Nanocrystalline Hydroxyapatite Loaded with Resveratrol in Colloidal Suspension Improves Viability, Metabolic Activity and Mitochondrial Potential in Human Adipose-Derived Mesenchymal Stromal Stem Cells (hASCs)
Abstract
1. Introduction
2. Materials and Methods
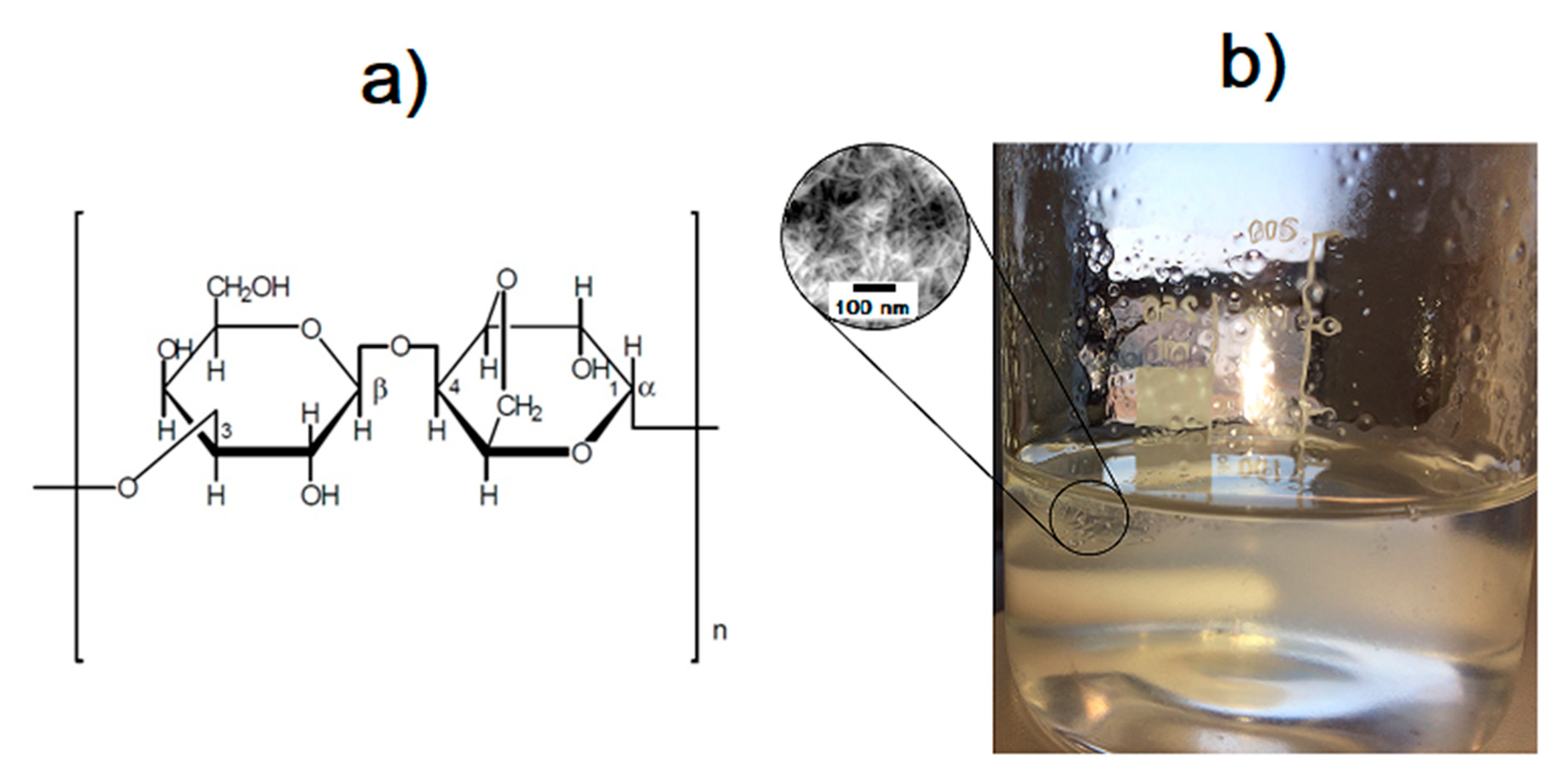
2.1. Synthesis of Hydroxyapatite Nanopowders
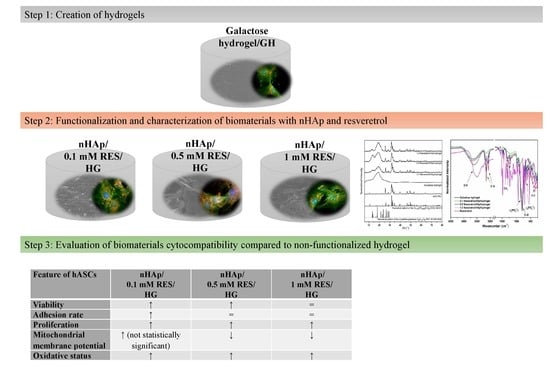
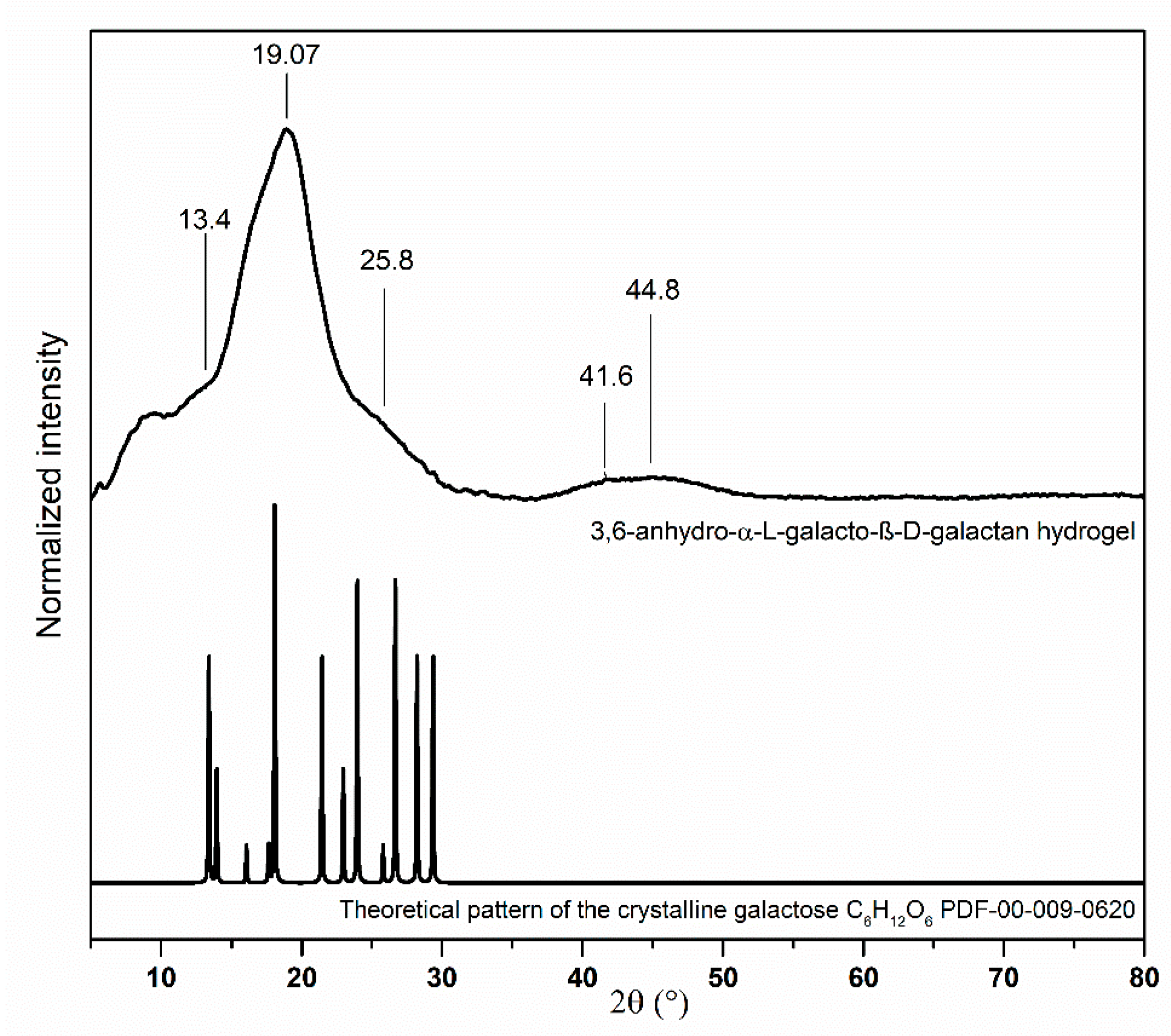
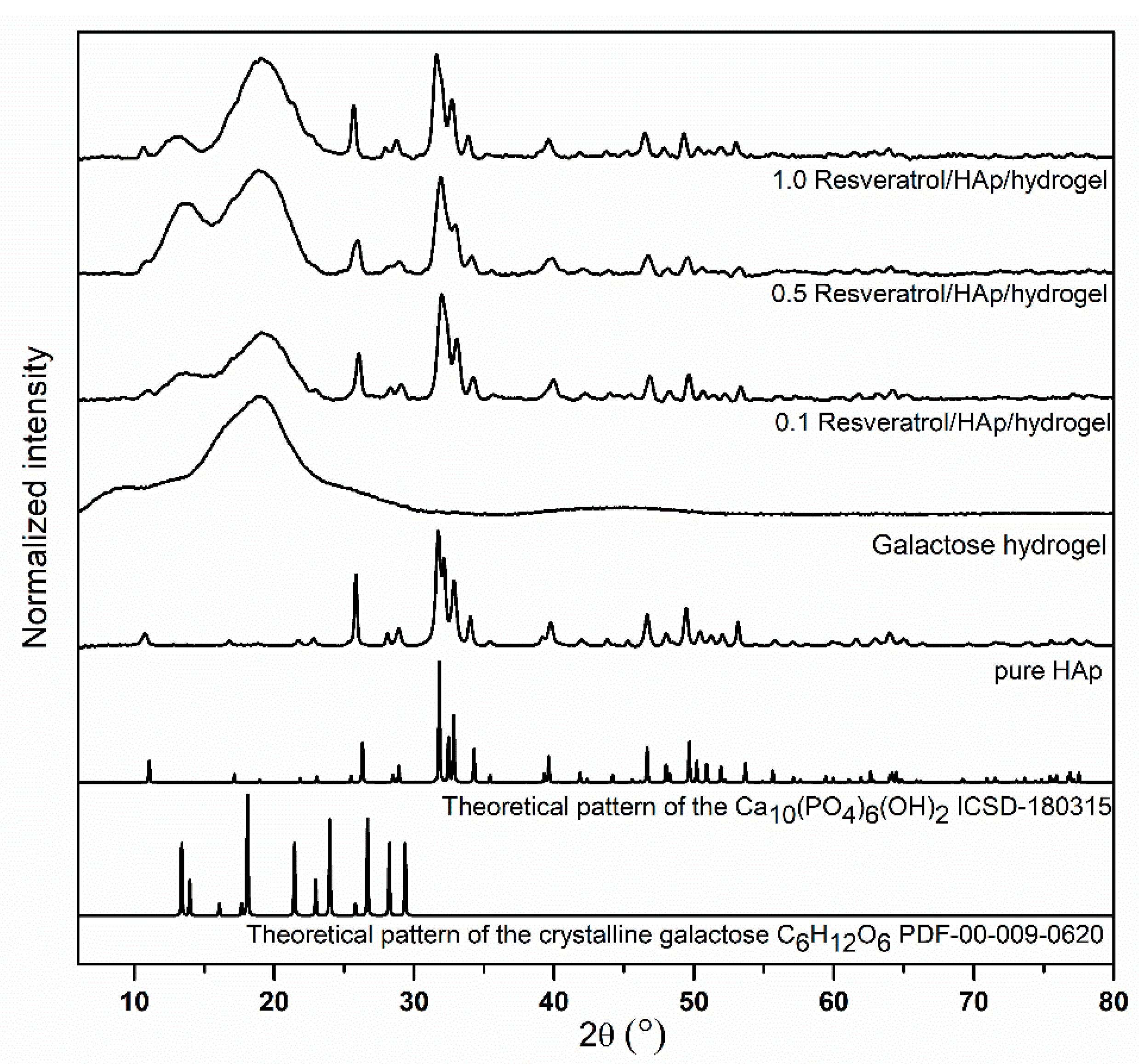
2.2. Preparation of 3,6-Anhydro-α-l-galacto-β-d-galactan Samples Filled with Hydroxyapatite and Resveratrol
2.3. Characterization
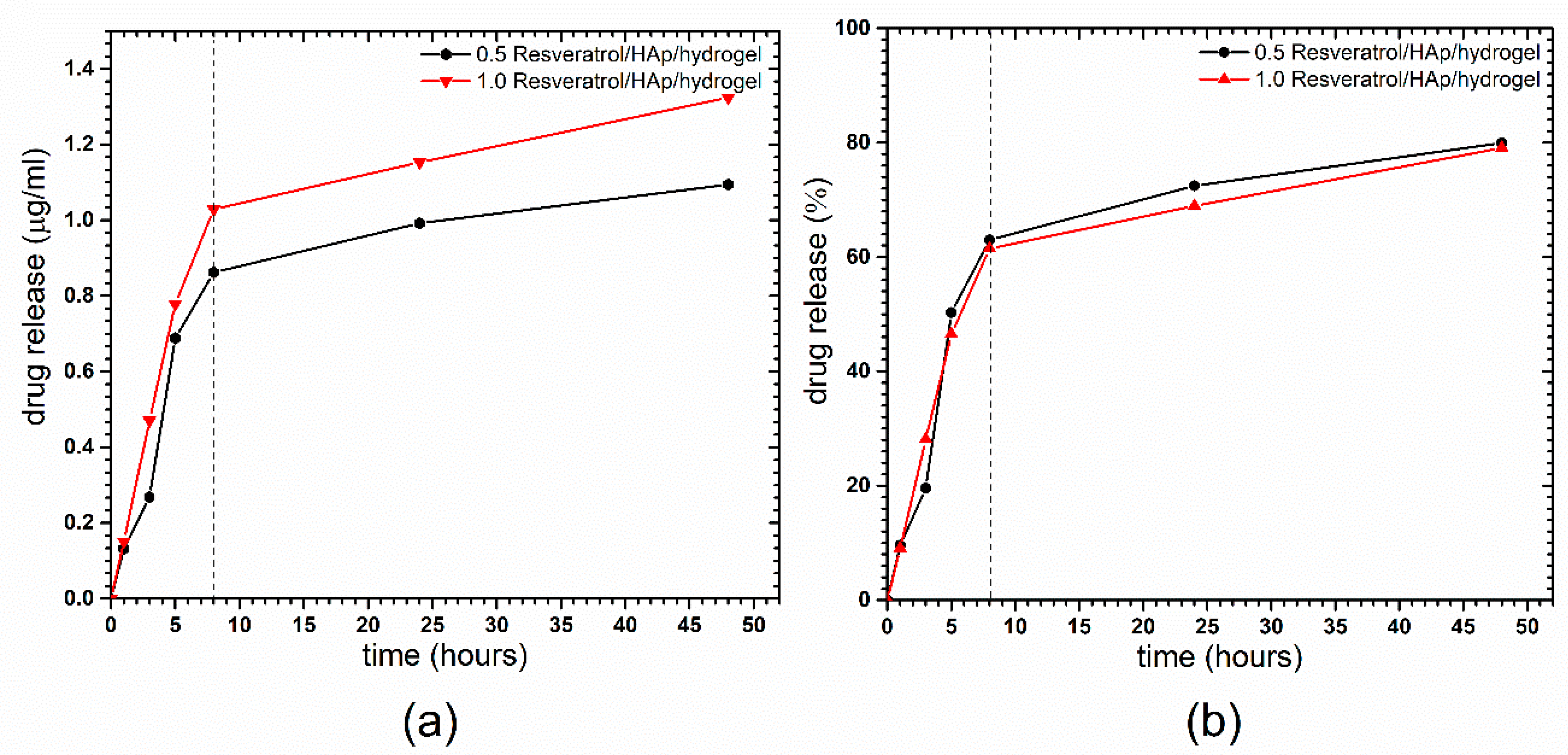
2.4. Determination of Resveratrol Release from 3,6-Anhydro-α-l-galacto-β-d-galactan Hydrogel Filled with Resveratrol and Hydroxyapatite
2.4.1. Dissolution Method
2.4.2. HPLC Method
2.5. Human Adipose-Derived Multipotent Stromal Cells (hASCs)
2.6. The Influence of Hydrogels Functionalized with nHAp and Resveratrol on Cultures of hASCs
2.6.1. The Analysis of Metabolic and Proliferative Activity of Human ASCs. The Alamar Blue Test and Bromodeoxyuridine hASC Proliferation Assay
2.6.2. Cytotoxicity of Hydrogels Functionalized with nHAp and Resveratrol toward hASCs
2.6.3. The Analysis of Biomaterials Cytotoxicity Based on Mitochondrial Membrane Potential of hASCs
2.6.4. The Analysis of Human ASCs Morphology Using Scanning Electron Microscope (SEM)
2.6.5. Real-Time Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.7. Statistical Analysis
3. Results and Discussion
3.1. Structural Analysis of Nanocomposites
3.2. Spectroscopic Properties
3.3. Resveratrol Release from 3,6-Anhydro-α-l-galacto-β-d-galactan Hydrogel Filled with Resveratrol and Hydroxyapatite
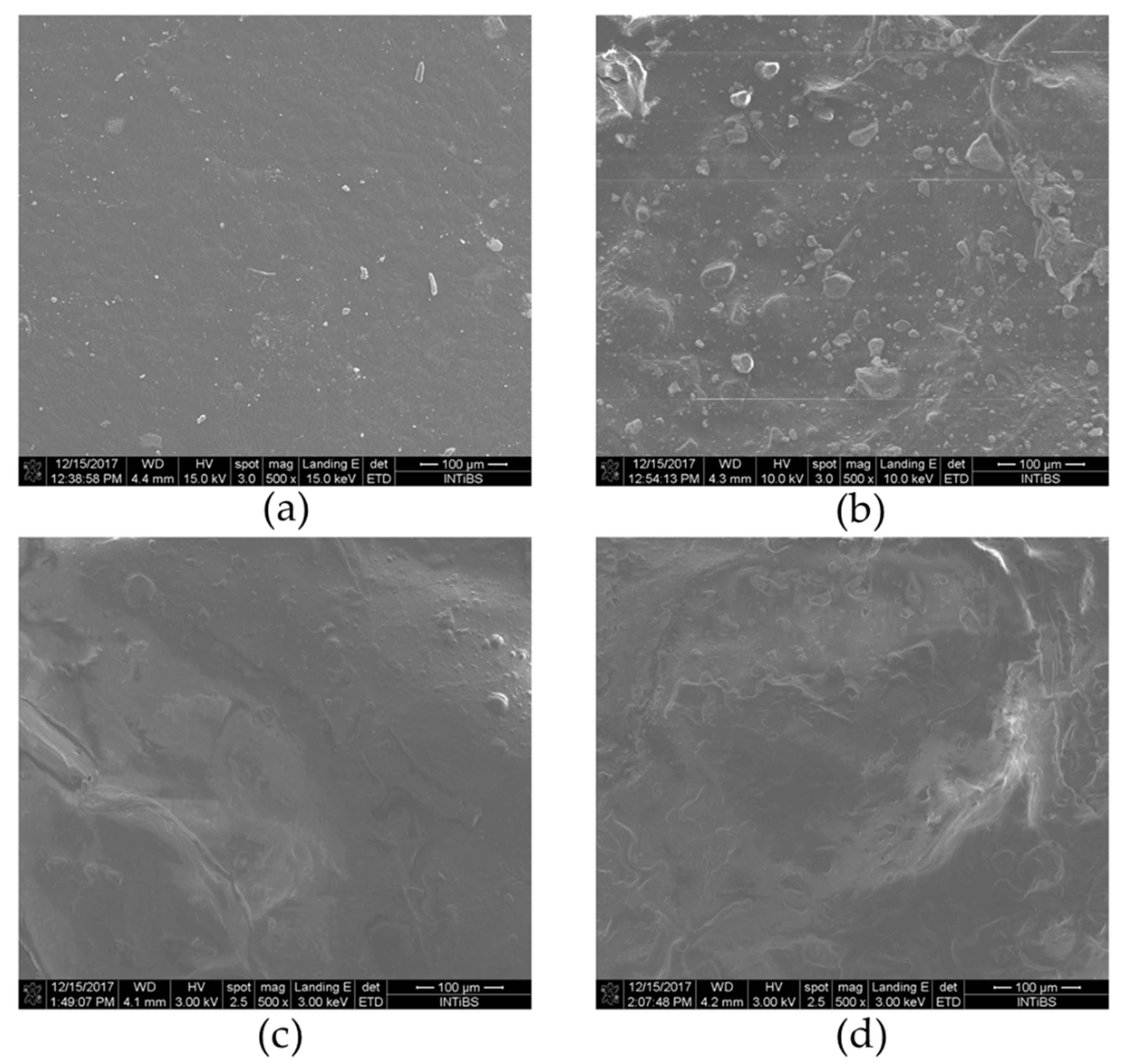
3.4. SEM Analysis of Nanocrystalline Hydroxyapatite Loaded with Resveratrol in Colloidal Suspension Structure
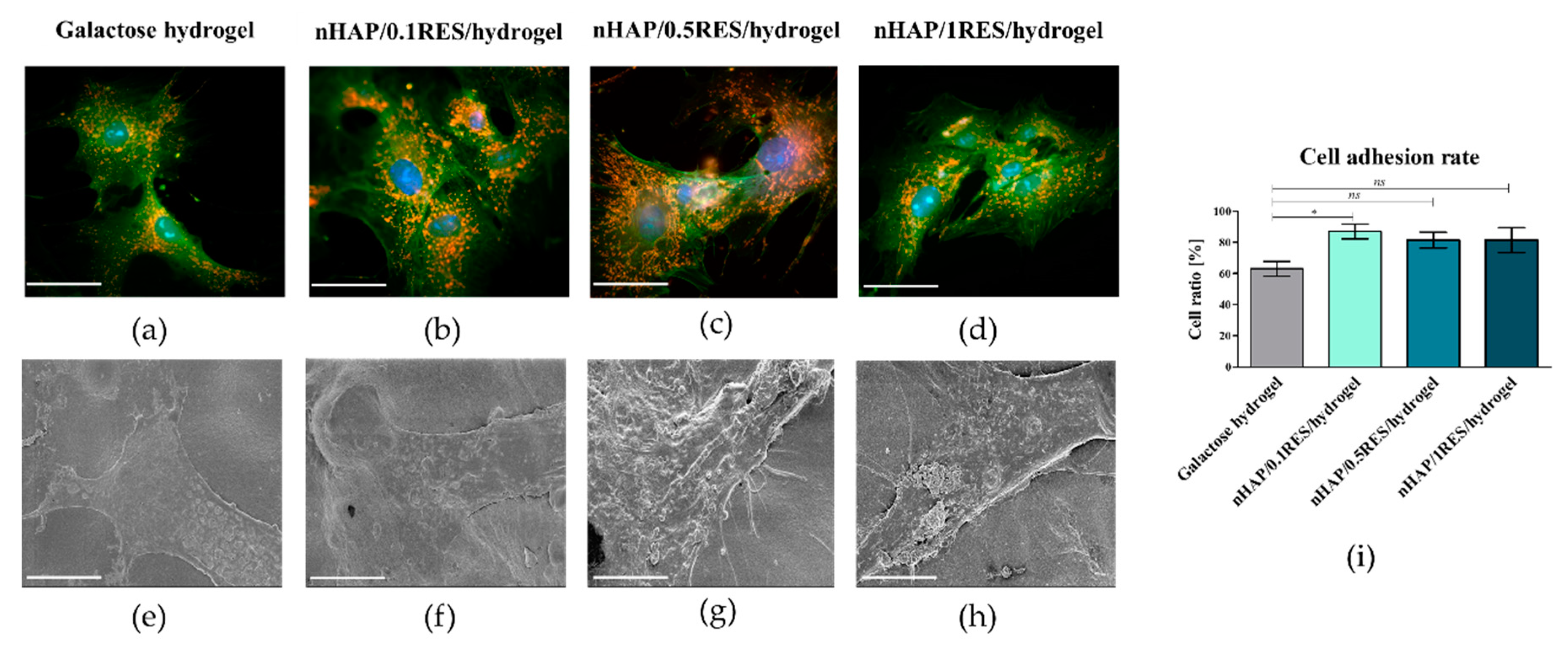
3.5. The Influence of Hydrogels Functionalized with nHAp and Resveratrol on hASC Morphology, Proliferation, and Metabolic Activity
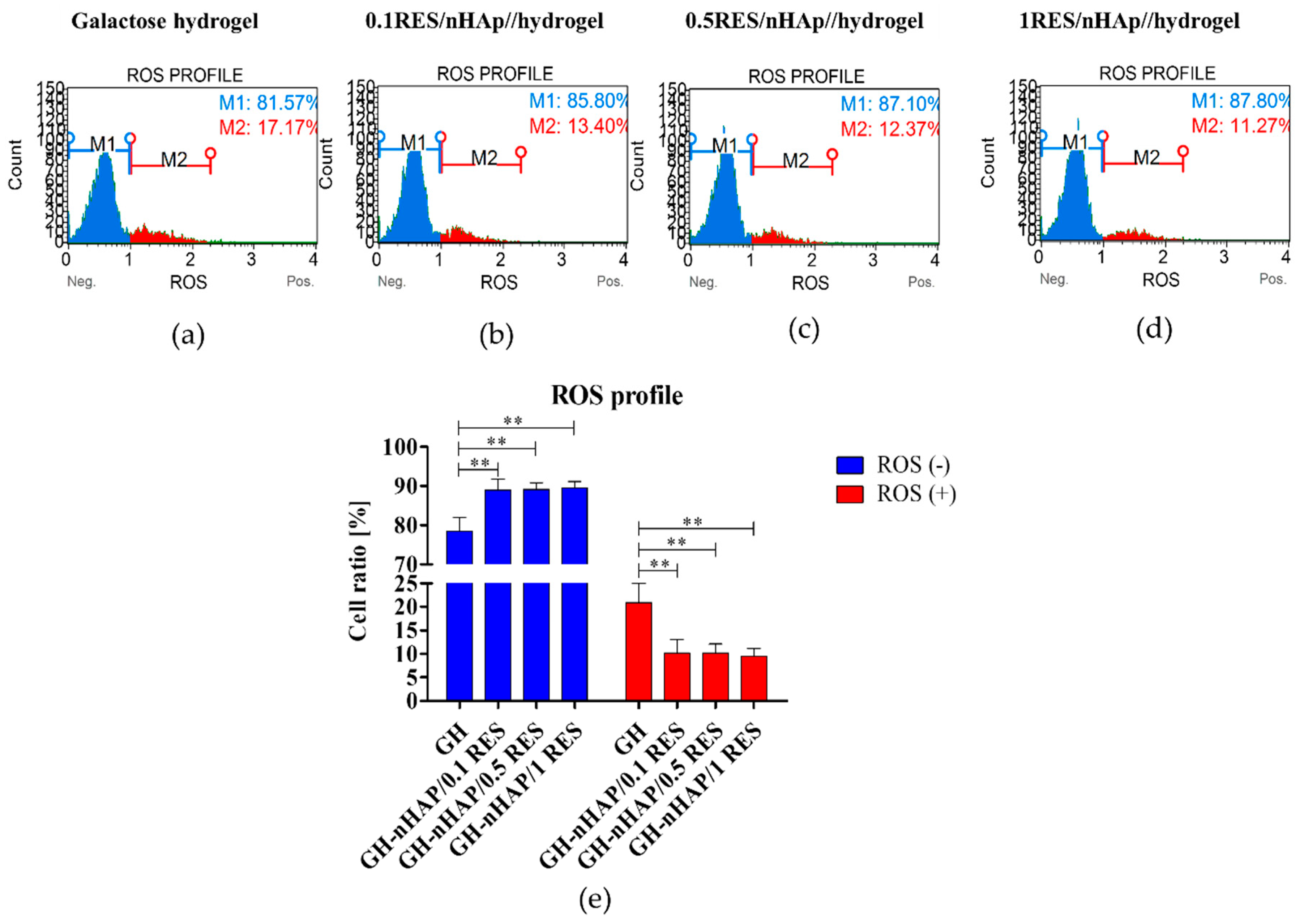
3.6. The Influence of Hydrogels Functionalized with nHAp and Resveratrol on hASCs Reactive Oxygen Species and Mitochondria Potential
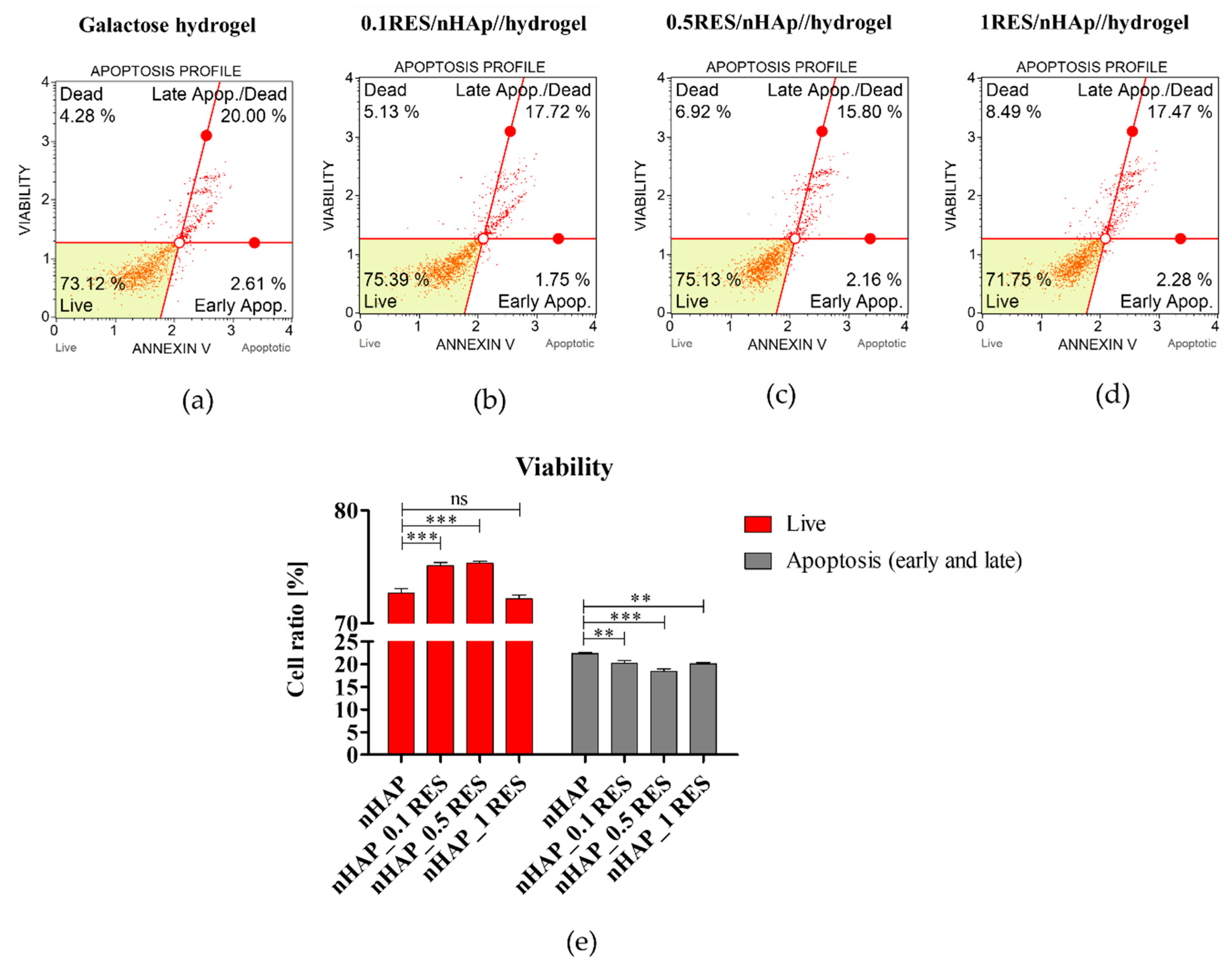
3.7. Apoptosis Analysis of hASCs with Resveratrol/nHAp/Hydrogels
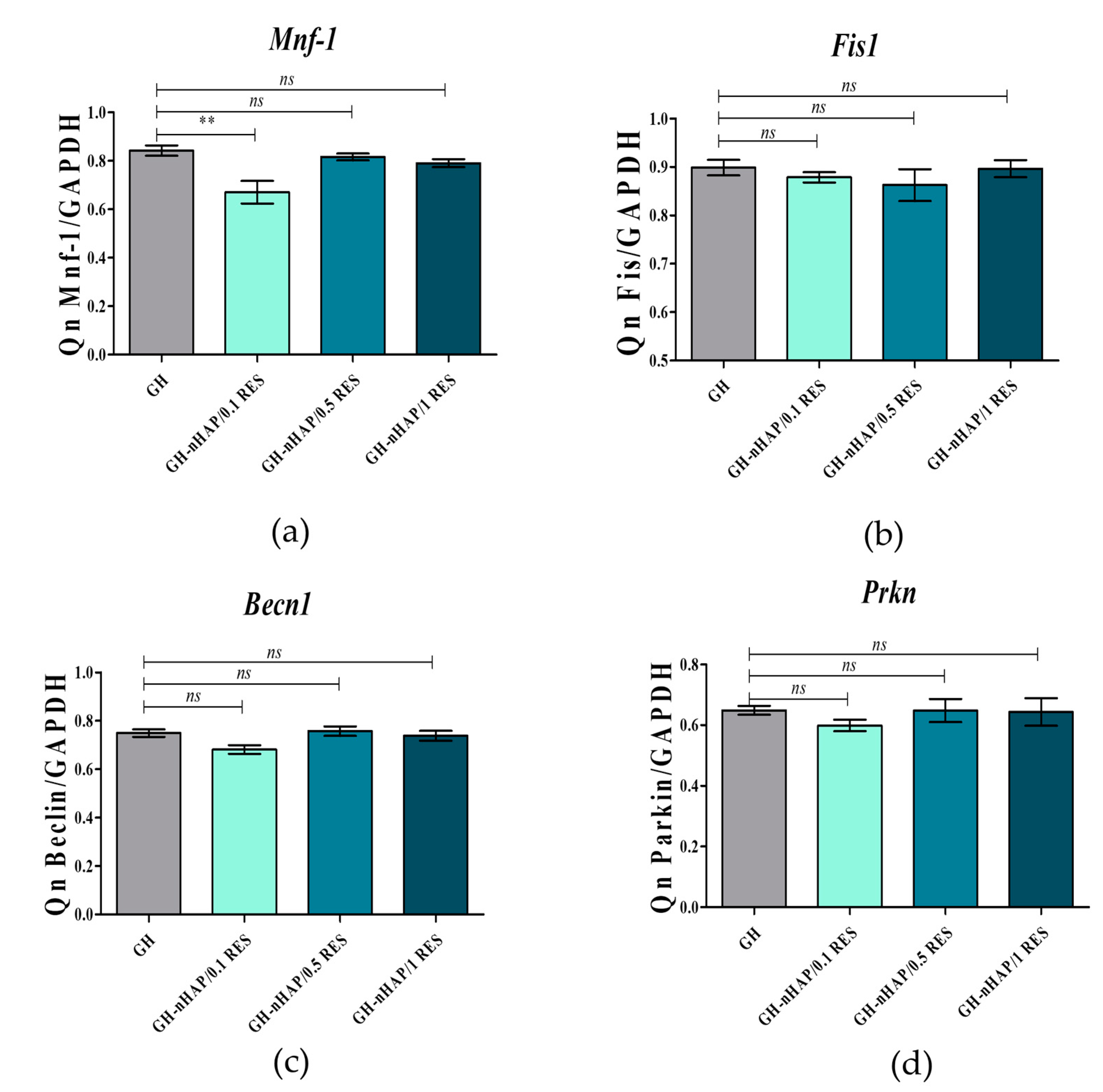
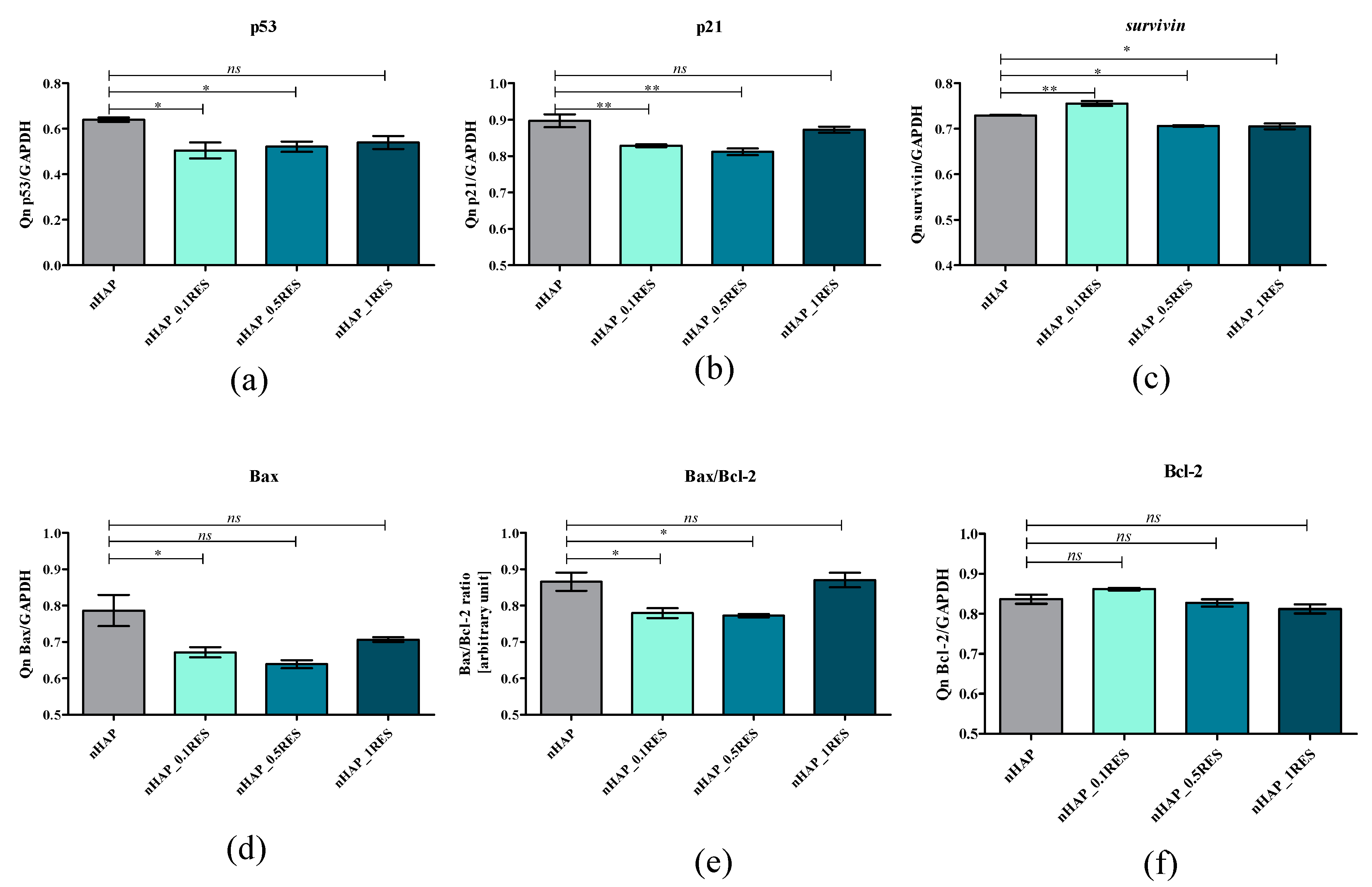
3.8. The Role of microRNAs after hASCs’ Resveratrol/NHAp/Hydrogels Treatment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pasparakis, G.; Vamvakaki, M. Multiresponsive polymers: nano-sized assemblies, stimuli-sensitive gels and smart surfaces. Polym. Chem. 2011, 2, 1234–1248. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2–11. [Google Scholar] [CrossRef]
- Baldari, S.; Di Rocco, G.; Piccoli, M.; Pozzobon, M.; Muraca, M.; Toietta, G. Challenges and Strategies for Improving the Regenerative Effects of Mesenchymal Stromal Cell-Based Therapies. Int. J. Mol. Sci. 2017, 18, 2087. [Google Scholar] [CrossRef] [PubMed]
- Bogdanowicz, D.R.; Lu, H.H. Designing the stem cell microenvironment for guided connective tissue regeneration. Ann. N. Y. Acad. Sci. 2017, 1410, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Castorina, A.; Szychlinska, M.A.; Marzagalli, R.; Musumeci, G. Mesenchymal stem cells-based therapy as a potential treatment in neurodegenerative disorders: is the escape from senescence an answer? Neural Regen. Res. 2015, 10, 850–858. [Google Scholar] [CrossRef]
- Schäfer, R.; Spohn, G.; Baer, P.C. Mesenchymal Stem/Stromal Cells in Regenerative Medicine: Can Preconditioning Strategies Improve Therapeutic Efficacy? Transfus. Med. Hemother. 2016, 43, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Barui, A.; Chowdhury, F.; Pandit, A.; Datta, P. Rerouting mesenchymal stem cell trajectory towards epithelial lineage by engineering cellular niche. Biomaterials 2018, 156, 28–44. [Google Scholar] [CrossRef]
- Szarek, D.; Laska, J.; Jarmundowicz, W.; Blazewicz, S.; Tabakow, P.; Marycz, K.; Wozniak, Z.; Mierzwa, J. Influence of Alginates on Tube Nerve Grafts of Different Elasticity—Preliminary in Vivo Study. J. Biomater. Nanobiotechnol. 2011, 2012. [Google Scholar]
- Grzesiak, J.; Marycz, K.; Szarek, D.; Bednarz, P.; Laska, J. Polyurethane/polylactide-based biomaterials combined with rat olfactory bulb-derived glial cells and adipose-derived mesenchymal stromal cells for neural regenerative medicine applications. Mater. Sci. Eng. C 2015, 52, 163–170. [Google Scholar] [CrossRef]
- Marycz, K.; Marędziak, M.; Grzesiak, J.; Lis, A.; Śmieszek, A. Biphasic Polyurethane/Polylactide Sponges Doped with Nano-Hydroxyapatite (nHAp) Combined with Human Adipose-Derived Mesenchymal Stromal Stem Cells for Regenerative Medicine Applications. Polymers 2016, 8, 339. [Google Scholar] [CrossRef]
- Censi, R.; Dubbini, A.; Matricardi, P. Bioactive hydrogel scaffolds-advances in cartilage regeneration through controlled drug delivery. Curr. Pharm. Des. 2015, 21, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Zimoch-Korzycka, A.; Śmieszek, A.; Jarmoluk, A.; Nowak, U.; Marycz, K. Potential Biomedical Application of Enzymatically Treated Alginate/Chitosan Hydrosols in Sponges—Biocompatible Scaffolds Inducing Chondrogenic Differentiation of Human Adipose Derived Multipotent Stromal Cells. Polymers 2016, 8, 320. [Google Scholar] [CrossRef]
- Kulig, D.; Zimoch-Korzycka, A.; Jarmoluk, A.; Marycz, K. Study on Alginate–Chitosan Complex Formed with Different Polymers Ratio. Polymers 2016, 8, 167. [Google Scholar] [CrossRef]
- Ravichandran, R.; Islam, M.M.; Alarcon, E.I.; Samanta, A.; Wang, S.; Lundström, P.; Hilborn, J.; Griffith, M.; Phopase, J. Functionalised type-I collagen as a hydrogel building block for bio-orthogonal tissue engineering applications. J. Mater. Chem. B 2015, 4, 318–326. [Google Scholar] [CrossRef]
- Fichman, G.; Gazit, E. Self-assembly of short peptides to form hydrogels: Design of building blocks, physical properties and technological applications. Acta Biomater. 2014, 10, 1671–1682. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Attalla, R.; Sadowski, L.P.; Chen, M.; Majcher, M.J.; Urosev, I.; Yin, D.-C.; Selvaganapathy, P.R.; Filipe, C.D.M.; Hoare, T. Autonomously Self-Adhesive Hydrogels as Building Blocks for Additive Manufacturing. Biomacromolecules 2018, 19, 62–70. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. Weinheim 2009, 21, 3307–3329. [Google Scholar] [CrossRef]
- Trohatou, O.; Roubelakis, M.G. Mesenchymal Stem/Stromal Cells in Regenerative Medicine: Past, Present, and Future. Cell Reprogram 2017, 19, 217–224. [Google Scholar] [CrossRef]
- Frese, L.; Dijkman, P.E.; Hoerstrup, S.P. Adipose Tissue-Derived Stem Cells in Regenerative Medicine. Transfus. Med. Hemother. 2016, 43, 268–274. [Google Scholar] [CrossRef]
- Hofer, H.R.; Tuan, R.S. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res. Ther. 2016, 7, 131. [Google Scholar] [CrossRef]
- Kornicka, K.; Marycz, K.; Tomaszewski, K.A.; Marędziak, M.; Śmieszek, A. The Effect of Age on Osteogenic and Adipogenic Differentiation Potential of Human Adipose Derived Stromal Stem Cells (hASCs) and the Impact of Stress Factors in the Course of the Differentiation Process. Oxid. Med. Cell. Longev. 2015, 2015, 309169. [Google Scholar] [CrossRef]
- Marędziak, M.; Marycz, K.; Tomaszewski, K.A.; Kornicka, K.; Henry, B.M. The Influence of Aging on the Regenerative Potential of Human Adipose Derived Mesenchymal Stem Cells. Available online: https://www.hindawi.com/journals/sci/2016/2152435/ (accessed on 6 January 2019).
- Kornicka, K.; Nawrocka, D.; Lis-Bartos, A.; Marędziak, M.; Marycz, K. Polyurethane–polylactide-based material doped with resveratrol decreases senescence and oxidative stress of adipose-derived mesenchymal stromal stem cell (ASCs). RSC Adv. 2017, 7, 24070–24084. [Google Scholar] [CrossRef]
- Kornicka, K.; Szłapka-Kosarzewska, J.; Śmieszek, A.; Marycz, K. 5-Azacytydine and resveratrol reverse senescence and ageing of adipose stem cells via modulation of mitochondrial dynamics and autophagy. J. Cell. Mol. Med. 2019, 23, 237–259. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Liu, S.; Liu, Y. Resveratrol protects bone marrow mesenchymal stem cell derived chondrocytes cultured on chitosan-gelatin scaffolds from the inhibitory effect of interleukin-1β. Acta Pharmacol. Sin. 2008, 29, 1350–1356. [Google Scholar] [CrossRef]
- Tseng, P.-C.; Hou, S.-M.; Chen, R.-J.; Peng, H.-W.; Hsieh, C.-F.; Kuo, M.-L.; Yen, M.-L. Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating RUNX2 gene expression via the SIRT1/FOXO3A axis. J. Bone Miner. Res. 2011, 26, 2552–2563. [Google Scholar] [CrossRef]
- Nuss, K.M.R.; von Rechenberg, B. Biocompatibility issues with modern implants in bone—A review for clinical orthopedics. Open Orthop. J. 2008, 2, 66–78. [Google Scholar] [CrossRef]
- Salgado, C.L.; Grenho, L.; Fernandes, M.H.; Colaço, B.J.; Monteiro, F.J. Biodegradation, biocompatibility, and osteoconduction evaluation of collagen-nanohydroxyapatite cryogels for bone tissue regeneration. J. Biomed. Mater. Res. A 2016, 104, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Szymonowicz, M.; Korczynski, M.; Dobrzynski, M.; Zawisza, K.; Mikulewicz, M.; Karuga-Kuzniewska, E.; Zywickab, B.; Rybak, Z.; Wiglusz, R.J. Cytotoxicity Evaluation of High-Temperature Annealed Nanohydroxyapatite in Contact with Fibroblast Cells. Materials 2017, 10, 590. [Google Scholar] [CrossRef]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Marycz, K.; Pazik, R.; Zawisza, K.; Wiglusz, K.; Maredziak, M.; Sobierajska, P.; Wiglusz, R.J. Multifunctional nanocrystalline calcium phosphates loaded with Tetracycline antibiotic combined with human adipose derived mesenchymal stromal stem cells (hASCs). Mater. Sci. Eng. C 2016, 69, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Sobierajska, P.; Smieszek, A.; Maredziak, M.; Wiglusz, K.; Wiglusz, R.J. Li+ activated nanohydroxyapatite doped with Eu3+ ions enhances proliferative activity and viability of human stem progenitor cells of adipose tissue and olfactory ensheathing cells. Further perspective of nHAP:Li+, Eu3+ application in theranostics. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Smieszek, A.; Marycz, K.; Szustakiewicz, K.; Kryszak, B.; Targonska, S.; Zawisza, K.; Watras, A.; Wiglusz, R.J. New approach to modification of poly(l-lactic acid) with nano-hydroxyapatite improving functionality of human adipose-derived stromal cells (hASCs) through increased viability and enhanced mitochondrial activity. Mater. Sci. Eng. C 2019, 98, 213–226. [Google Scholar] [CrossRef]
- Sobierajska, P.; Zawisza, K.; Kowalski, R.M.; Renaudin, G.; Nedelec, J.-M.; Zienkiewicz, J.; Wiglusz, R.J. Preparation of up-converting nano-biphasic calcium phosphate. RSC Adv. 2017, 7, 30086–30095. [Google Scholar] [CrossRef]
- Suchanek, W.; Yoshimura, M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J. Mater. Res. 1998, 13, 94–117. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphates in Nature, Biology and Medicine. Materials 2009, 2, 399–498. [Google Scholar] [CrossRef]
- Śmieszek, A.; Szydlarska, J.; Mucha, A.; Chrapiec, M.; Marycz, K. Enhanced cytocompatibility and osteoinductive properties of sol-gel-derived silica/zirconium dioxide coatings by metformin functionalization. J. Biomater. Appl. 2017, 32, 570–586. [Google Scholar]
- Śmieszek, A.; Tomaszewski, K.A.; Kornicka, K.; Marycz, K. Metformin Promotes Osteogenic Differentiation of Adipose-Derived Stromal Cells and Exerts Pro-Osteogenic Effect Stimulating Bone Regeneration. J. Clin. Med. 2018, 7, 482. [Google Scholar]
- Marędziak, M.; Marycz, K.; Smieszek, A.; Lewandowski, D.; Toker, N.Y. The influence of static magnetic fields on canine and equine mesenchymal stem cells derived from adipose tissue. In Vitro Cell. Dev. Biol. Anim. 2014, 50, 562–571. [Google Scholar] [CrossRef]
- Śmieszek, A.; Czyrek, A.; Basinska, K.; Trynda, J.; Skaradzińska, A.; Siudzińska, A.; Marędziak, M.; Marycz, K. Effect of Metformin on Viability, Morphology, and Ultrastructure of Mouse Bone Marrow-Derived Multipotent Mesenchymal Stromal Cells and Balb/3T3 Embryonic Fibroblast Cell Line. Available online: https://www.hindawi.com/journals/bmri/2015/769402/ (accessed on 26 December 2018).
- Marycz, K.; Krzak-Roś, J.; Donesz-Sikorska, A.; Śmieszek, A. The morphology, proliferation rate, and population doubling time factor of adipose-derived mesenchymal stem cells cultured on to non-aqueous SiO2, TiO2, and hybrid sol-gel-derived oxide coatings. J. Biomed. Mater. Res. A 2014, 102, 4017–4026. [Google Scholar] [CrossRef]
- Doubling Time—Online computing with 2 points. Available online: http://www.doubling-time.com/compute.php (accessed on 6 January 2019).
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Kornicka, K.; Walczak, R.; Mucha, A.; Marycz, K. Released from ZrO2/SiO2 coating resveratrol inhibits senescence and oxidative stress of human adipose-derived stem cells (ASC). Open Chem. 2018, 16, 481–495. [Google Scholar] [CrossRef]
- Śmieszek, A.; Giezek, E.; Chrapiec, M.; Murat, M.; Mucha, A.; Michalak, I.; Marycz, K. The Influence of Spirulina platensis Filtrates on Caco-2 Proliferative Activity and Expression of Apoptosis-Related microRNAs and mRNA. Mar. Drugs 2017, 15, 65. [Google Scholar]
- Hendrawan; Khoerunnisa, F.; Sonjaya, Y.; Chotimah, N. Physical and chemical characteristics of alginate-poly(vinyl alcohol) based controlled release hydrogel. J. Environ. Chem. Eng. 2016, 4(PB), 4863–4869. [Google Scholar] [CrossRef]
- Kanmani, P.; Rhim, J.-W. Antimicrobial and physical-mechanical properties of agar-based films incorporated with grapefruit seed extract. Carbohydr. Polym. 2014, 102, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Ren, X.; Zhang, X.; Hou, T.; Chalamaiah, M.; Ma, H.; Xu, B. Effect of ultrasound on the preparation of resveratrol-loaded zein particles. J. Food Eng. 2018, 221, 88–94. [Google Scholar] [CrossRef]
- Kawamura, K.; Yasuda, T.; Hatanaka, T.; Hamahiga, K.; Matsuda, N.; Ueshima, M.; Nakai, K. In situ UV–VIS spectrophotometry within the second time scale as a research tool for solid-state catalyst and liquid-phase reactions at high temperatures: Its application to the formation of HMF from glucose and cellulose. Chem. Eng. J. 2017, 307, 1066–1075. [Google Scholar] [CrossRef]
- Yin, Z.-C.; Wang, Y.-L.; Wang, K. A pH-responsive composite hydrogel beads based on agar and alginate for oral drug delivery. J. Drug Deliv. Sci. Technol. 2018, 43, 12–18. [Google Scholar] [CrossRef]
- Wu, Y.; Geng, F.; Chang, P.R.; Yu, J.; Ma, X. Effect of agar on the microstructure and performance of potato starch film. Carbohydr. Polym. 2009, 76, 299–304. [Google Scholar] [CrossRef]
- Meng, X.; Tian, F.; Yang, J.; He, C.-N.; Xing, N.; Li, F. Chitosan and alginate polyelectrolyte complex membranes and their properties for wound dressing application. J. Mater. Sci. Mater. Med. 2010, 21, 1751–1759. [Google Scholar] [CrossRef]
- Le-Tien, C.; Millette, M.; Mateescu, M.-A.; Lacroix, M. Modified alginate and chitosan for lactic acid bacteria immobilization. Biotechnol. Appl. Biochem. 2004, 39, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Filová, E.; Suchý, T.; Sucharda, Z.; Šupová, M.; Žaloudková, M.; Balík, K.; Lisá, V.; Šlouf, M.; Bačáková, L. Support for the initial attachment, growth and differentiation of MG-63 cells: a comparison between nano-size hydroxyapatite and micro-size hydroxyapatite in composites. Int. J. Nanomed. 2014, 9, 3687–3706. [Google Scholar]
- Zhou, G.S.; Su, Z.Y.; Cai, Y.R.; Liu, Y.K.; Dai, L.C.; Tang, R.K.; Zhang, M. Different effects of nanophase and conventional hydroxyapatite thin films on attachment, proliferation and osteogenic differentiation of bone marrow derived mesenchymal stem cells. Biomed. Mater. Eng. 2007, 17, 387–395. [Google Scholar]
- Peltz, L.; Gomez, J.; Marquez, M.; Alencastro, F.; Atashpanjeh, N.; Quang, T.; Bach, T.; Zhao, Y. Resveratrol Exerts Dosage and Duration Dependent Effect on Human Mesenchymal Stem Cell Development. PLOS ONE 2012, 7, e37162. [Google Scholar] [CrossRef] [PubMed]
- Denu, R.A.; Hematti, P. Effects of Oxidative Stress on Mesenchymal Stem Cell Biology. Oxid. Med. Cell. Longev. 2016, 2016, 2989076. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011, 18, 571–580. [Google Scholar] [CrossRef]
- Narendra, D.; Tanaka, A.; Suen, D.-F.; Youle, R.J. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J. Cell Biol. 2008, 183, 795–803. [Google Scholar] [CrossRef]
- Gomes, L.C.; Scorrano, L. High levels of Fis1, a pro-fission mitochondrial protein, trigger autophagy. Biochim. Biophys. Acta 2008, 1777, 860–866. [Google Scholar] [CrossRef]
- Seo, A.Y.; Joseph, A.-M.; Dutta, D.; Hwang, J.C.Y.; Aris, J.P.; Leeuwenburgh, C. New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J. Cell. Sci. 2010, 123, 2533–2542. [Google Scholar] [CrossRef]
- Vogel, M.W. Cell death, Bcl-2, Bax, and the cerebellum. Cerebellum 2002, 1, 277–287. [Google Scholar] [CrossRef]
- Yang, M.Y.; Rajamahendran, R. Expression of Bcl-2 and Bax proteins in relation to quality of bovine oocytes and embryos produced in vitro. Anim. Reprod. Sci. 2002, 70, 159–169. [Google Scholar] [CrossRef]
- Singh, P.; Fukuda, S.; Saunders, M.R.; Pelus, L.M. Survivin is a master regulator of mesenchymal stem cell functions and potential therapeutic target for mesenchymal stem cell expansion. Blood 2014, 124, 774. [Google Scholar]
- Jing, D.; Hao, J.; Shen, Y.; Tang, G.; Li, M.-L.; Huang, S.-H.; Zhao, Z.-H. The role of microRNAs in bone remodeling. Int. J. Oral Sci. 2015, 7, 131–143. [Google Scholar] [CrossRef]
- Foroutan, T.; Farhadi, A.; Abroun, S.; Mohammad Soltani, B. Adipose Derived Stem Cells Affect miR-145 and p53 Expressions of Co-Cultured Hematopoietic Stem Cells. Cell J. 2018, 19, 654–659. [Google Scholar]
- Wang, X.; Zhang, Y. Resveratrol alleviates LPS-induced injury in human keratinocyte cell line HaCaT by up-regulation of miR-17. Biochem. Biophys. Res. Commun. 2018, 501, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Yuan, X.; Liu, M.; Xue, B. miRNA-223-3p regulates NLRP3 to promote apoptosis and inhibit proliferation of hep3B cells. Exp. Ther. Med. 2018, 15, 2429–2435. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Su, C.; Deng, T. miR-223 decreases cell proliferation and enhances cell apoptosis in acute myeloid leukemia via targeting FBXW7. Oncol. Lett. 2016, 12, 3531–3536. [Google Scholar] [CrossRef] [PubMed]
- Song, C.-L.; Liu, B.; Diao, H.-Y.; Shi, Y.-F.; Zhang, J.-C.; Li, Y.-X.; Liu, N.; Yu, Y.-P.; Wang, G.; Wang, J.-P.; et al. Down-regulation of microRNA-320 suppresses cardiomyocyte apoptosis and protects against myocardial ischemia and reperfusion injury by targeting IGF-1. Oncotarget 2016, 7, 39740–39757. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Dong, Q.; Wang, E. MicroRNA-320 inhibits invasion and induces apoptosis by targeting CRKL and inhibiting ERK and AKT signaling in gastric cancer cells. Onco Targets Ther. 2017, 10, 1049–1058. [Google Scholar] [CrossRef] [PubMed]





| miRNA | Specific Primer Sequence | Accession Number |
|---|---|---|
| hsa-miR-17-5p | CAAAGTGCTTACAGTGCAGGTAG | MIMAT0000070 |
| hsa-miR-145-5p | GTCCAGTTTTCCCAGGAATCCCT | MIMAT0000437 |
| hsa-miR-223-3p | TGTCAGTTTGTCAAATACCCCA | MIMAT0000280 |
| hsa-miR-320a-3p | AAAAGCTGGGTTGAGAGGGCGA | MIMAT0000510 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marycz, K.; Smieszek, A.; Trynda, J.; Sobierajska, P.; Targonska, S.; Grosman, L.; Wiglusz, R.J. Nanocrystalline Hydroxyapatite Loaded with Resveratrol in Colloidal Suspension Improves Viability, Metabolic Activity and Mitochondrial Potential in Human Adipose-Derived Mesenchymal Stromal Stem Cells (hASCs). Polymers 2019, 11, 92. https://doi.org/10.3390/polym11010092
Marycz K, Smieszek A, Trynda J, Sobierajska P, Targonska S, Grosman L, Wiglusz RJ. Nanocrystalline Hydroxyapatite Loaded with Resveratrol in Colloidal Suspension Improves Viability, Metabolic Activity and Mitochondrial Potential in Human Adipose-Derived Mesenchymal Stromal Stem Cells (hASCs). Polymers. 2019; 11(1):92. https://doi.org/10.3390/polym11010092
Chicago/Turabian StyleMarycz, Krzysztof, Agnieszka Smieszek, Justyna Trynda, Paulina Sobierajska, Sara Targonska, Lukasz Grosman, and Rafal J. Wiglusz. 2019. "Nanocrystalline Hydroxyapatite Loaded with Resveratrol in Colloidal Suspension Improves Viability, Metabolic Activity and Mitochondrial Potential in Human Adipose-Derived Mesenchymal Stromal Stem Cells (hASCs)" Polymers 11, no. 1: 92. https://doi.org/10.3390/polym11010092
APA StyleMarycz, K., Smieszek, A., Trynda, J., Sobierajska, P., Targonska, S., Grosman, L., & Wiglusz, R. J. (2019). Nanocrystalline Hydroxyapatite Loaded with Resveratrol in Colloidal Suspension Improves Viability, Metabolic Activity and Mitochondrial Potential in Human Adipose-Derived Mesenchymal Stromal Stem Cells (hASCs). Polymers, 11(1), 92. https://doi.org/10.3390/polym11010092