Foaming Behavior and Microcellular Morphologies of Incompatible SAN/CPE Blends with Supercritical Carbon Dioxide as a Physical Blowing Agent
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SAN/CPE Blends
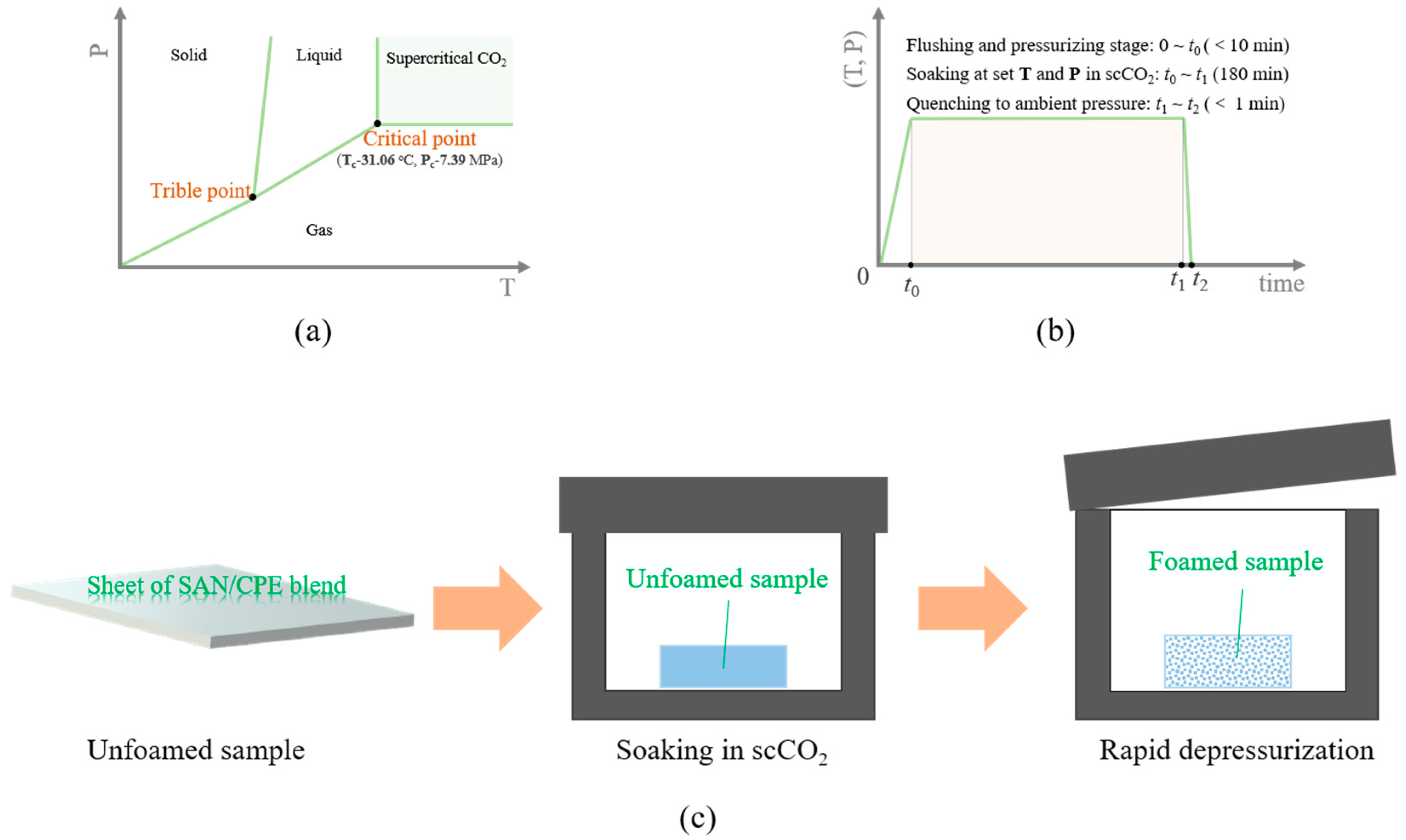
2.3. Foaming of SAN/CPE Blends with Supercritical CO2
2.4. Characterization
2.4.1. Melt Flow Index (MFI)
2.4.2. Thermogravimetric Analysis (TGA)
2.4.3. Differential Scanning Calorimetry (DSC)
2.4.4. Dynamic Thermomechanical Analysis (DMA)
2.4.5. Field-Emission Scanning Electron Microscopy (FSEM)
3. Results and Discussion
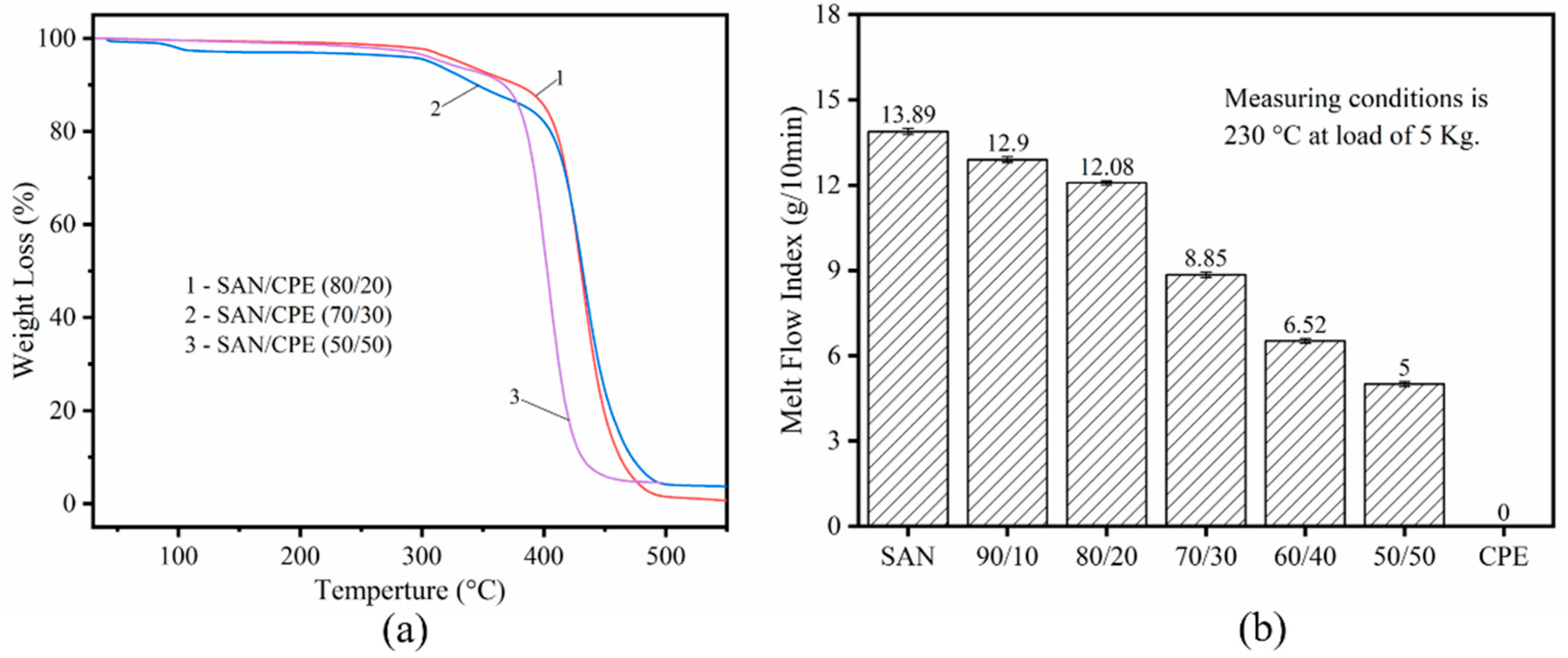
3.1. Thermogravimetric Analysis and Melt Flow Index of SAN/CPE Blends
3.2. Compatibility of SAN/CPE Blends
3.3. Porous Structures of Foamed SAN/CPE Samples
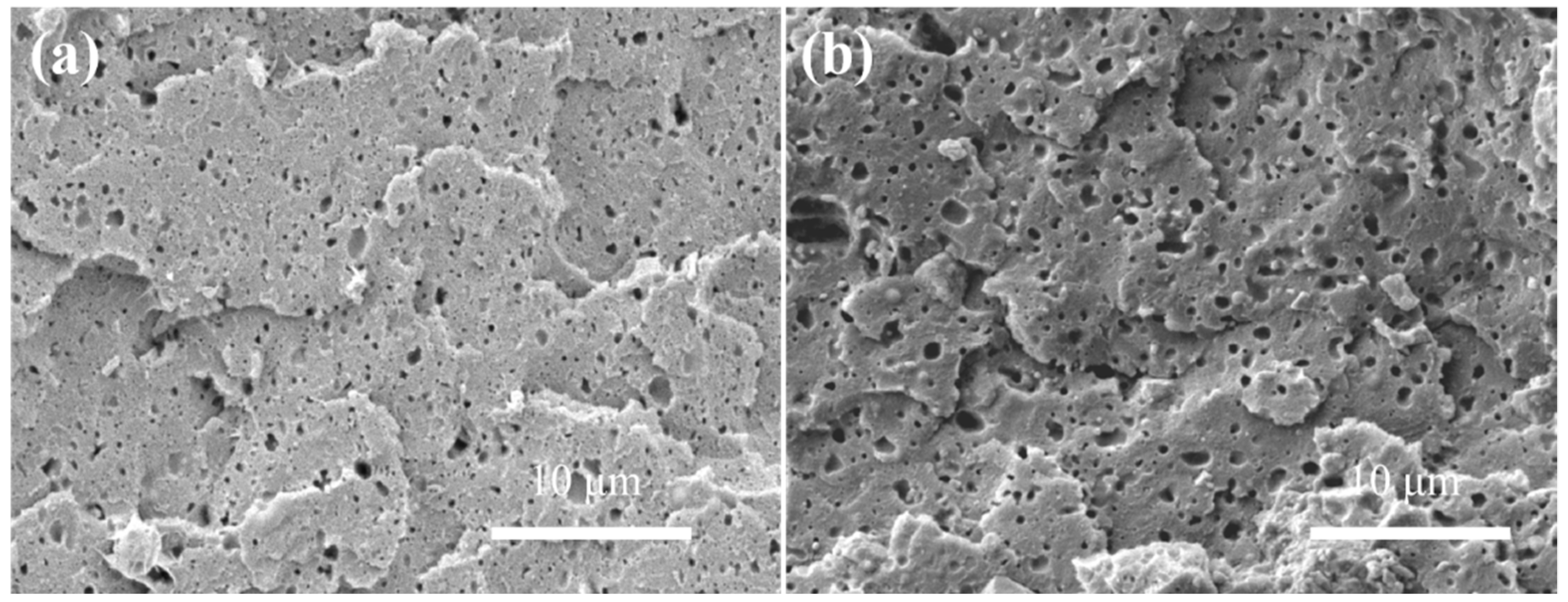
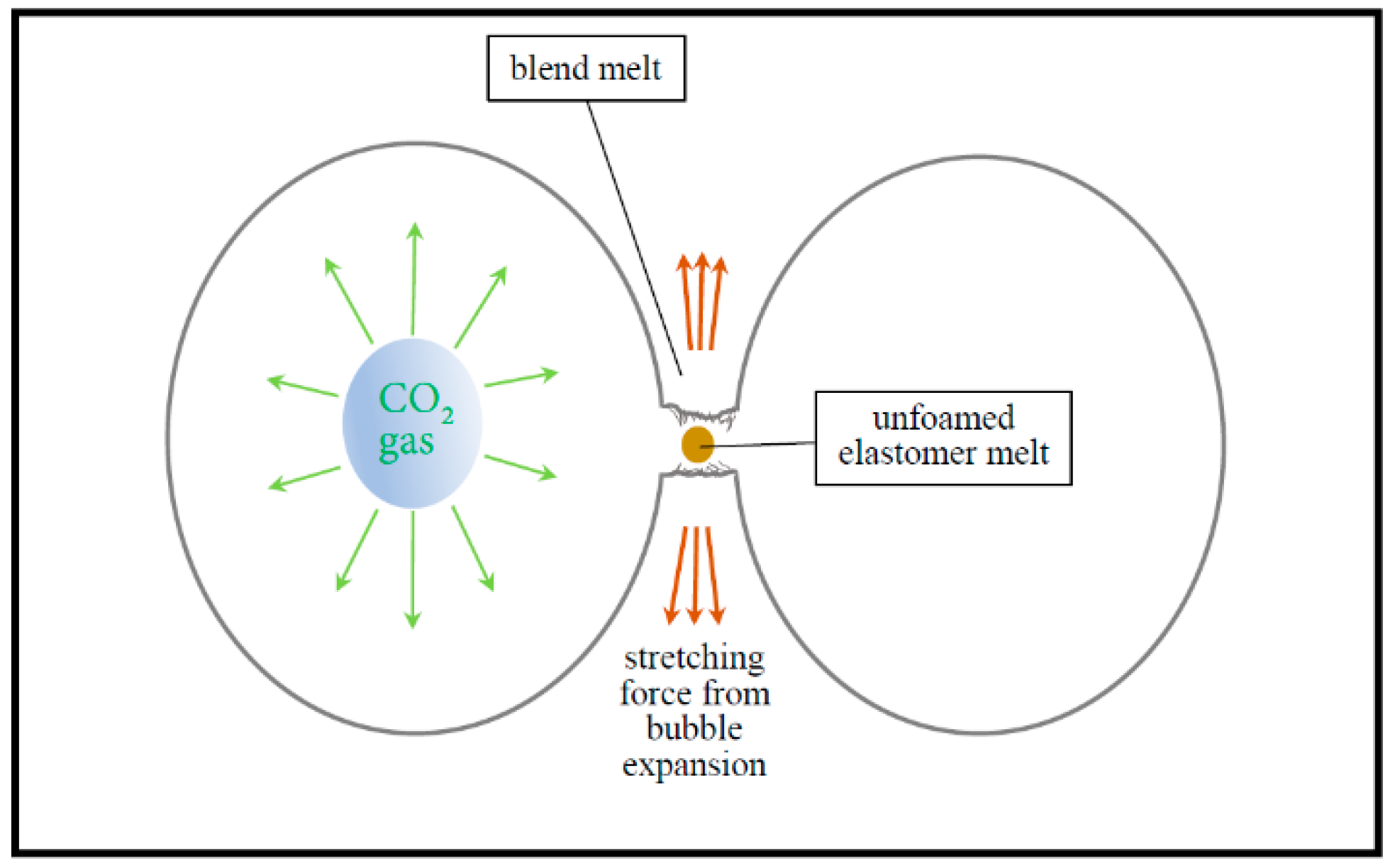
3.3.1. Foaming Behavior of Neat SAN and Neat CPE
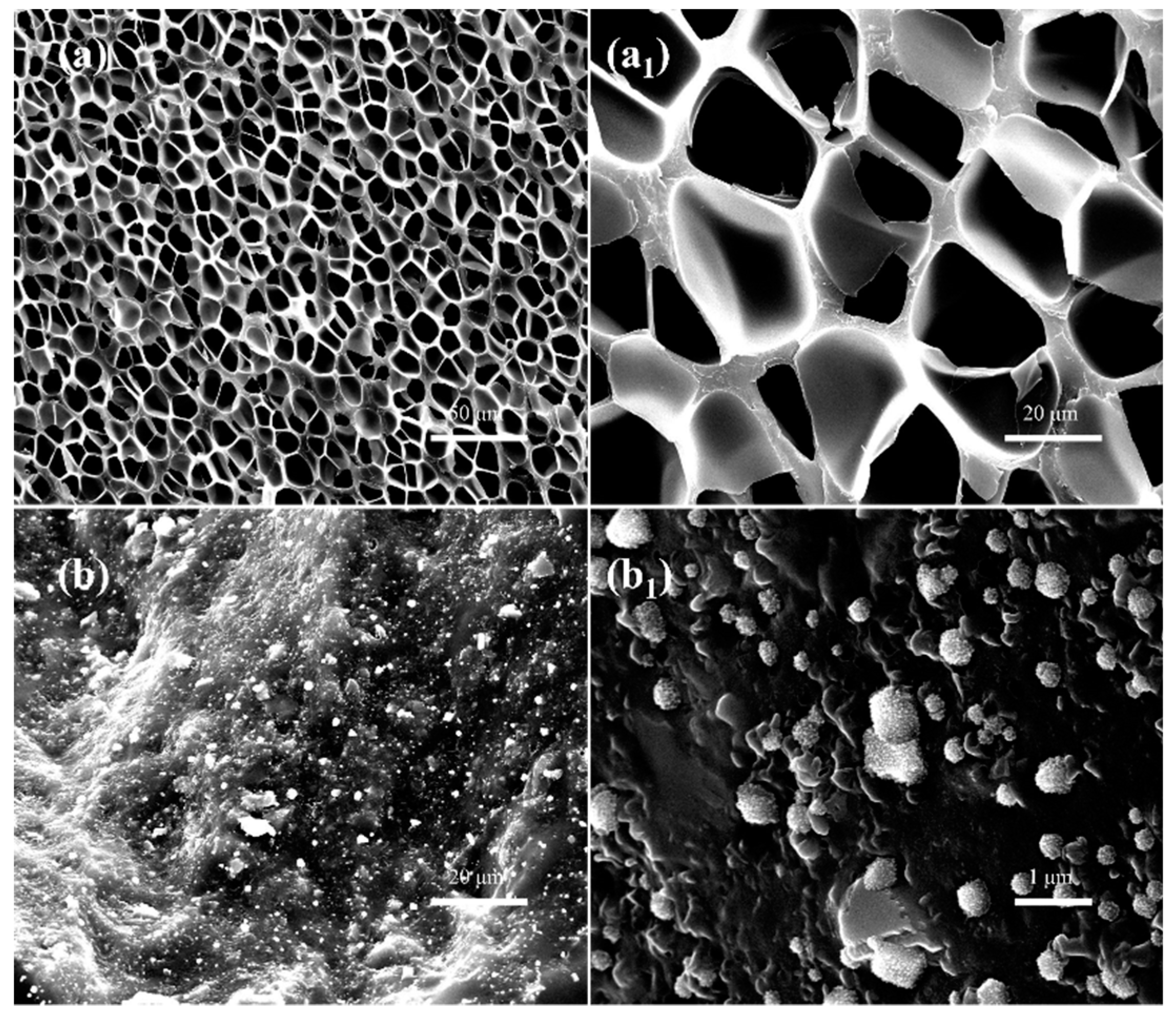
3.3.2. Microcellular Morphologies of SAN/CPE Blend Foams
3.3.3. Interconnected Porous Structures in SAN/CPE Foams
3.4. Mechanical Properties of SAN/CPE Microcellular Foams
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, G.; Zhao, G.; Dong, G.; Mu, Y.; Park, C.B. Lightweight and strong microcellular injection molded PP/talc nanocomposite. Compos. Sci. Technol. 2018, 168, 38–46. [Google Scholar] [CrossRef]
- Nofar, M.; Park, C.B. Poly (lactic acid) foaming. Prog. Polym. Sci. 2014, 39, 1721–1741. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, C.; Gong, W.; Ji, Y.; Qin, S.; He, L. Preparation of microcellular epoxy foams through a limited-foaming process: A contradiction with the time–temperature–transformation cure diagram. Adv. Mater. 2018, 30, 1703992. [Google Scholar] [CrossRef] [PubMed]
- Okolieocha, C.; Raps, D.; Subramaniam, K.; Altstädt, V. Microcellular to nanocellular polymer foams: Progress (2004–2015) and future directions—A review. Eur. Polym. J. 2015, 73, 500–519. [Google Scholar] [CrossRef]
- Kumar, V.; Suh, N.P. A process for making microcellular thermoplastic parts. Polym. Eng. Sci. 1990, 30, 1323–1329. [Google Scholar] [CrossRef]
- Kumar, V.; VanderWel, M.; Weller, J.; Seeler, K.A. Experimental characterization of the tensile behavior of microcellular polycarbonate foams. J. Eng. Mater. Technol. 1994, 116, 439–445. [Google Scholar] [CrossRef]
- Goel, S.K.; Beckman, E.J. Generation of microcellular polymeric foams using supercritical carbon dioxide. I: Effect of pressure and temperature on nucleation. Polym. Eng. Sci. 1994, 34, 1137–1147. [Google Scholar] [CrossRef]
- Yilmaz, G.; Ellingham, T.; Turng, L.-S. Improved processability and the processing-structure-properties relationship of ultra-high molecular weight polyethylene via supercritical nitrogen and carbon dioxide in injection molding. Polymers 2018, 10, 36. [Google Scholar] [CrossRef]
- Arora, K.A.; Lesser, A.J.; McCarthy, T.J. Preparation and characterization of microcellular polystyrene foams processed in supercritical carbon dioxide. Macromolecules 1998, 31, 4614–4620. [Google Scholar] [CrossRef]
- Tsivintzelis, I.; Sanxaridou, G.; Pavlidou, E.; Panayiotou, C. Foaming of polymers with supercritical fluids: A thermodynamic investigation. J. Supercrit. Fluids 2016, 110, 240–250. [Google Scholar] [CrossRef]
- Li, R.; Ye, N.; Shaayegan, V.; Fang, T. Experimental measurement of CO2 diffusion in PMMA and its effect on microcellular foaming. J. Supercrit. Fluids 2018, 135, 180–187. [Google Scholar] [CrossRef]
- Notario, B.; Pinto, J.; Rodríguez-Pérez, M.A. Towards a new generation of polymeric foams: PMMA nanocellular foams with enhanced physical properties. Polymer 2015, 63, 116–126. [Google Scholar] [CrossRef]
- Notario, B.; Pinto, J.; Solorzano, E.; de Saja, J.A.; Dumon, M.; Rodríguez-Pérez, M.A. Experimental validation of the knudsen effect in nanocellular polymeric foams. Polymer 2015, 56, 57–67. [Google Scholar] [CrossRef]
- Forest, C.; Chaumont, P.; Cassagnau, P.; Swoboda, B.; Sonntag, P. Nanofoaming of PMMA using a batch CO2 process: Influence of the PMMA viscoelastic behaviour. Polymer 2015, 77, 1–9. [Google Scholar] [CrossRef]
- Forest, C.; Chaumont, P.; Cassagnau, P.; Swoboda, B.; Sonntag, P. Polymer nano-foams for insulating applications prepared from CO2 foaming. Prog. Polym. Sci. 2015, 41, 122–145. [Google Scholar] [CrossRef]
- Pinto, J.; Dumon, M.; Rodriguez-Perez, M.A.; Garcia, R.; Dietz, C. Block copolymers self-assembly allows obtaining tunable micro or nanoporous membranes or depth filters based on PMMA; fabrication method and nanostructures. J. Phys. Chem. C 2014, 118, 4656–4663. [Google Scholar] [CrossRef]
- Salerno, A.; Zeppetelli, S.; Di Maio, E.; Iannace, S.; Netti, P.A. Design of bimodal PCL and PCL-HA nanocomposite scaffolds by two step depressurization during solid-state supercritical CO2 foaming. Macromol. Rapid Commun. 2011, 32, 1150–1156. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, Y.; Ye, L.; Gu, Y.; Qiu, J.; Tang, T.; Li, M. Unexpected foaming behavior of heterografted comb-like PS-g-(PS/PE) copolymers with high branching density at semi-solid state under CO2 batching foam. Polymer 2018, 146, 304–311. [Google Scholar] [CrossRef]
- Kuang, T.-R.; Mi, H.-Y.; Fu, D.-J.; Jing, X.; Chen, B.-y.; Mou, W.-J.; Peng, X.-F. Fabrication of poly(lactic acid)/graphene oxide foams with highly oriented and elongated cell structure via unidirectional foaming using supercritical carbon dioxide. Ind. Eng. Chem. Res. 2015, 54, 758–768. [Google Scholar] [CrossRef]
- Hwang, I.J.; Kim, B.K. Effect of the type of SAN in SAN/CPE blend: Morphology, mechanical, and rheological properties. J. Appl. Polym. Sci. 1998, 67, 27–36. [Google Scholar] [CrossRef]
- Mao, Z.; Zhang, J. Toughening effect of CPE on ASA/SAN binary blends at different temperatures. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Lee, K.-N.; Lee, H.-J.; Kim, J.-H. Preparation and morphology characterization of microcellular styrene-co-acrylonitrile (SAN) foam processed in supercritical CO2. Polym. Int. 2000, 49, 712–718. [Google Scholar] [CrossRef]
- Urbanczyk, L.; Calberg, C.; Detrembleur, C.; Jérôme, C.; Alexandre, M. Batch foaming of SAN/clay nanocomposites with scCO2: A very tunable way of controlling the cellular morphology. Polymer 2010, 51, 3520–3531. [Google Scholar] [CrossRef]
- Parks, K.L.; Beckman, E.J. Generation of microcellular polyurethane foams via polymerization in carbon dioxide. II: Foam formation and characterization. Polym. Eng. Sci. 1996, 36, 2417–2431. [Google Scholar] [CrossRef]
- Wu, C.; Akiyama, S.; Mabuchi, T.; Nitta, K.-H. Dynamic mechanical properties and morphologies of organic hybrids consisting of chlorinated polyethylene and hindered phenol. Polym. J. 2001, 33, 792. [Google Scholar] [CrossRef]
- Wu, C.; Yamagishi, T.-A.; Nakamoto, Y.; Ishida, S.; Nitta, K.-H.; Kubota, S. Organic hybrid of chlorinated polyethylene and hindered phenol. I. Dynamic mechanical properties. J. Polym. Sci. Part B: Polym. Phys. 2000, 38, 2285–2295. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Suzuki, K.I. Rheological properties and foam processability for blends of linear and crosslinked polyethylenes. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 2159–2167. [Google Scholar] [CrossRef]
- Abe, S.; Yamaguchi, M. Study on the foaming of crosslinked polyethylene. J. Appl. Polym. Sci. 2001, 79, 2146–2155. [Google Scholar] [CrossRef]
- Colton, J.S.; Suh, N.P. The nucleation of microcellular thermoplastic foam with additives: Part I: Theoretical considerations. Polym. Eng. Sci. 1987, 27, 485–492. [Google Scholar] [CrossRef]
- Di Maio, E.; Kiran, E. Foaming of polymers with supercritical fluids and perspectives on the current knowledge gaps and challenges. J. Supercrit. Fluids 2018, 134, 157–166. [Google Scholar] [CrossRef]
- Zhai, W.; Yu, J.; Wu, L.; Ma, W.; He, J. Heterogeneous nucleation uniformizing cell size distribution in microcellular nanocomposites foams. Polymer 2006, 47, 7580–7589. [Google Scholar] [CrossRef]
- Xu, Q.; Ren, X.; Chang, Y.; Wang, J.; Yu, L.; Dean, K. Generation of microcellular biodegradable polycaprolactone foams in supercritical carbon dioxide. J. Appl. Polym. Sci. 2004, 94, 593–597. [Google Scholar] [CrossRef]
- Siddiq, A.R.; Kennedy, A.R. Porous poly-ether ether ketone (PEEK) manufactured by a novel powder route using near-spherical salt bead porogens: Characterisation and mechanical properties. Mater. Sci. Eng. C 2015, 47, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Zhang, Q.; Shi, S.; Zhu, S. Highly porous poly(high internal phase emulsion) membranes with “open-cell” structure and CO2-switchable wettability used for controlled oil/water separation. Langmuir 2017, 33, 11936–11944. [Google Scholar] [CrossRef]
- Goel, S.K.; Beckman, E.J. Generation of microcellular polymeric foams using supercritical carbon dioxide. II: Cell growth and skin formation. Polym. Eng. Sci. 1994, 34, 1148–1156. [Google Scholar] [CrossRef]
- Collias, D.I.; Baird, D.G. Impact behavior of microcellular foams of polystyrene and styrene-acrylonitrile copolymer, and single-edge-notched tensile toughness of microcellular foams of polystyrene, styrene-acrylonitrile copolymer, and palycarbonate. Polym. Eng. Sci. 1995, 35, 1178–1183. [Google Scholar] [CrossRef]
- Gibson, L.; Ashby, M. The mechanics of foams: Basic results. In Cellular Solids: Structure and Properties, 2nd ed.; Cambridge University Press: Cambridge, UK, 1997; pp. 175–234. ISBN 9781139878326. [Google Scholar]

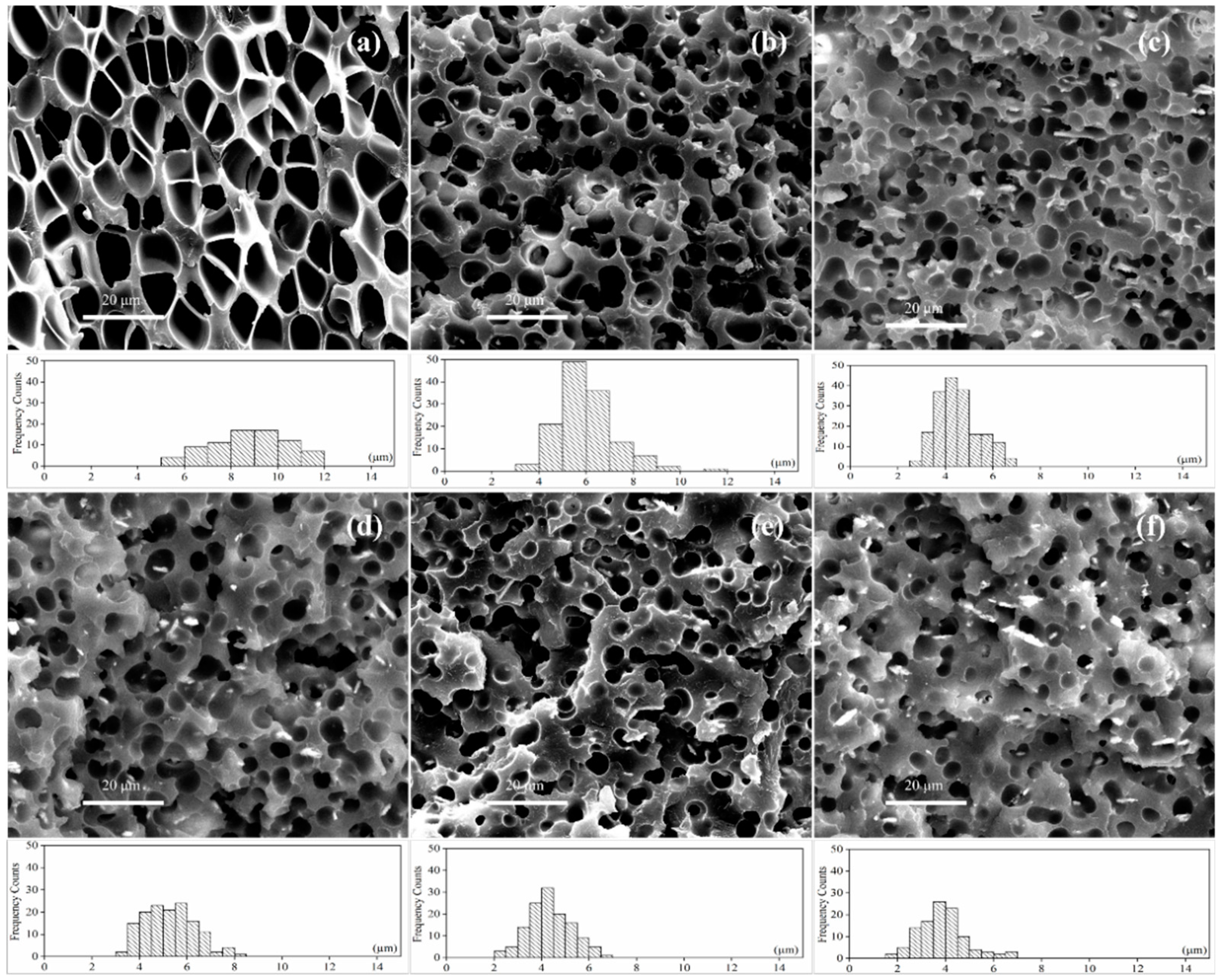
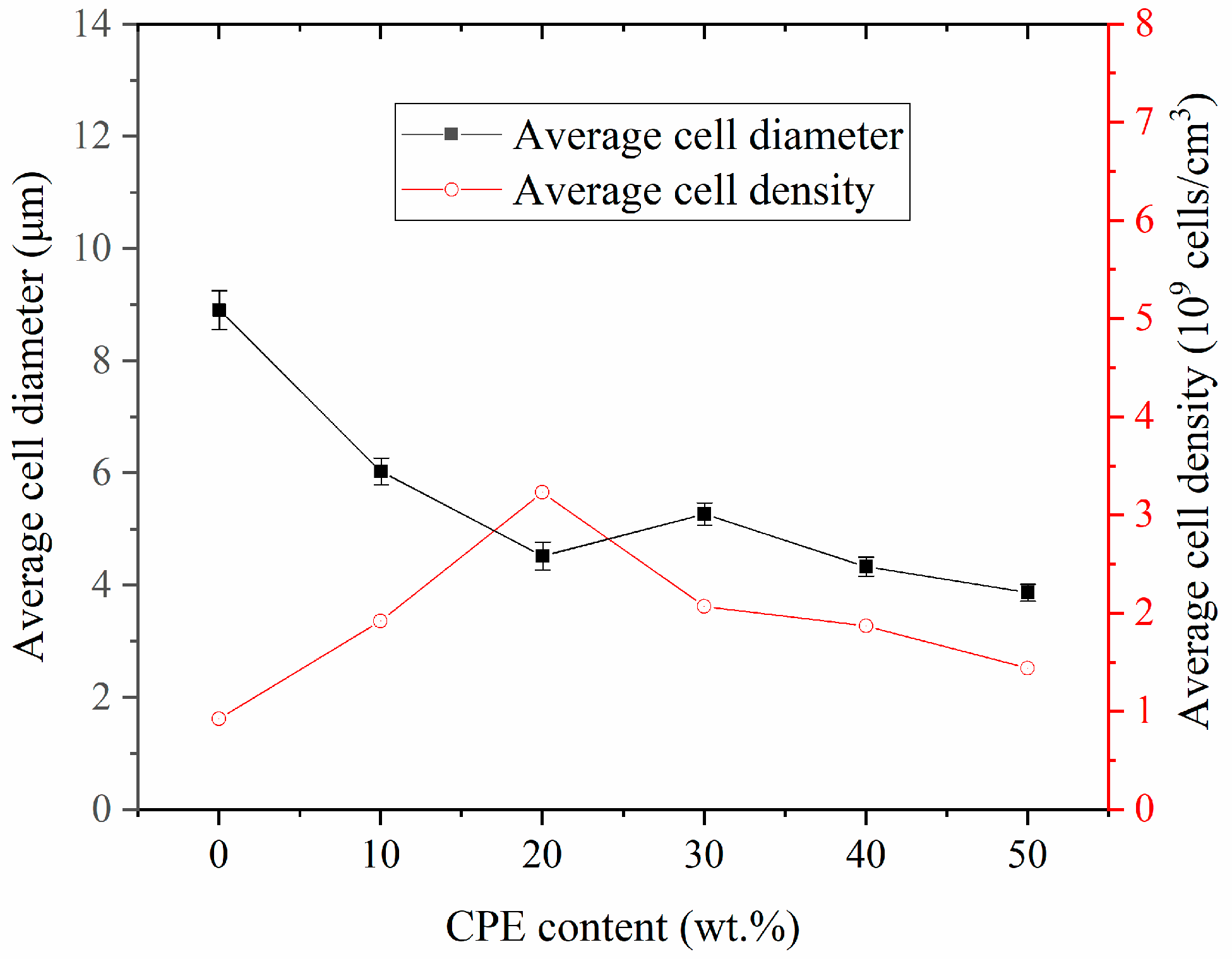


| Temperature (°C) | Average Cell Diameter (μm) | Cell Density (Cells/cm3) |
|---|---|---|
| 70 | 2.31 ± 0.16 | 1.32 × 109 |
| 90 | 3.67 ± 0.09 | 1.52 × 109 |
| 110 | 3.83 ± 0.12 | 1.68 × 109 |
| 130 | 4.02 ± 0.11 | 2.07 × 109 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.-C.; Yu, C.-N.; Liang, Y.; Lin, G.-X.; Meng, C. Foaming Behavior and Microcellular Morphologies of Incompatible SAN/CPE Blends with Supercritical Carbon Dioxide as a Physical Blowing Agent. Polymers 2019, 11, 89. https://doi.org/10.3390/polym11010089
Zhang H-C, Yu C-N, Liang Y, Lin G-X, Meng C. Foaming Behavior and Microcellular Morphologies of Incompatible SAN/CPE Blends with Supercritical Carbon Dioxide as a Physical Blowing Agent. Polymers. 2019; 11(1):89. https://doi.org/10.3390/polym11010089
Chicago/Turabian StyleZhang, Hai-Chen, Chun-Na Yu, Yong Liang, Gui-Xiang Lin, and Cong Meng. 2019. "Foaming Behavior and Microcellular Morphologies of Incompatible SAN/CPE Blends with Supercritical Carbon Dioxide as a Physical Blowing Agent" Polymers 11, no. 1: 89. https://doi.org/10.3390/polym11010089
APA StyleZhang, H.-C., Yu, C.-N., Liang, Y., Lin, G.-X., & Meng, C. (2019). Foaming Behavior and Microcellular Morphologies of Incompatible SAN/CPE Blends with Supercritical Carbon Dioxide as a Physical Blowing Agent. Polymers, 11(1), 89. https://doi.org/10.3390/polym11010089






