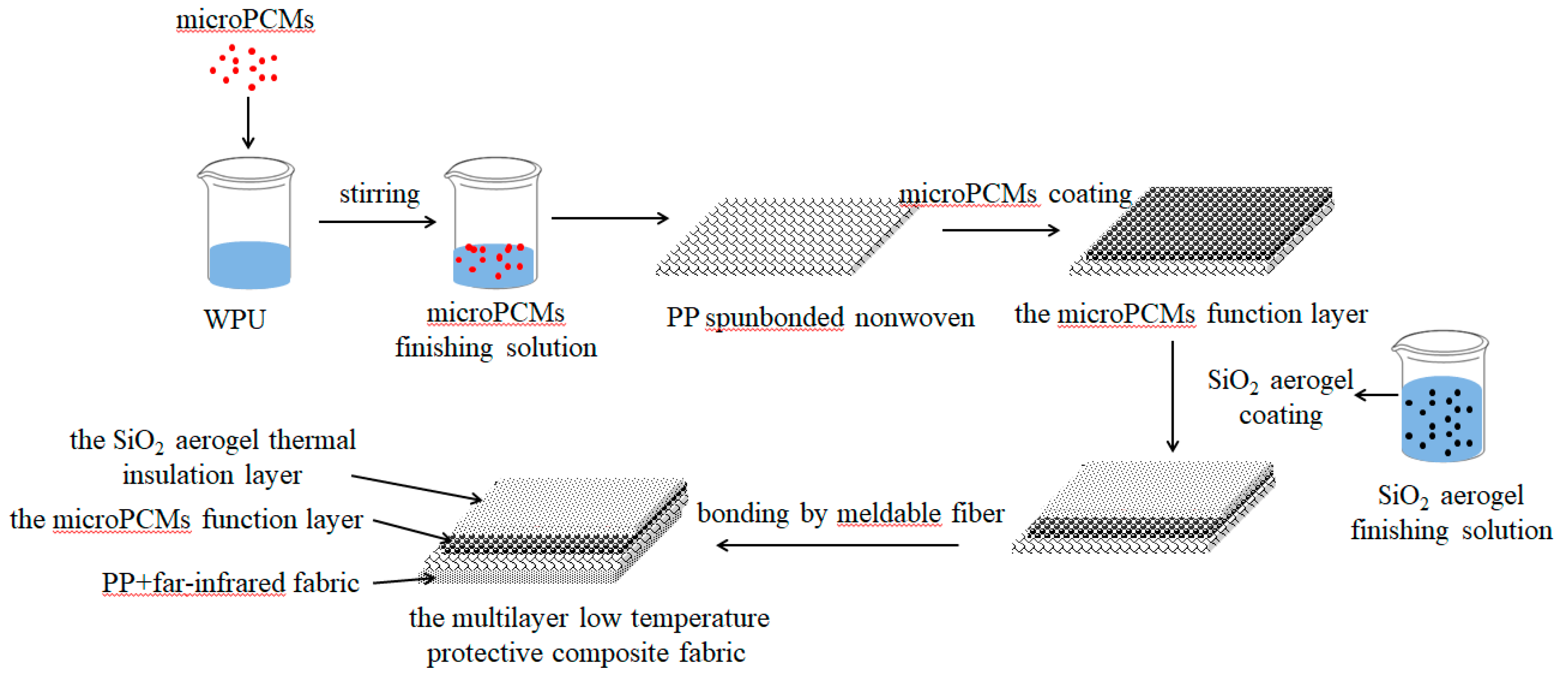
2.4. Preparation of the MPF
2.5. Characterization
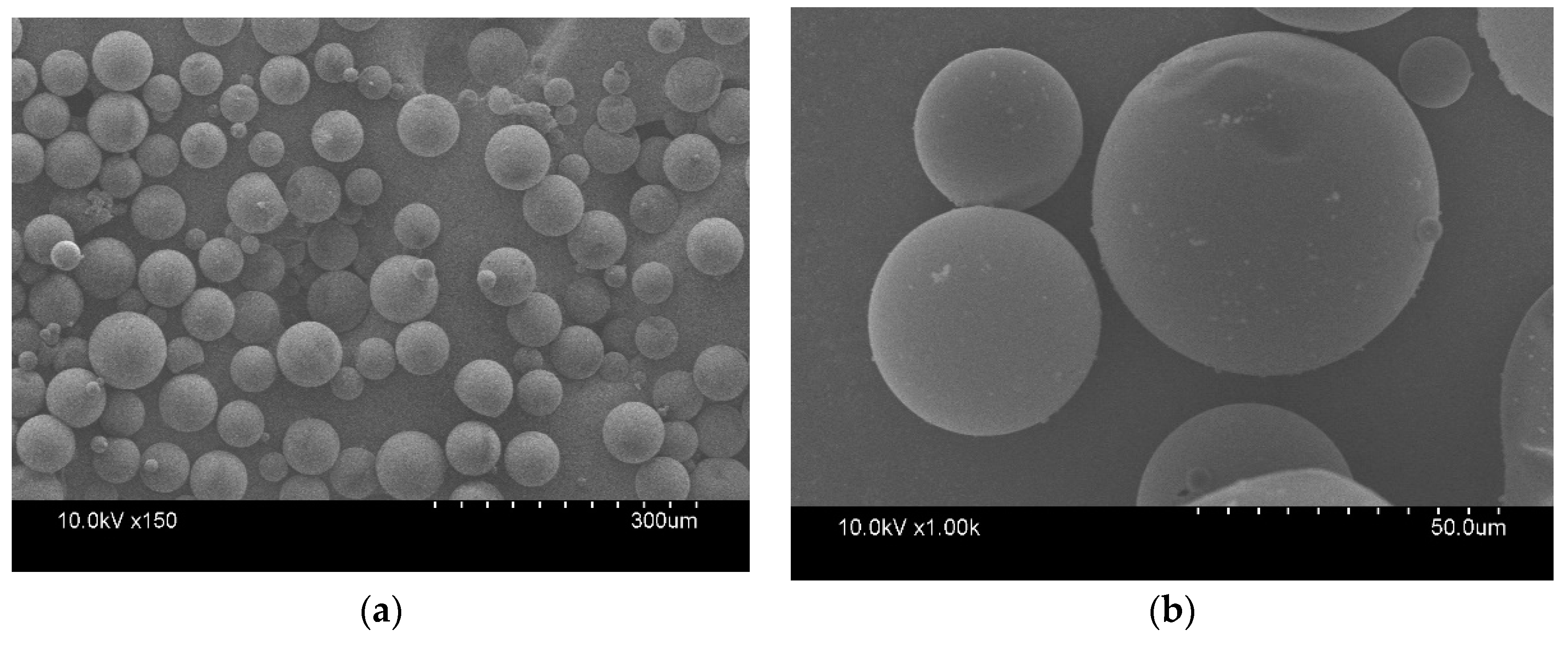
2.5.1. Morphology
2.5.2. Particle Size Distribution
2.5.3. Thermal Properties
2.5.4. Thermal Conductivity
2.5.5. Low-Temperature Resistance
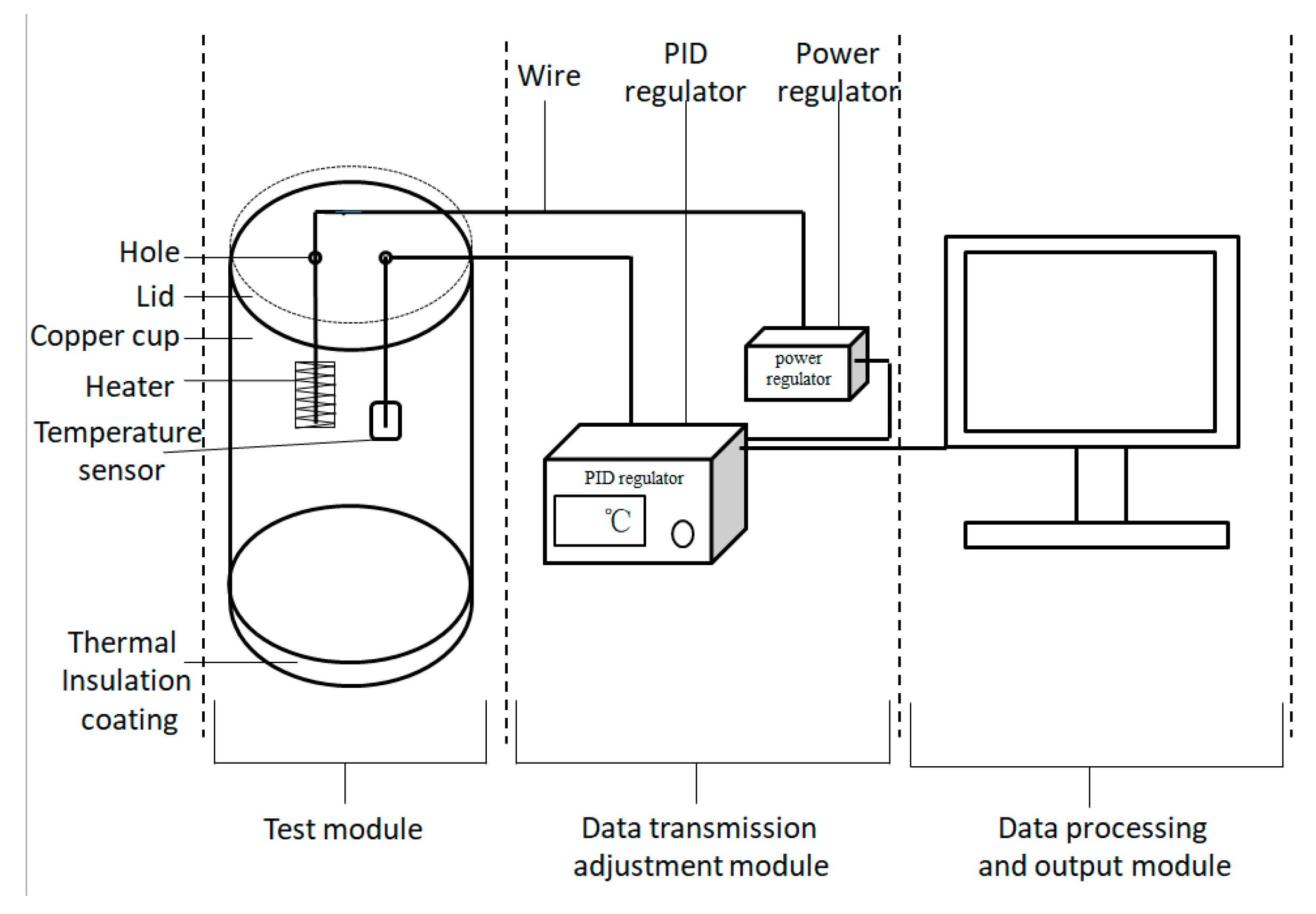
2.5.6. Thermal Insulation Performance
3. Results and Discussion
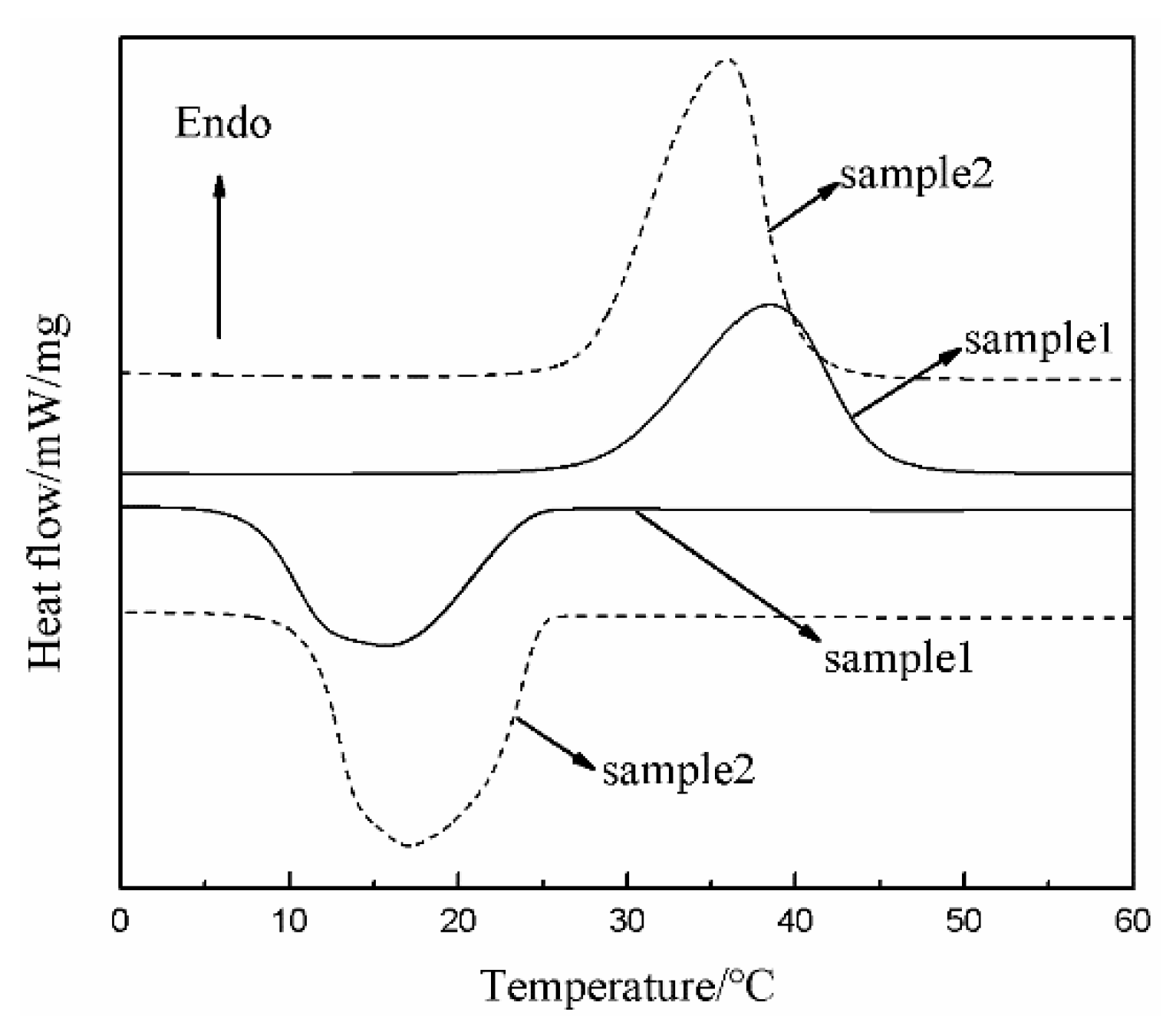
3.1. Morphology, Average Particle Size, and Thermal Properties of the microPCMs
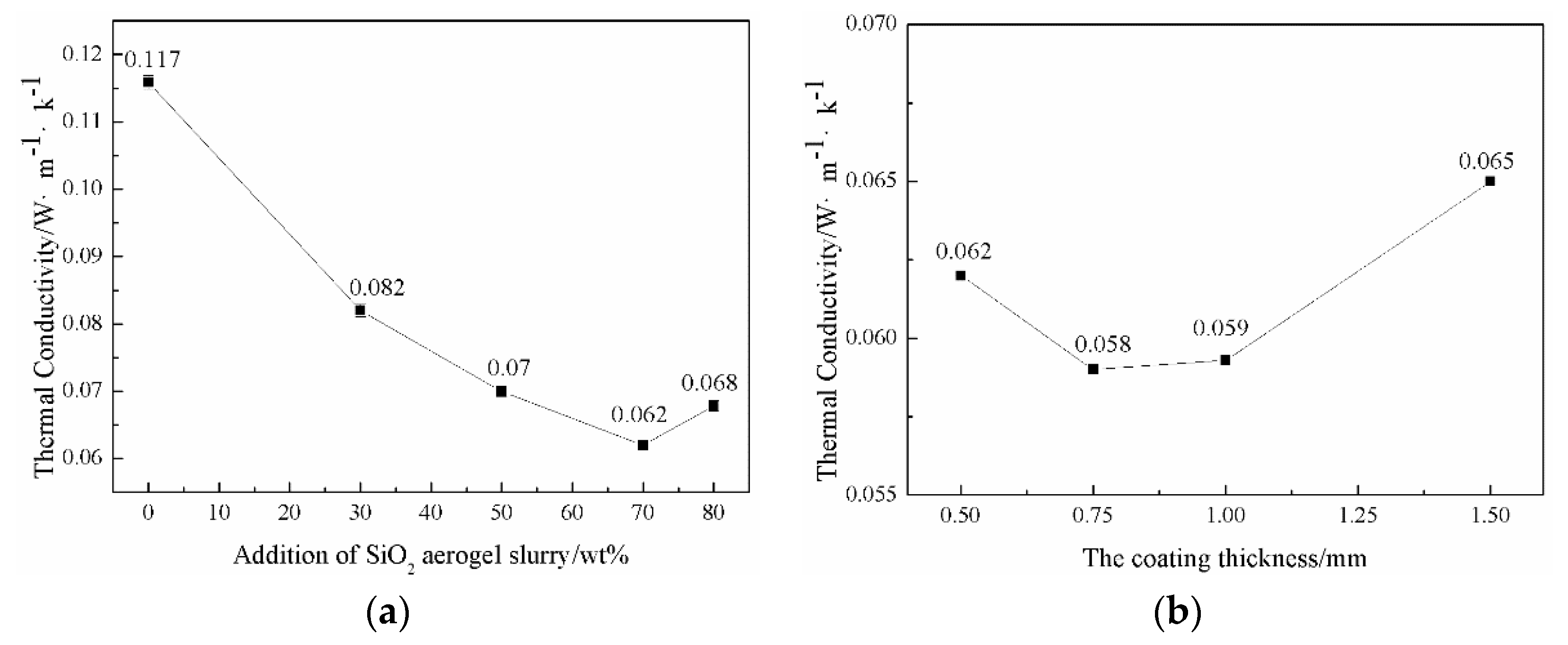
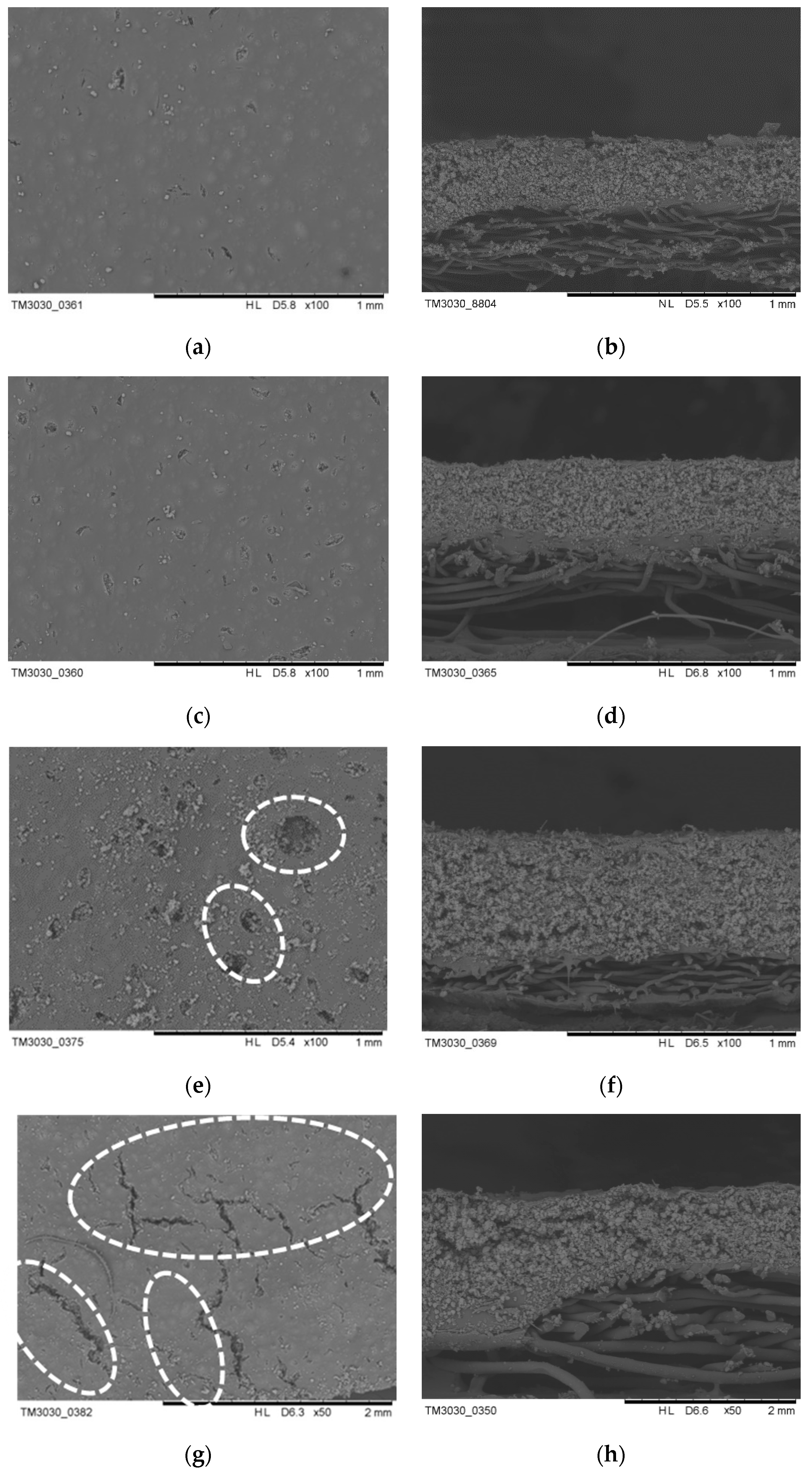
3.2. Effect of Aerogel Slurry Addition Amount on the Morphologies and Thermal Conductivity of the Thermal Insulation Layer
3.3. Effect of Coating Thickness on the Morphologies and Thermal Conductivity of the Thermal Insulation Layer
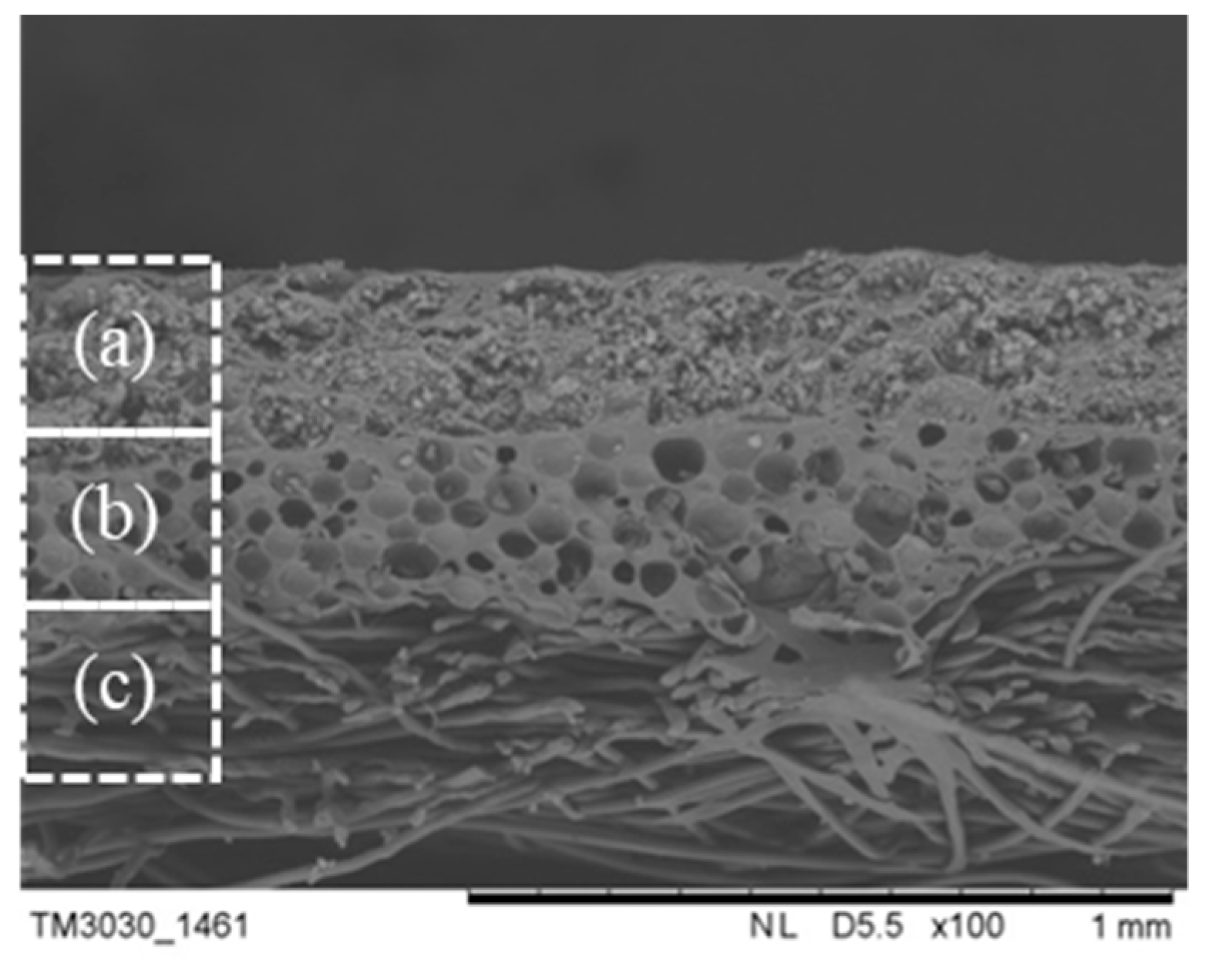
3.4. Cross-Section Morphology and Thermal Properties of the MPF
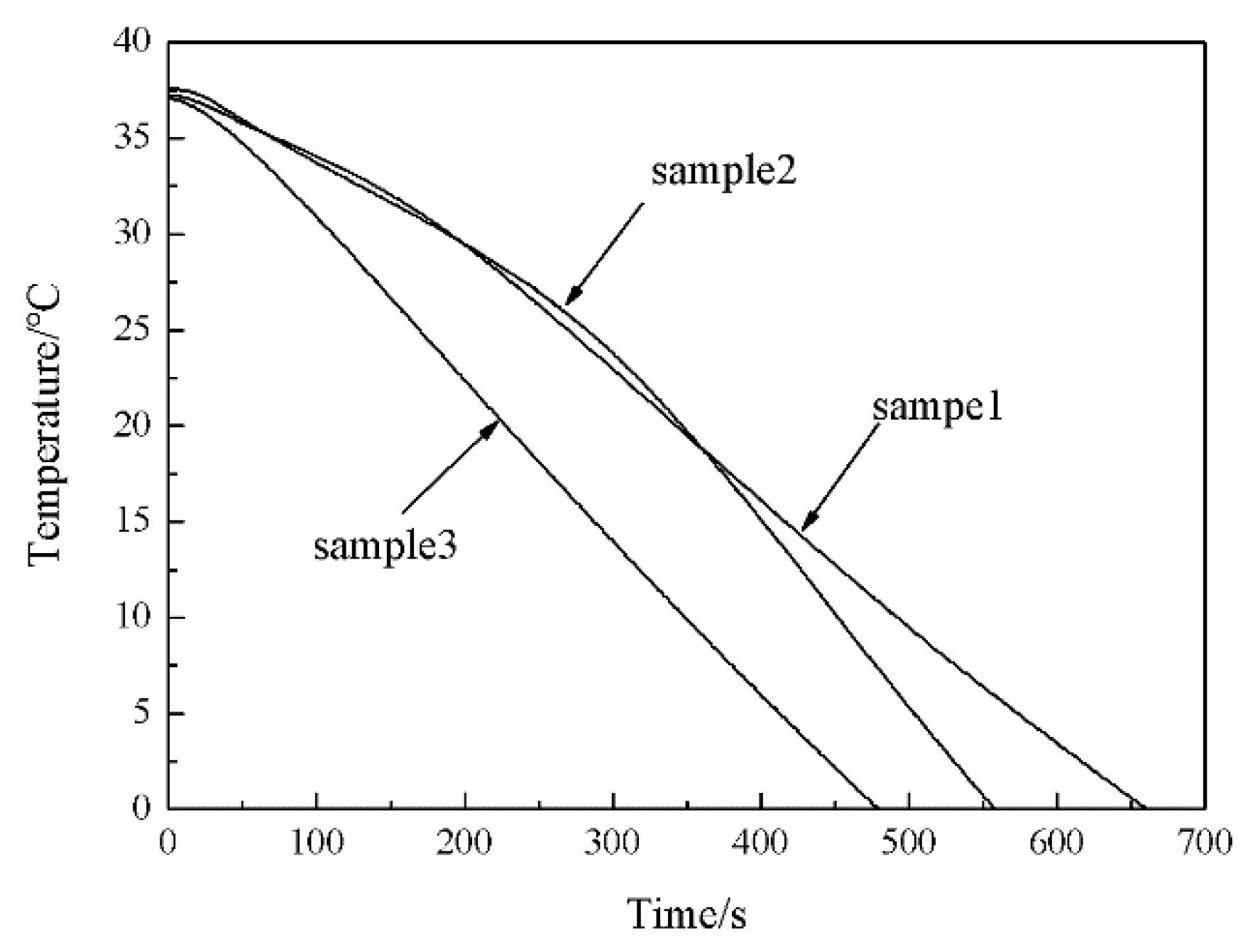
3.5. Low-Temperature Resistance of the MPF
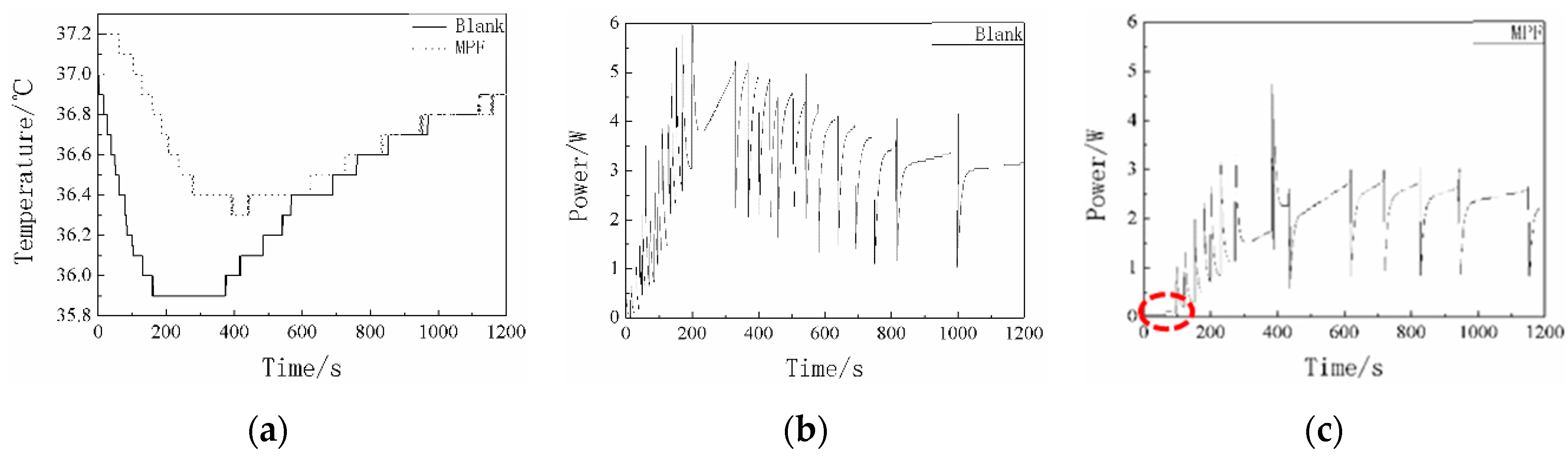
3.6. Thermal Conductivity and Thermal Insulation Performance in the Low-Temperature Environment of the MPF
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, X.X.; Sun, Y.C.; Xu, J.Y.; Chen, R.X. Thermal requirement analysis of phase change protective clothing in low temperature environment. Adv. Mater. Res. 2013, 796, 649–652. [Google Scholar] [CrossRef]
- Keatinge, W.R. Winter mortality and its causes. Int. J. Circumpol. Health 2012, 61, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Torossian, A.; Gerven, E.V.; Geertsen, K.; Horn, B.; Velde, M.V.D.; Raeder, J. Active perioperative patient warming using a self-warming blanket (BARRIER EasyWarm) is superior to passive thermal insulation: A multinational, multicenter, randomized trial. J. Clin. Anesth. 2016, 34, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Li, M.; Shang, K. Development of thermal insulation materials technology for spacesuit. J. Aeronaut. Mater. (Beijing, China) 2016, 36, 87–96. [Google Scholar]
- Pakdel, E.; Naebe, M.; Sun, L.; Wang, X. Advanced functional fibrous materials for enhanced thermoregulating performance. ACS Appl. Mater. Interfaces 2019, 11, 13039–13057. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xue, Y.S.; Chang, L.Z.; Chong, H.W.; Chang, X.Y.; Fu, T.L. Preparation and characterization of mullite fiber-reinforced Al2O3-SiO2 aerogel composites. Key Eng. Mater. 2016, 697, 360–363. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, Y.; Feng, J.; Feng, J.; Yue, C. Infrared-opacified Al2O3-SiO2 aerogel composites reinforced by SiC-coated mullite fibers for thermal insulations. Ceram. Int. 2015, 41, 437–442. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Z.; Pei, J.; Rui, L.; Zhang, J.; Cai, J.; Cui, J. A novel TiO2-SiO2 aerogel nanocomposite absorbent: Preparation, characterization and photocatalytic degradation effects on automobile exhaust. Mater. Res. Express 2018, 5, 025063. [Google Scholar] [CrossRef]
- Fei, Z.; Yang, Z.; Chen, G.; Li, K.; Shuang, Z.; Su, G. Preparation and characterization of glass fiber/polyimide/SiO2 composite aerogels with high specific surface area. J. Mater. Sci. 2018, 53, 12885–12893. [Google Scholar] [CrossRef]
- Wang, J. Preparation and properties of SiO2 aerogel and fabric composite based on polyurethane. Integr. Ferroelectr. 2018, 189, 36–43. [Google Scholar] [CrossRef]
- Cao, J.; Meng, J.G.; Li, L. Studies on properties of EKS heat-generating fiber and yarn. Appl. Mech. Mater. 2012, 184–185, 1216–1220. [Google Scholar] [CrossRef]
- Bai, Y.; Li, H.; Gan, S.; Li, Y.; Hao, L.; Li, C. Flexible heating fabrics with temperature perception based on fine copper wire and fusible interlining fabrics. Measurement 2018, 122, 192–200. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S. Characteristics of electrical heating elements coated with graphene nanocomposite on polyester fabric: Effect of different graphene contents and annealing temperatures. Fibers Polym. 2018, 19, 965–976. [Google Scholar] [CrossRef]
- Moury, R.; Demirci, U.B.; Ichikawa, T.; Filinchuk, Y.; Chiriac, R.; Lee, A.V.D.; Miele, P. Sodium hydrazinidoborane: A chemical hydrogen-storage material. ChemSusChem 2013, 6, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Myagmarjav, O.; Ryu, J.; Kato, Y. Lithium bromide-mediated reaction performance enhancement of a chemical heat-storage material for magnesium oxide/water chemical heat pumps. Appl. Therm. Eng. 2014, 63, 170–176. [Google Scholar] [CrossRef]
- Wang, X.; Jin, X. Thermal Storage Conditioning Fiber and its Application in Nonwovens Materials. Nonwovens 2006, 14, 33–35. [Google Scholar]
- Iqbal, K.; Khan, A.; Sun, D.; Ashraf, M.; Rehman, A.; Safdar, F.; Basit, A.; Maqsood, H. Phase change materials, their synthesis and application in textiles-a review. J. Text. Inst. 2019, 110, 625–638. [Google Scholar] [CrossRef]
- Mishra, L.; Sinha, A.; Gupta, R. Recent developments in latent heat energy storage systems using phase change materials (PCMs)-A review. In Springer Transactions in Civil and Environmental Engineering; Drück, H., Pillai, R., Tharian, M., Majeed, A., Eds.; Green Buildings and Sustainable Engineering; Springer Transactions in Civil and Environmental Engineering: Singapore, 2018; pp. 25–37. [Google Scholar]
- Aftab, W.; Huang, X.; Wu, W.; Liang, Z.; Zou, R. Nanoconfined phase change materials for thermal energy applications. Energ. Environ. Sci. 2018, 11, 1392–1424. [Google Scholar] [CrossRef]
- Wei, G.; Gang, W.; Chao, X.; Xing, J.; Xing, L.; Du, X.; Yang, Y. Selection principles and thermophysical properties of high temperature phase change materials for thermal energy storage: A review. Renew. Sustain. Energ. Rev. 2018, 81, 1768–1771. [Google Scholar] [CrossRef]
- Sadrameli, S.M.; Motaharinejad, F.; Mohammadpour, M.; Dorkoosh, F. An experimental investigation to the thermal conductivity enhancement of paraffin wax as a phase change material using diamond nanoparticles as a promoting factor. Heat Mass Transfer 2019, 55, 1801–1808. [Google Scholar] [CrossRef]
- Senthil, R.; Sundaram, P.; Kumar, M. Experimental investigation on packed bed thermal energy storage using paraffin wax for concentrated solar collector. Mater. Today 2018, 5, 8916–8922. [Google Scholar] [CrossRef]
- Cao, V.D.; Salas-Bringas, C.; Schüller, R.B.; Szczotok, A.M.; Hiorth, M.; Carmona, M.; Rodriguez, J.F.; Kjøniksen, A.L. Rheological and thermal properties of suspensions of microcapsules containing phase change materials. Colloid. Polym. Sci. 2018, 296, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Jian, L.; Chen, L.; Fang, X.; Zhang, Z. Preparation of graphite nanoparticles-modified phase change microcapsules and their dispersed slurry for direct absorption solar collectors. Sol. Energy Mater. Sol. Cells 2017, 159, 159–166. [Google Scholar]
- Yu, Q.; Tchuenbou-Magaia, F.; Al-Duri, B.; Zhang, Z.; Ding, Y.; Li, Y. Thermo-mechanical analysis of microcapsules containing phase change materials for cold storage. Appl. Energy 2018, 211, 1190–1202. [Google Scholar] [CrossRef]
- Li, T.; Wu, B.; Liu, X. Synthesis of N-Alkane mixture microcapsule and its application in low-temperature protective fabric. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2017, 32, 29–35. [Google Scholar]
- Sun, Y.; Wang, R.; Liu, X.; Fang, S.; Li, B. Design of a novel multilayer low-temperature protection composite based on phase change microcapsules. J. Appl. Polym. Sci. 2019, 136, 47534. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, R.; Liu, X.; Li, M.; Yang, H.; Li, B. Improvements in the thermal conductivity and mechanical properties of phase-change microcapsules with oxygen-plasma-modified multiwalled carbon nanotubes. J. Appl. Polym. Sci. 2017, 134, 45269. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, R.; Liu, X.; Xu, L.; Yang, H. Preparation and properties of composite fabric for light-weight low temperature protective gloves. J. Text. Res. 2017, 38, 58–63. [Google Scholar]
- He, W.; Wang, J.P.; Wang, X.; Wei, L.; Zhang, X. Preparation and properties of microencapsulated phase change materials containing two-phase core materials. Ind. Eng. Chem. Res. 2013, 52, 14706–14712. [Google Scholar]
- You, M.; Zhang, X.; Wang, J.; Wang, X. Polyurethane foam containing microencapsulated phase-change materials with styrene-divinybenzene co-polymer shells. J. Mater. Sci. 2009, 44, 3141–3147. [Google Scholar] [CrossRef]
- Qian, Y.M.; Wu, Q.; Zhou, Y.D. High-performance heat-insulated coating fabric and its technology for producing. Key Eng. Mater. 2011, 464, 429–433. [Google Scholar] [CrossRef]
- Xie, A.; Meer, L.V.D.; Austin, R.H. Excited-State Lifetimes of Far-Infrared Collective Modes in Proteins. J. Biol. Phys. 2002, 28, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.Y.; Chiu, J.H.; Yang, S.D.; Hsu, Y.C.; Lui, W.Y.; Wu, C.W. Biological effect of far-infrared therapy on increasing skin microcirculation in rats. Photodermatol. Photoimmunol. Photomed. 2006, 22, 78–86. [Google Scholar] [CrossRef] [PubMed]


| Samples | Average Particle Size (μm) | Tm (°C) | ΔHm (J/g) | Tc (°C) | ΔHc (J/g) | η (%) |
|---|---|---|---|---|---|---|
| C18 | 27.2 | 307.1 | 25.5 | 298.8 | ||
| microPCMs | 42.63 | 26.8 | 240.6 | 25.7 | 241.5 | 79.57 |
| Sample | Power at Stable Temperature/W | The Power Consumption/J | |
|---|---|---|---|
| 10min | 20min | ||
| Blank | 3.3 | 1647 | 3472 |
| MPF | 2.5 | 629 | 1872 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Wang, R.; Li, B.; Fan, W. The Design and Manufacture of a Multilayer Low-Temperature Protective Composite Fabric Based on Active Heating Materials and Passive Insulating Materials. Polymers 2019, 11, 1616. https://doi.org/10.3390/polym11101616
Sun Y, Wang R, Li B, Fan W. The Design and Manufacture of a Multilayer Low-Temperature Protective Composite Fabric Based on Active Heating Materials and Passive Insulating Materials. Polymers. 2019; 11(10):1616. https://doi.org/10.3390/polym11101616
Chicago/Turabian StyleSun, Yanli, Rui Wang, Bo Li, and Wei Fan. 2019. "The Design and Manufacture of a Multilayer Low-Temperature Protective Composite Fabric Based on Active Heating Materials and Passive Insulating Materials" Polymers 11, no. 10: 1616. https://doi.org/10.3390/polym11101616
APA StyleSun, Y., Wang, R., Li, B., & Fan, W. (2019). The Design and Manufacture of a Multilayer Low-Temperature Protective Composite Fabric Based on Active Heating Materials and Passive Insulating Materials. Polymers, 11(10), 1616. https://doi.org/10.3390/polym11101616







